Abstract
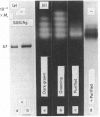
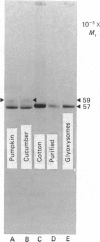
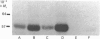
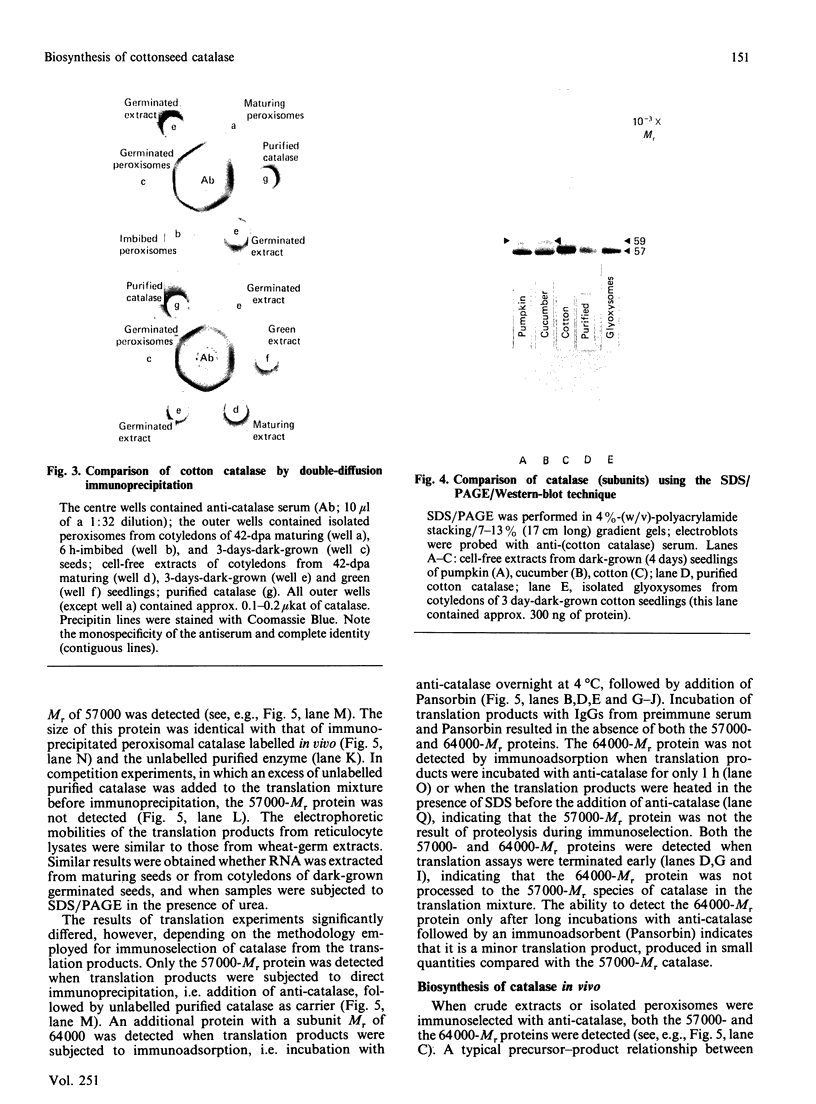
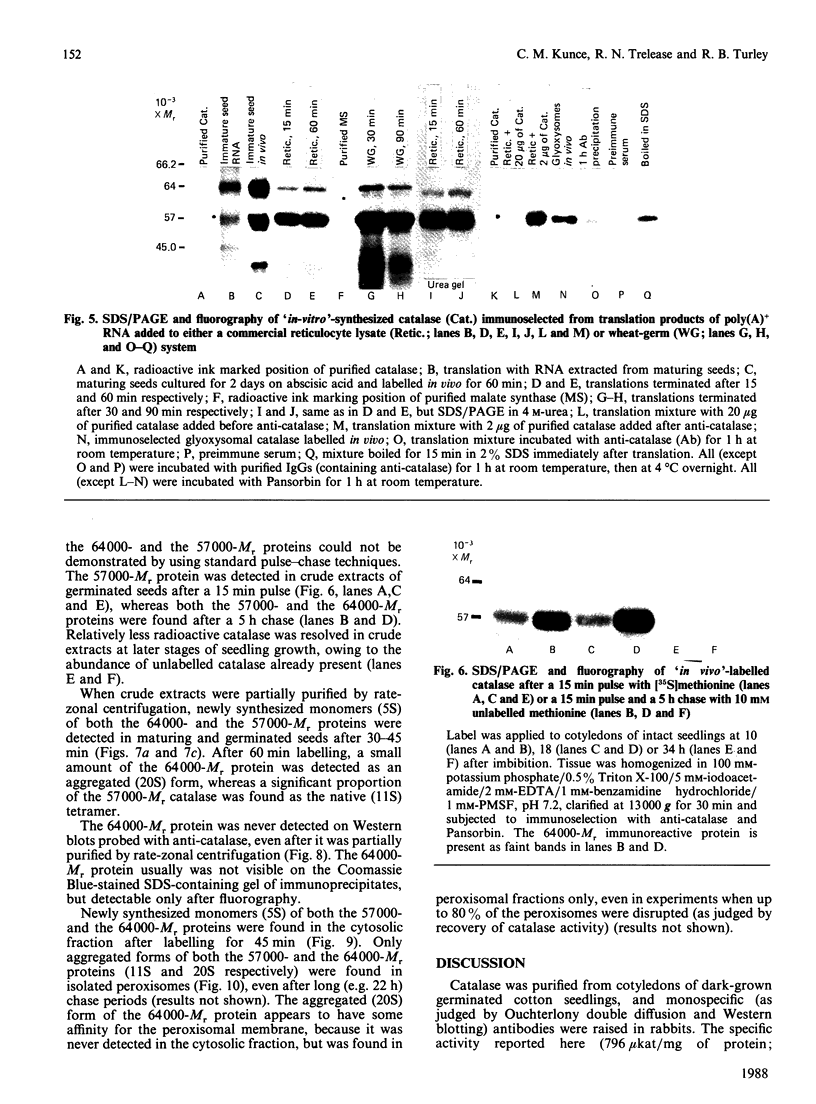
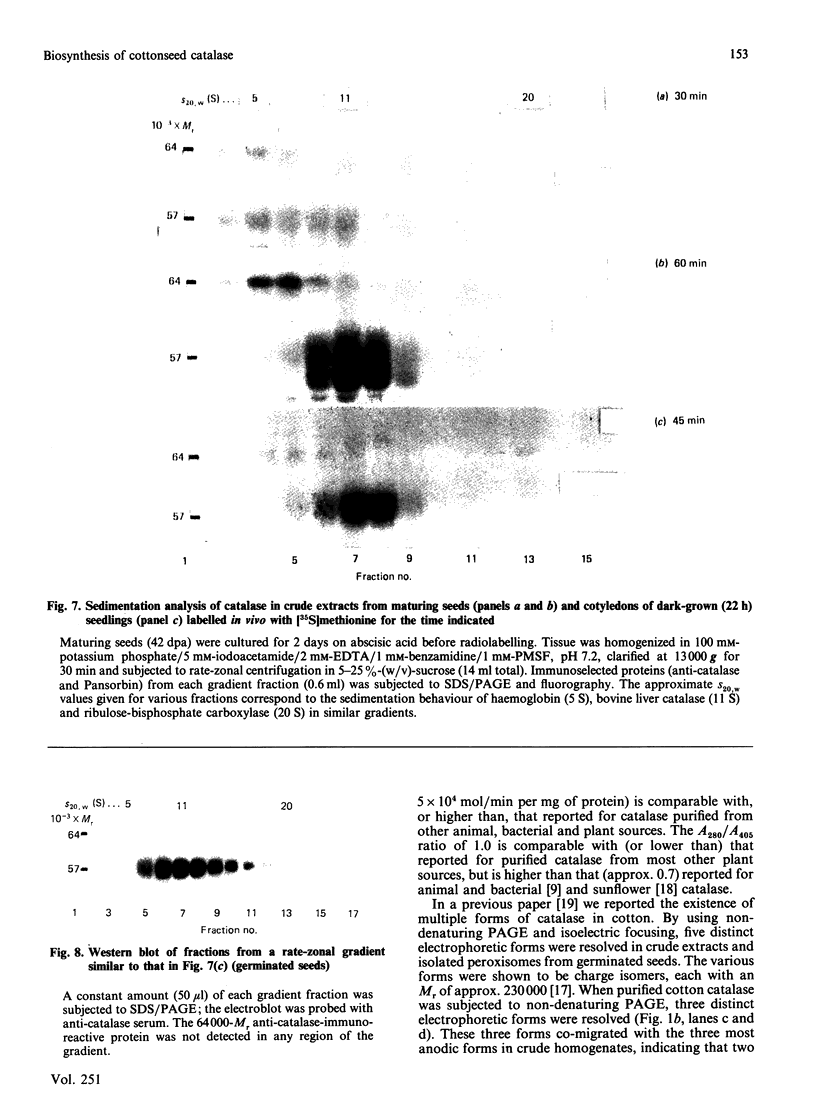
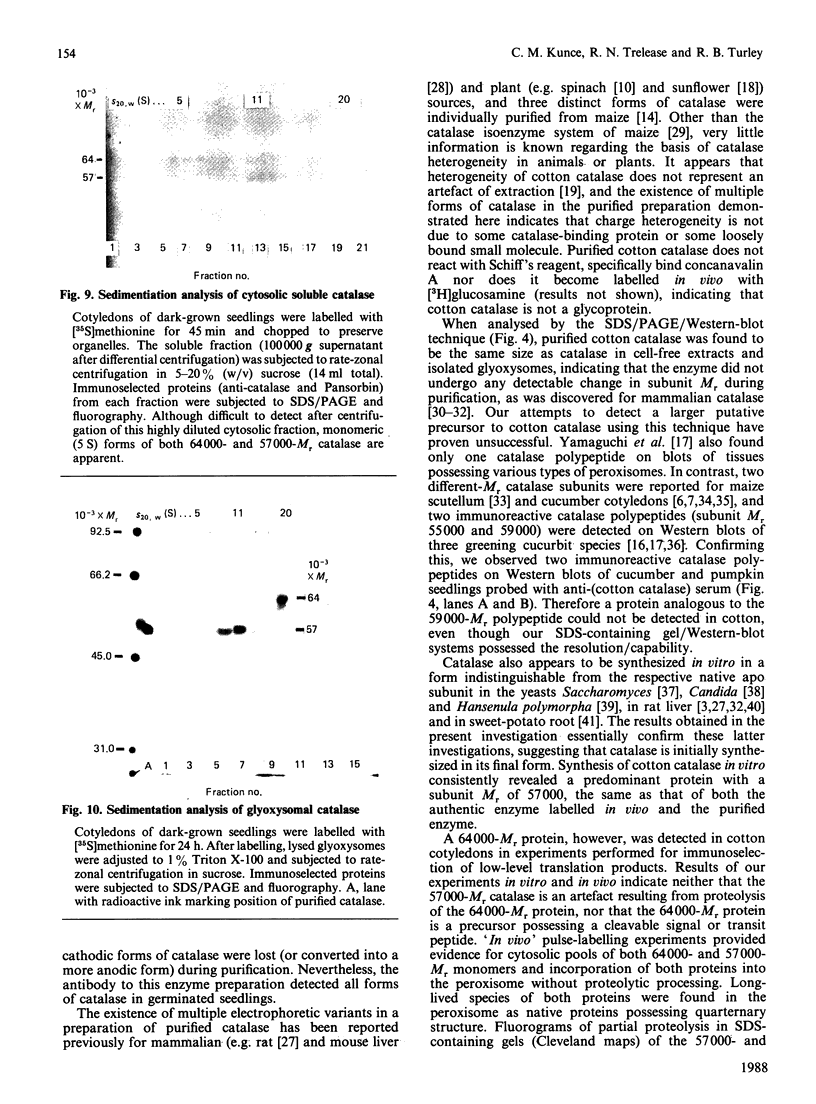
As part of our research on peroxisome biogenesis, catalase was purified from cotyledons of dark-grown cotton (Gossypium hirsutum L.) seedlings and monospecific antibodies were raised in rabbits. Purified catalase appeared as three distinct electrophoretic forms in non-denaturing gels and as a single protein band (with a subunit Mr of 57,000) on silver-stained SDS/polyacrylamide gels. Western blots of crude extracts and isolated peroxisomes from cotton revealed one immunoreactive polypeptide with the same Mr (57,000) as the purified enzyme, indicating that catalase did not undergo any detectable change in Mr during purification. Synthesis in vitro, directed by polyadenylated RNA isolated from either maturing seeds or cotyledons of dark-grown cotton seedlings, revealed a predominant immunoreactive translation product with a subunit Mr of 57,000 and an additional minor immunoreactive product with a subunit Mr of 64000. Labelling studies in vivo revealed newly synthesized monomers of both the 64000- and 57,000-Mr proteins present in the cytosol and incorporation of both proteins into the peroxisome without proteolytic processing. Within the peroxisome, the 57,000-Mr catalase was found as an 11S tetramer; whereas the 64,000-Mr protein was found as a relatively long-lived 20S aggregate (native Mr approx. 