Abstract
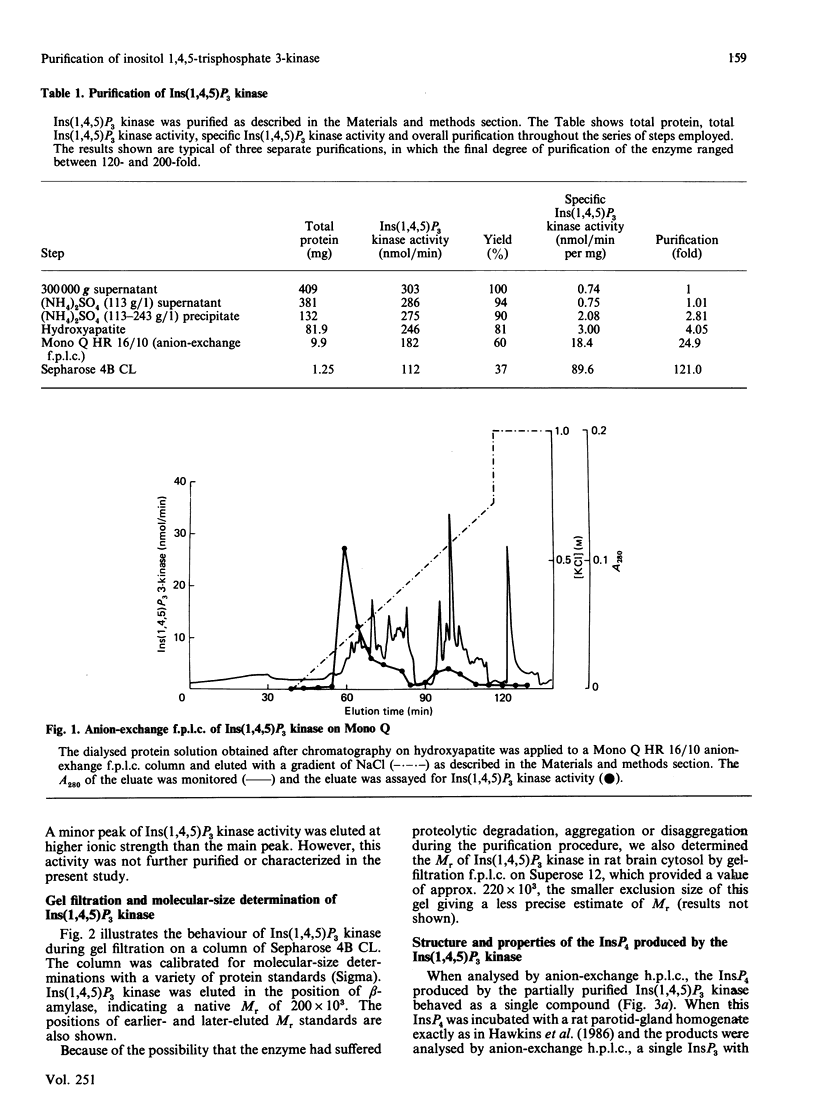
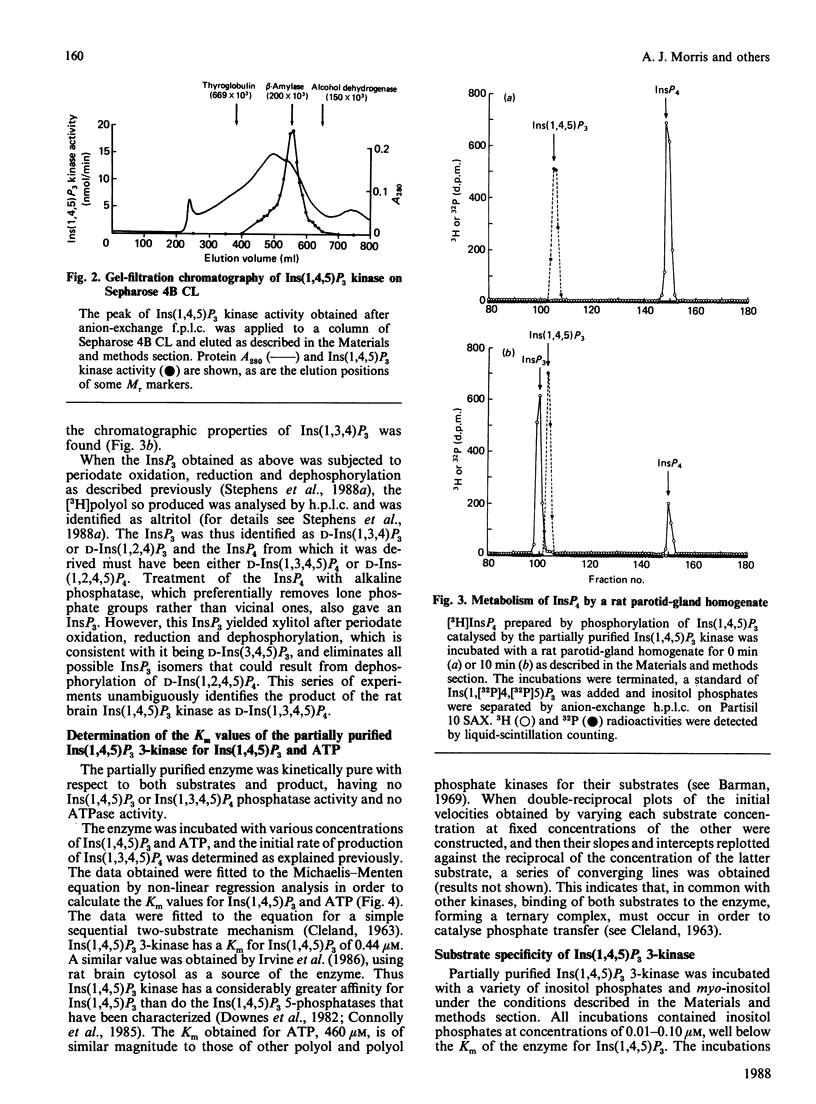
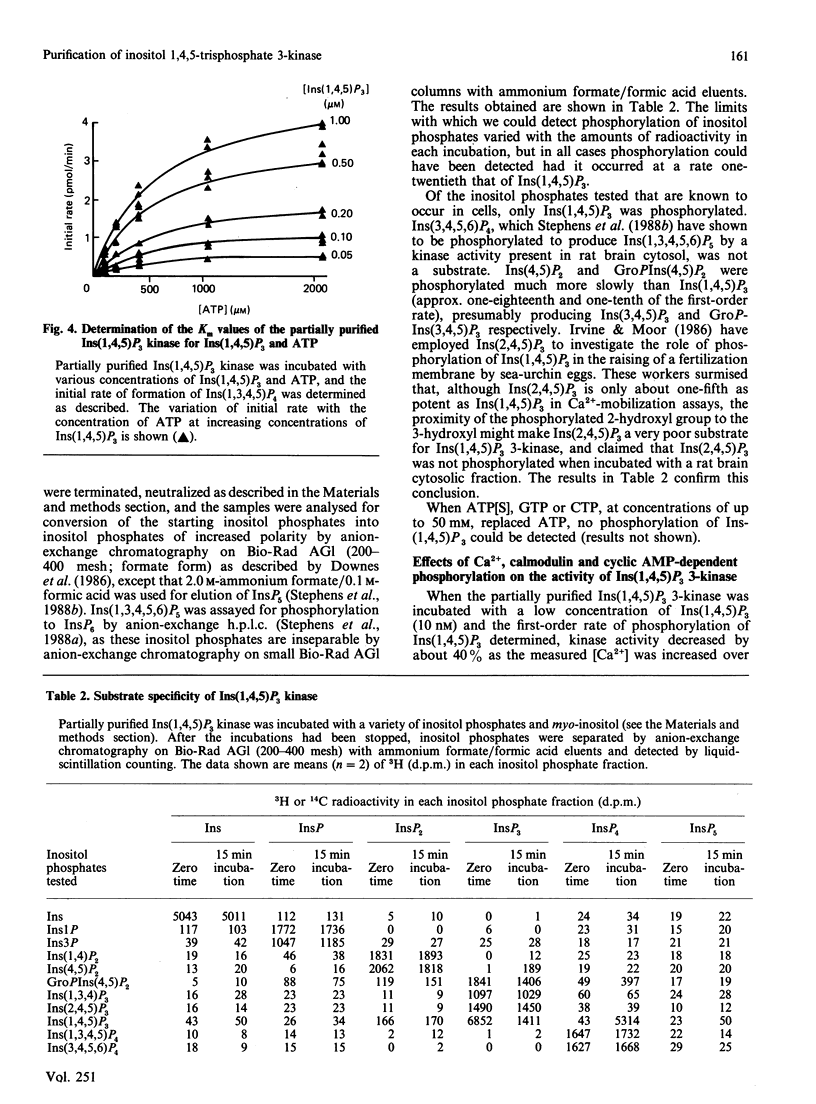
An enzyme which catalyses the ATP-dependent phosphorylation of inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] was purified approx. 180-fold from rat brain cytosol by (NH4)2SO4 precipitation, chromatography through hydroxyapatite, anion-exchange fast protein liquid chromatography and gel-filtration chromatography. Gel filtration on Sepharose 4B CL gives an Mr of 200 x 10(3) for the native enzyme. The inositol tetrakisphosphate (InsP4) produced by the enzyme has the chromatographic, chemical and metabolic properties of Ins(1,3,4,5)P4. Ins(1,4,5)P3 3-kinase displays simple Michaelis-Menten kinetics for both its substrates, having Km values of 460 microM and 0.44 microM for ATP and Ins(1,4,5)P3 respectively. When many of the inositol phosphates known to occur in cells were tested, only Ins(1,4,5)P3 was a substrate for the enzyme; the 2,4,5-trisphosphate was not phosphorylated. Inositol 4,5-bisphosphate and glycerophosphoinositol 4,5-bisphosphate were phosphorylated much more slowly than Ins(1,4,5)P3. CTP, GTP and adenosine 5'-[gamma-thio]triphosphate were unable to substitute for ATP. When assayed under conditions of first-order kinetics, Ins(1,4,5)P3 kinase activity decreased by about 40% as the [Ca2+] was increased over the physiologically relevant range. This effect was insensitive to the presence of calmodulin and appeared to be the result of an increase in the Km of the enzyme for Ins(1,4,5)P3. Preincubation