Abstract
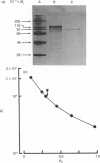
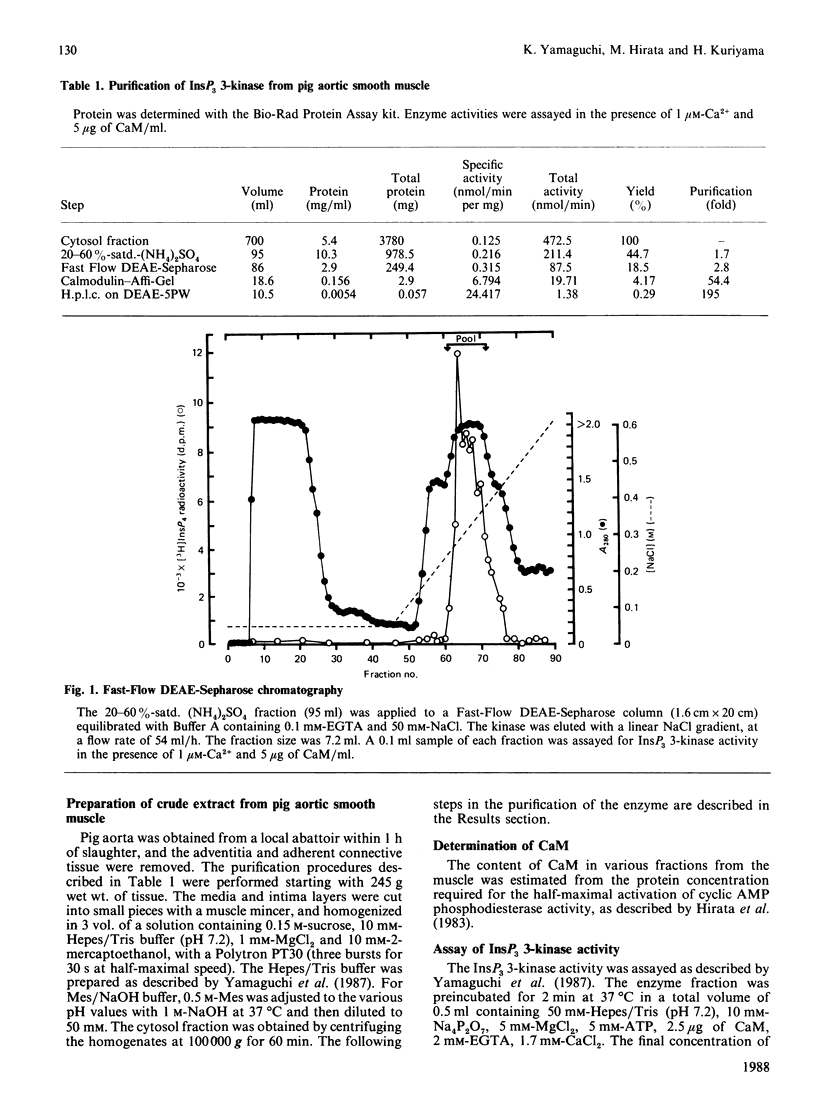
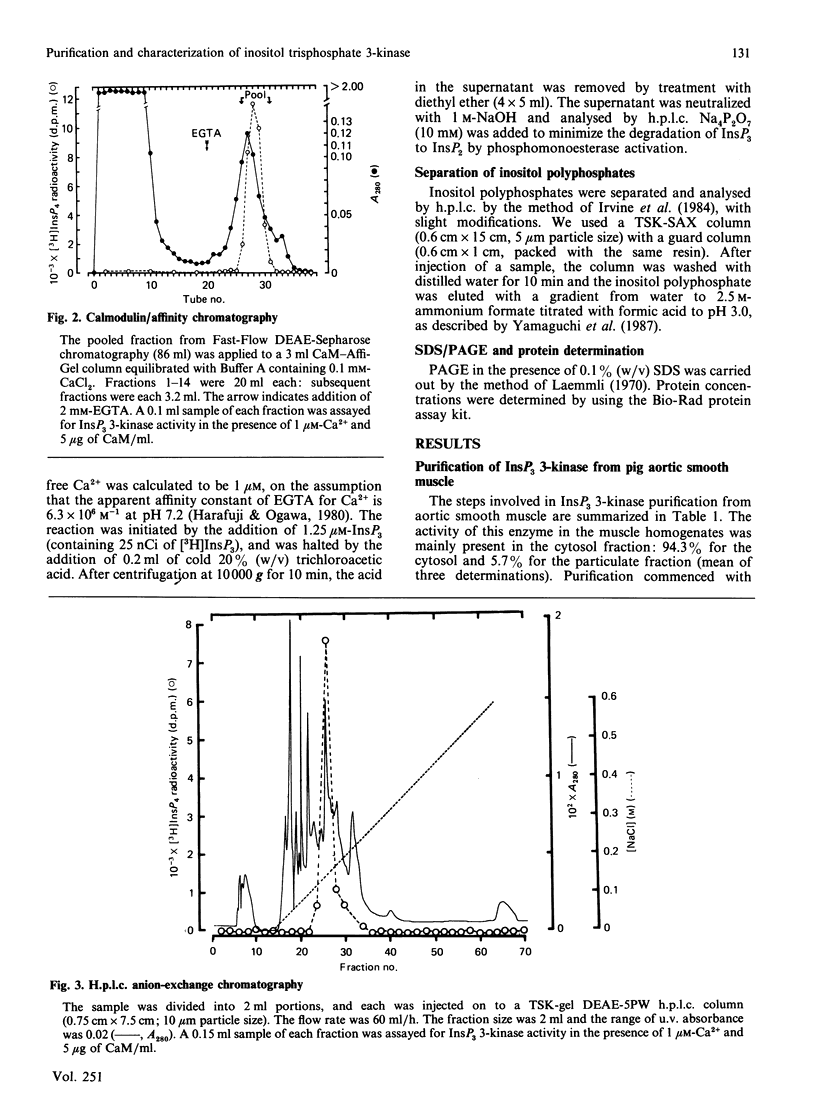
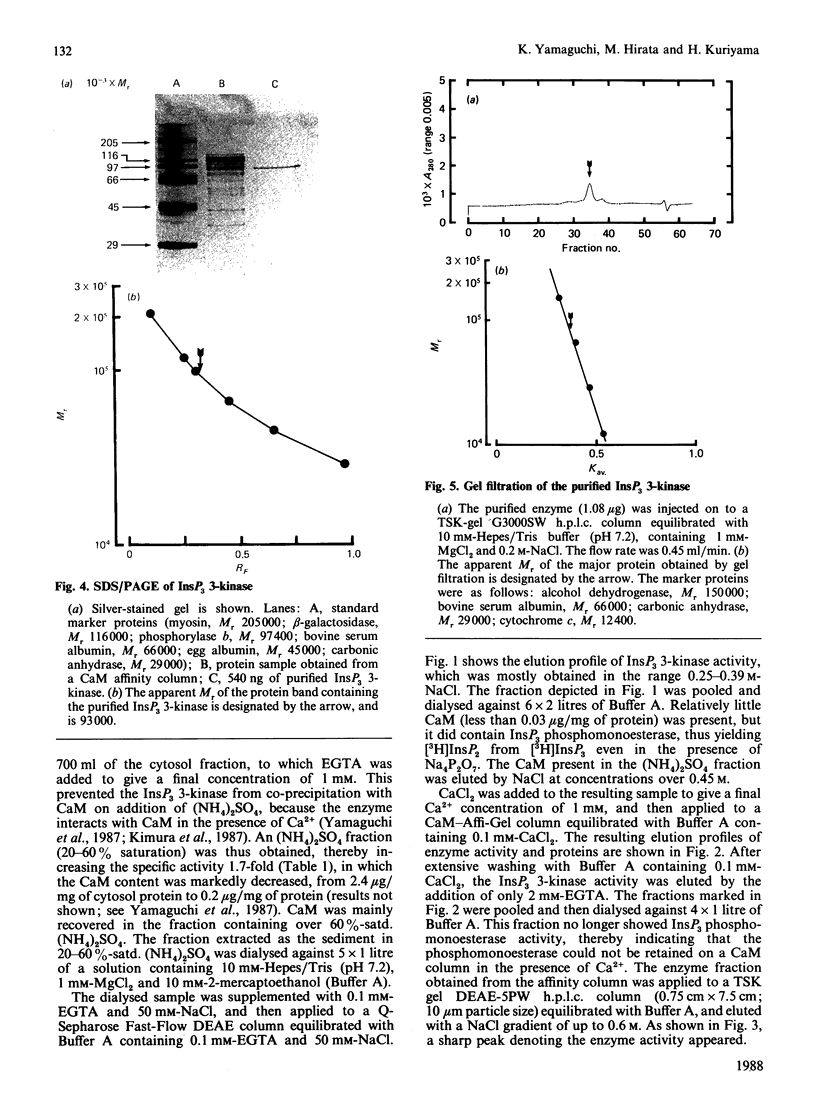
Inositol 1,4,5-trisphosphate (InsP3) 3-kinase, which phosphorylates InsP3 to form inositol 1,3,4,5-tetrakisphosphate, was purified to apparent homogeneity by (NH4)2SO4 fractionation and sequential chromatographic steps on DEAE-sepharose, calmodulin-Affi-Gel and DEAE-5PW h.p.l.c. The purified enzyme had a specific activity of 24.4 nmol of inositol tetrakisphosphate formed/min per mg of protein, which represented a purification of approx. 195-fold with a 0.29% recovery, compared with the cytosol fraction of the muscle. SDS/polyacrylamide-gel electrophoresis showed a single protein-staining band of Mr 93,000. Moreover, the major protein peak, of Mr 84,000, was detected by TSK gel G3000SW gel-permeation chromatography of the purified sample. As this value was approximately consistent with the Mr determined by SDS/polyacrylamide-gel-electrophoretic analysis, the InsP3 3-kinase might be a monomeric enzyme. The purified enzyme had a Km for InsP3 of 0.4 microM, with an optimum pH range of 5.8-7.7. The enzyme was maximally activated by calmodulin, with a stoichiometry of 1:1.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification and characterization of smooth muscle myosin light chain kinase. J Biol Chem. 1981 Jul 25;256(14):7501–7509. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRADO C., BALLOU C. E. Myo-inositol phosphates obtained by alkaline hydrolysis of beef brain phosphoinositide. J Biol Chem. 1961 Jan;236:54–60. [PubMed] [Google Scholar]