600,000-800,000). The results strongly indicate that the 64,000-Mr protein (catalase?) is not a precursor to the 57,000-Mr catalase and that cotton catalase is translated on cytosolic ribosomes without a cleavable transit or signal sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G., Richter K., Hartter E., Ruis H. Synthesis of Saccharomyces cerevisiae catalase A in vitro. Eur J Biochem. 1981 Jan;113(2):327–331. doi: 10.1111/j.1432-1033.1981.tb05070.x. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Borst P. How proteins get into microbodies (peroxisomes, glyoxysomes, glycosomes). Biochim Biophys Acta. 1986 May 5;866(4):179–203. doi: 10.1016/0167-4781(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman B. M., Blobel G. Biogenesis of peroxisomes: intracellular site of synthesis of catalase and uricase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5066–5070. doi: 10.1073/pnas.75.10.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. L., Masters C. J. On the nature and characteristics of the multiple forms of catalase in mouse liver. Arch Biochem Biophys. 1975 Jul;169(1):7–21. doi: 10.1016/0003-9861(75)90311-2. [DOI] [PubMed] [Google Scholar]
- Kindl H. The biosynthesis of microbodies (peroxisomes, glyoxysomes). Int Rev Cytol. 1982;80:193–229. doi: 10.1016/s0074-7696(08)60370-8. [DOI] [PubMed] [Google Scholar]
