Abstract
Cervical cancer, despite being preventable through primary and secondary prevention strategies, remains one of the leading causes of morbidity and mortality among women in Brazil. This study aimed to analyze the temporal, spatial, and space-time patterns of cervical cancer mortality in Brazil. An ecological study was conducted using temporal, spatial, and space-time analysis techniques, using death certificates with cervical cancer as the underlying cause or associated condition among females in Brazil from 2000 to 2021. Death certificate and population data were provided by the Department of Health Informatics of the Unified Health System (DATASUS) and the Brazilian Institute of Geography and Statistics (IBGE), respectively. A total of 123,306 deaths associated with cervical cancer among women were registered during the study period. A rising trend in mortality was detected since 2014 onwards, after 14 years of decline. Particularly, an increase in mortality was observed among the younger age groups, and in the North and Northeast regions regardless of age. Heterogeneity in the spatial distribution of cervical cancer mortality was observed, with high mortality clusters around the country, but mostly concentrated in the North and Northeast regions. These findings suggest a need and an opportunity to develop efficient and effective health policies targeting those regions and groups of women at higher risk which in turn will allow for fast and significant reductions in cervical cancer mortality in Brazil.
Keywords: Cervical cancer, Mortality, Time series studies, Spatial analysis, Spatio-temporal analysis
Subject terms: Cancer, Cancer epidemiology
Introduction
Cervical cancer, although preventable through primary prevention by Human Papillomavirus (HPV) vaccination, and secondary prevention by screening strategies, is one of the leading causes of cancer morbidity and mortality among women worldwide1. It ranks as the fourth most common cancer and the fourth leading cause of death by cancer in females1. In 2020, approximately 604,000 new cases and 342,000 deaths were recorded globally due to this disease1. In this context, the World Health Organization (WHO) issued a call to all countries to direct efforts towards the elimination of cervical cancer as a public health problem2.
Despite significant efforts and resources invested, most countries including Brazil have continued to experience high morbidity and mortality rates3. According to Brazil’s National Cancer Institute (INCA), excluding non-melanoma skin tumors, cervical cancer in Brazil ranks third in the number of cases and fourth in deaths among women3. In 2020, the mortality rate for cervical cancer in the country, age-adjusted to the World Standard Population, was 4.6 deaths per 100,000 women, representing 6.1% of the total deaths in the female population3. Historically, deaths from cervical cancer in Brazil are rare among women under 30 years old. Mortality progressively increases from the fourth decade of life3.
The main risk factor for the development of cervical cancer is having a persistent infection with an oncogenic type of HPV, which is linked to virtually all cases of the disease4. HPV is the most common sexually transmitted agent, and most infections are controlled quickly, or at least within 1–2 years, without causing disease. Behavioral and other risk factors (e.g., smoking, prolonged use of oral contraceptives, multiparity) are minor influences on risk of causing precancer/cancer5. Immunosuppression due to Human Immunodeficiency Virus (HIV) is an important risk factor because it diminishes the typical cell-mediated clearance mechanism6,7. Additionally, the literature highlights other factors related to social determinants of health, such as low socioeconomic status, difficulty in accessing healthcare services, social inequality, and lower Human Development Index (HDI)1.
Brazil, with its continental dimensions and local-regional disparities, faces a complex scenario regarding cervical cancer, with incidence and mortality varying considerably between regions, along with specific challenges related to access to services and feasibility for implementation of preventive measures8.
In this context, it becomes necessary to conduct studies that investigate the temporal, spatial, and spatio-temporal aspects of mortality related to cervical cancer. Such studies can help create tools and strategies to faster reduce the incidence and mortality of cervical cancer9. Specifically, practical tools to classify risk areas can optimize the allocation of resources and efforts, enabling more effective interventions focused on the regions and populations that need them most.
Materials and methods
Study type
Population-based ecological study using cervical cancer deaths among females recorded in Brazil from 2000 to 2021. Temporal, spatial, and space-time analysis techniques were used with the 5,570 Brazilian municipalities as the analysis unit.
Study area and notification form
Brazil is divided into five regions (North, Northeast, Midwest, Southeast, and South), 27 states (federal units)8. In 2022, the female population of Brazil constituted 51.5% (104,548,325) of the total population of the country10. This distribution is similar across Brazilian regions; however, there are significant variations in socioeconomic conditions, health, and access to essential services10. The North and Northeast face greater challenges in terms of access to good quality education and health services, reflected in distinct social and health indicators for the female population compared to other regions11–13.
Since 1975, all deaths in the country have been recorded in the Mortality Information System (SIM) of the Ministry of Health14. In 1999, a standard Death Certificate (DC) form was implemented to be filled out by the physician who is certifying the death14. This document is made available on a federal website for public domain, facilitating access to the data and subsequent in-depth analyses of deaths in Brazil14.