- Hansen C. A., Mah S., Williamson J. R. Formation and metabolism of inositol 1,3,4,5-tetrakisphosphate in liver. J Biol Chem. 1986 Jun 25;261(18):8100–8103. [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Hirata M., Itoh T., Kanmura Y., Kuriyama H. Inositol 1,4,5-trisphosphate activates pharmacomechanical coupling in smooth muscle of the rabbit mesenteric artery. J Physiol. 1986 Jan;370:605–618. doi: 10.1113/jphysiol.1986.sp015953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida H., Brown D. A. Membrane current responses to intracellular injections of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in NG108-15 hybrid cells. FEBS Lett. 1986 Nov 24;208(2):283–286. doi: 10.1016/0014-5793(86)81033-x. [DOI] [PubMed] [Google Scholar]
- Hirata M., Sasaguri T., Hamachi T., Hashimoto T., Kukita M., Koga T. Irreversible inhibition of Ca2+ release in saponin-treated macrophages by the photoaffinity derivative of inositol-1, 4, 5-trisphosphate. Nature. 1985 Oct 24;317(6039):723–725. doi: 10.1038/317723a0. [DOI] [PubMed] [Google Scholar]
- Hirata M., Suematsu E., Koga T. Effect of calmodulin and calmodulin antagonists on the Ca2+ uptake by the intracellular Ca2+-accumulating system of guinea pig peritoneal macrophages treated with saponin. Mol Pharmacol. 1983 Jan;23(1):78–85. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Downes C. P. Inositol trisphosphates in carbachol-stimulated rat parotid glands. Biochem J. 1984 Oct 1;223(1):237–243. doi: 10.1042/bj2230237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Greengard P. Two calcium/calmodulin-dependent protein kinases, which are highly concentrated in brain, phosphorylate protein I at distinct sites. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1293–1297. doi: 10.1073/pnas.78.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y., Hirata M., Yamaguchi K., Koga T. Activation by calmodulin of inositol-1,4,5-trisphosphate 3-kinase in guinea pig peritoneal macrophages. Arch Biochem Biophys. 