with ATP and the purified catalytic subunit of cyclic AMP-dependent protein kinase did not affect the rate of phosphorylation of Ins(1,4,5)P3 when the enzyme was assayed at saturating concentrations of Ins(1,4,5)P3 or at concentrations close to its Km for this substrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Comte M., Cox J. A., Wollheim C. B. Calcium-calmodulin stimulates inositol 1,4,5-trisphosphate kinase activity from insulin-secreting RINm5F cells. J Biol Chem. 1987 Jul 15;262(20):9437–9440. [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Connolly T. M., Bross T. E., Majerus P. W. Isolation of a phosphomonoesterase from human platelets that specifically hydrolyzes the 5-phosphate of inositol 1,4,5-trisphosphate. J Biol Chem. 1985 Jul 5;260(13):7868–7874. [PubMed] [Google Scholar]
- Dedman J. R., Kaetzel M. A. Calmodulin purification and fluorescent labeling. Methods Enzymol. 1983;102:1–8. doi: 10.1016/s0076-6879(83)02003-0. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Moor R. M. Micro-injection of inositol 1,3,4,5-tetrakisphosphate activates sea urchin eggs by a mechanism dependent on external Ca2+. Biochem J. 1986 Dec 15;240(3):917–920. doi: 10.1042/bj2400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. J., Downes C. P., Harden T. K., Michell R. H. Turkey erythrocytes possess a membrane-associated inositol 1,4,5-trisphosphate 3-kinase that is activated by Ca2+ in the presence of calmodulin. Biochem J. 1987 Dec 1;248(2):489–493. doi: 10.1042/bj2480489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Reimann E. M., Beham R. A. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:51–55. doi: 10.1016/0076-6879(83)99039-0. [DOI] [PubMed] [Google Scholar]