Data source and variables
All the data used in this study are publicly available. Sociodemographic characteristics including age, race/color, marital status, education, and municipality of residence for deaths recorded in SIM were obtained through the Department of Health Informatics of the Unified Health System (DATASUS)15. Population data and digital cartographic meshes were obtained from the Brazilian Institute of Geography and Statistics, with information generated based on the National Population Census (2000 and 2010) and intercensal estimates (2001–2009 and 2011–2021)15,16.
Study population
All deaths certificates associated with cervical cancer were included, i.e., all having cervical cancer either as the underlying cause or as associated condition, including the following 10th International Classification of Diseases (ICD-10) codes: C53 (malignant neoplasm of the cervix uteri), C53.0 (malignant neoplasm of the endocervix), C53.1 (malignant neoplasm of the exocervix), C53.8 (malignant neoplasm of the cervix uteri with invasive lesion), and C53.9 (malignant neoplasm of the cervix uteri, unspecified).
The reallocation of ill-defined deaths and uterine cancer (unspecified) was not performed in this study, as we chose to use the data directly reported by Brazil’s Mortality Information System (SIM). While reallocation may adjust estimates, we believe there is no guarantee that the proportional distribution of deaths according to the portion of the uterus is uniform across all regions studied. Implementing this redistribution could introduce additional uncertainties, as regional variations may affect the accuracy of this approach. Moreover, by maintaining the original data, we ensure comparability with other epidemiological studies that utilize the same official data sources.
Statistical analysis
Crude mortality rates (MR) were calculated by considering the number of deaths associated with cervical cancer in the numerator and the reference population of women, stratified by age group, for the same location and period in the denominator. These rates were then age-standardized using the direct method with the World Standard Population as the reference (ASMR)17. The Local Empirical Bayesian Model was applied to smooth the rates, aiming to reduce random fluctuations and enhance visualization on a broader scale18.
Choropleth maps were created based on stratified rates using the Jenks Natural Breaks algorithm, to reduce within-class differences and maximize interclass distinctions19.
The Global Moran Index (Moran I) was calculated to assess the presence of global spatial autocorrelation20. Moran I values range from − 1 to + 1, with negative values suggesting negative spatial autocorrelation, positive values indicating positive spatial autocorrelation, and values equal to zero indicating an absence of spatial autocorrelation20.
In the presence of evidence of global spatial autocorrelation, the Local Indicators of Spatial Association (LISA) were calculated21. LISA classify locations based on the degree of spatial dependence on their neighbors and allow the identification of spatial association patterns classified as: high-high (hot spots) indicating locations with high rates surrounded by neighbors with high rates; low-low (cold spots) indicating locations with low rates surrounded by neighbors with low rates; high-low indicating locations with high rates surrounded by neighbors with low rates, and low-high indicating locations with low rates surrounded by neighbors with high rates21. Both high-low and low-high can be classified as transition areas21.
To identify space-time clusters of high rates, the retrospective space-time scanning statistic of Kulldorff was applied22. The criteria included a one-year aggregation time, no geographic or temporal overlap of clusters, circular clusters, a maximum cluster size equal to 50% of the at-risk population, and a significance level of 5%22. Clusters were detected using the log-likelihood ratio test (LLR), considering the Poisson probability model, 999 Monte Carlo permutations, and a statistical significance of 5%22.
For the analysis of temporal trends, the segmented linear regression method (Joinpoint) was applied23. This method allows the examination of both temporal trends and changes in trends over the years by adjusting data to the smallest number of inflection points, with statistically significant additional joinpoints23. The Weighted Bayesian Information Criterion (WBIC) was used to choose the best-fit model24. The Annual Percent Change (APC) was presented for each line segment, and the Average Annual Percent Change (AAPC) was calculated to quantify the trend over the entire analyzed time interval, with respective 95% confidence intervals, calculated by the Empirical Quantile Confidence Interval method24. Increasing trends were considered when APC or AAPC values were positive and significant (APC/AAPC is significantly different from zero at the alpha = 0.05 level), decreasing trends when negative and significant, and stationary trends otherwise24.
Software
R 4.3.1 and Microsoft Excel 365 were used for descriptive analysis, graph and table construction. QGIS 3.32.2 was used for map creation, GeoDa 1.20 for spatial analyses, SaTScan™ 10.1 for space-time scanning analysis, and Joinpoint Regression Software™ 5.0.2 for temporal trend analysis.
Ethical considerations
This study utilized only anonymized, aggregated, and publicly available secondary data. As participant identification is not possible due to the ecological nature of the study, informed consent is deemed unnecessary. Therefore, ethical review by a Research Ethics Committee was not required.