1987 Sep;257(2):363–369. doi: 10.1016/0003-9861(87)90578-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Rossier M. F., Dentand I. A., Lew P. D., Capponi A. M., Vallotton M. B. Interconversion of inositol (1,4,5)-trisphosphate to inositol (1,3,4,5)-tetrakisphosphate and (1,3,4)-trisphosphate in permeabilized adrenal glomerulosa cells is calcium-sensitive and ATP-dependent. Biochem Biophys Res Commun. 1986 Aug 29;139(1):259–265. doi: 10.1016/s0006-291x(86)80107-3. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Bond M., Somlyo A. P., Scarpa A. Inositol trisphosphate-induced calcium release and contraction in vascular smooth muscle. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5231–5235. doi: 10.1073/pnas.82.15.5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Hashimoto T., Kuriyama H. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun. 1984 Apr 30;120(2):481–485. doi: 10.1016/0006-291x(84)91279-8. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Sasaguri T., Hashimoto T., Kuriyama H. Roles of Ca2+ on the inositol 1,4,5-trisphosphate-induced release of Ca2+ from saponin-permeabilized single cells of the porcine coronary artery. Comp Biochem Physiol A Comp Physiol. 1985;82(3):645–649. doi: 10.1016/0300-9629(85)90446-3. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Hirata M., Kuriyama H. Calmodulin activates inositol 1,4,5-trisphosphate 3-kinase activity in pig aortic smooth muscle. Biochem J. 1987 Jun 15;244(3):787–791. doi: 10.1042/bj2440787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., van Breemen C. Inositol-1,4,5-trisphosphate releases calcium from skinned cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):270–274. doi: 10.1016/0006-291x(85)90412-7. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Sakuma M., Yagi K. Calmodulins from muscles of marine invertebrates, scallop and sea anemone. J Biochem. 1980 May;87(5):1313–1320. doi: 10.1093/oxfordjournals.jbchem.a132869. [DOI] [PubMed] [Google Scholar]