- Kunce C. M., Trelease R. N. Heterogeneity of catalase in maturing and germinated cotton seeds. Plant Physiol. 1986 Aug;81(4):1134–1139. doi: 10.1104/pp.81.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. E., Riezman H., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 2. Isolation and Immunological Detection of Isocitrate Lyase and Catalase. Plant Physiol. 1978 Nov;62(5):754–760. doi: 10.1104/pp.62.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarow P. B., Fujiki Y. Biogenesis of peroxisomes. Annu Rev Cell Biol. 1985;1:489–530. doi: 10.1146/annurev.cb.01.110185.002421. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B., Robbi M., Fujiki Y., Wong L. Biogenesis of peroxisomal proteins in vivo and in vitro. Ann N Y Acad Sci. 1982;386:285–300. doi: 10.1111/j.1749-6632.1982.tb21423.x. [DOI] [PubMed] [Google Scholar]
- Mainferme F., Wattiaux R. Effect of lysosomes on rat-liver catalase. Eur J Biochem. 1982 Oct;127(2):343–346. doi: 10.1111/j.1432-1033.1982.tb06877.x. [DOI] [PubMed] [Google Scholar]
- Masters C. J. On the turnover and multiplicity of peroxisomal catalases. Ann N Y Acad Sci. 1982;386:301–313. doi: 10.1111/j.1749-6632.1982.tb21424.x. [DOI] [PubMed] [Google Scholar]
- Rachubinski R. A., Fujiki Y., Mortensen R. M., Lazarow P. B. Acyl-Coa oxidase and hydratase-dehydrogenase, two enzymes of the peroxisomal beta-oxidation system, are synthesized on free polysomes of clofibrate-treated rat liver. J Cell Biol. 1984 Dec;99(6):2241–2246. doi: 10.1083/jcb.99.