- Rossier M. F., Dentand I. A., Lew P. D., Capponi A. M., Vallotton M. B. Interconversion of inositol (1,4,5)-trisphosphate to inositol (1,3,4,5)-tetrakisphosphate and (1,3,4)-trisphosphate in permeabilized adrenal glomerulosa cells is calcium-sensitive and ATP-dependent. Biochem Biophys Res Commun. 1986 Aug 29;139(1):259–265. doi: 10.1016/s0006-291x(86)80107-3. [DOI] [PubMed] [Google Scholar]
- Sharps E. S., McCarl R. L. A high-performance liquid chromatographic method to measure 32P incorporation into phosphorylated metabolites in cultured cells. Anal Biochem. 1982 Aug;124(2):421–424. doi: 10.1016/0003-2697(82)90059-8. [DOI] [PubMed] [Google Scholar]
- Stephens L. R., Hawkins P. T., Morris A. J., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate (3-hydroxy)kinase. Biochem J. 1988 Jan 1;249(1):283–292. doi: 10.1042/bj2490283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L., Hawkins P. T., Carter N., Chahwala S. B., Morris A. J., Whetton A. D., Downes P. C. L-myo-inositol 1,4,5,6-tetrakisphosphate is present in both mammalian and avian cells. Biochem J. 1988 Jan 1;249(1):271–282. doi: 10.1042/bj2490271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Hirata M., Kuriyama H. Calmodulin activates inositol 1,4,5-trisphosphate 3-kinase activity in pig aortic smooth muscle. Biochem J. 1987 Jun 15;244(3):787–791. doi: 10.1042/bj2440787. [DOI] [PMC free article] [PubMed] [Google Scholar]