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H., Weir E. M., Leaver C. J., Titus D. E., Becker W. M. Regulation of Glyoxysomal Enzymes during Germination of Cucumber: 3. IN VITRO TRANSLATION AND CHARACTERIZATION OF FOUR GLYOXYSOMAL ENZYMES. Plant Physiol. 1980 Jan;65(1):40–46. doi: 10.1104/pp.65.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa M., Blobel G. Biosynthesis of peroxisomal enzymes in the methylotrophic yeast Hansenula polymorpha. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6872–6876. doi: 10.1073/pnas.80.22.6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbi M., Lazarow P. B. Peptide mapping of peroxisomal catalase and its precursor. Comparison to the primary wheat germ translation product. J Biol Chem. 1982 Jan 25;257(2):964–970. [PubMed] [Google Scholar]
- Robbi M., Lazarow P. B. Synthesis of catalase in two cell-free protein-synthesizing systems and in rat liver. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4344–4348. doi: 10.1073/pnas.75.9.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer S., Teifel W., Kindl H. Plant microbody proteins, I. Purification and characterization of catalase from leaves of lens culinaris. Hoppe Seylers Z Physiol Chem. 1976 Feb;357(2):163–175. doi: 10.1515/bchm2.1976.357.1.163. [DOI] [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Evidence for processing of maize catalase 2 and purification of its messenger RNA aided by translation of antibody-bound polysomes. Biochemistry. 1986 Apr 22;25(8):2027–2032. doi: 10.1021/bi00356a029. [DOI] [PubMed] [Google Scholar]
- Sugita Y., Tobe T., Sakamoto T., Higashi T. Immature precursor catalase in subcellular fractions of rat liver. J Biochem. 1982 Aug;92(2):509–515. doi: 10.1093/oxfordjournals.jbchem.a133958. [DOI] [PubMed] [Google Scholar]
- Trelease R. N., Hermerath C. A., Turley R. B., Kunce C. M. Cottonseed malate synthase : purification and immunochemical characterization. Plant Physiol. 1987 Aug;84(4):1343–1349. doi: 10.1104/pp.84.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yamada T., Tanaka A., Horikawa S., Numa S., Fukui S. Cell-free translation and regulation of Candida tropicalis catalase messenger RNA. Eur J Biochem. 1982 Dec 15;129(2):251–255. doi: 10.1111/j.1432-1033.1982.tb07046.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M., Akazawa T. Maturation of catalase precursor proceeds to a different extent in glyoxysomes and leaf peroxisomes of pumpkin cotyledons. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4809–4813. doi: 10.1073/pnas.81.15.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M., Akazawa T. Purification and characterization of heme-containing low-activity form of catalase from greening pumpkin cotyledons. Eur J Biochem. 1986 Sep 1;159(2):315–322. doi: 10.1111/j.1432-1033.1986.tb09870.x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J., Nishimura M. Purification of glyoxysomal catalase and immunochemical comparison of glyoxysomal and leaf peroxisomal catalase in germinating pumpkin cotyledons. Plant Physiol. 1984 Feb;74(2):261–267. doi: 10.1104/pp.74.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]