Results
Sociodemographic characteristics of women whose deaths were associated with cervical cancer and temporal distribution of mortality
From 2000 to 2021, 123,306 deaths associated with cervical cancer were recorded among women in Brazil. This disease was mentioned as the underlying cause in 115,193 (93.4%) death certificates. As shown in Table 1, the majority of deaths occurred in residents of the populous Southeast region with 44,080 (35.7%), followed by the Northeast region with 37,167 (30.1%). Cervical cancer related deaths were more common among women 50 to 59 years old (22.1%), white race/color (44.4%), married or in a stable union marital status (34.5%), and education level of 1 to 3 years (21.9%).
Table 1.
Sociodemographic characteristics of women whose deaths were associated with cervical cancer according to region of residence. Brazil, 2000 to 2021.
| Variables | Brazil (N = 123,306) | North (N = 14,327) | Northeast (N = 37,167) | Southeast (N = 44,080) | South (N = 18,391) | Midwest (N = 9,341) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age group (years) | ||||||||||||
| 0–19 | 55 | 0.0 | 12 | 0.1 | 18 | 0.0 | 17 | 0.0 | 5 | 0.0 | 3 | 0.0 |
| 20–29 | 3306 | 2.7 | 464 | 3.2 | 952 | 2.6 | 1103 | 2.5 | 545 | 3.0 | 242 | 2.6 |
| 30–39 | 14,785 | 12.0 | 2095 | 14.6 | 4332 | 11.7 | 4672 | 10.6 | 2483 | 13.5 | 1203 | 12.9 |
| 40–49 | 25,180 | 20.4 | 3320 | 23.2 | 7401 | 19.9 | 8635 | 19.6 | 3796 | 20.6 | 2028 | 21.7 |
| 50–59 | 27,274 | 22.1 | 3154 | 22.0 | 8116 | 21.8 | 9803 | 22.2 | 4120 | 22.4 | 2081 | 22.3 |
| 60–69 | 23,842 | 19.3 | 2545 | 17.8 | 7090 | 19.1 | 8974 | 20.4 | 3485 | 18.9 | 1748 | 18.7 |
| 70–79 | 17,695 | 14.4 | 1726 | 12.0 | 5473 | 14.7 | 6660 | 15.1 | 2538 | 13.8 | 1298 | 13.9 |
| 80 or more | 11,169 | 9.1 | 1011 | 7.1 | 3785 | 10.2 | 4216 | 9.6 | 1419 | 7.7 | 738 | 7.9 |
| Race/color | ||||||||||||
| White | 54,787 | 44.4 | 2399 | 16.7 | 8725 | 23.5 | 24,539 | 55.7 | 15,368 | 83.6 | 3756 | 40.2 |
| Black | 9780 | 7.9 | 729 | 5.1 | 3148 | 8.5 | 4422 | 10.0 | 861 | 4.7 | 620 | 6.6 |
| Yellow | 572 | 0.5 | 40 | 0.3 | 163 | 0.4 | 287 | 0.7 | 43 | 0.2 | 39 | 0.4 |
| Brown | 51,159 | 41.5 | 10,490 | 73.2 | 22,439 | 60.4 | 12,312 | 27.9 | 1437 | 7.8 | 4481 | 48.0 |
| Indigenous | 563 | 0.5 | 248 | 1.7 | 104 | 0.3 | 29 | 0.1 | 58 | 0.3 | 124 | 1.3 |
| Missing data | 6445 | 5.2 | 421 | 2.9 | 2588 | 7.0 | 2491 | 5.7 | 624 | 3.4 | 321 | 3.4 |
| Marital status | ||||||||||||
| Unmarried | 40,212 | 32.6 | 5560 | 38.8 | 13,144 | 35.4 | 13,774 | 31.2 | 4845 | 26.3 | 2889 | 30.9 |
| Married/stable union | 42,594 | 34.5 | 5498 | 38.4 | 12,807 | 34.5 | 14,214 | 32.2 | 6899 | 37.5 | 3176 | 34.0 |
| Widow | 25,241 | 20.5 | 2068 | 14.4 | 6765 | 18.2 | 10,380 | 23.5 | 4183 | 22.7 | 1845 | 19.8 |
| Divorced | 7537 | 6.1 | 454 | 3.2 | 1210 | 3.3 | 3583 | 8.1 | 1567 | 8.5 | 723 | 7.7 |
| Missing data | 7722 | 6.3 | 747 | 5.2 | 3241 | 8.7 | 2129 | 4.8 | 897 | 4.9 | 708 | 7.6 |
| Schooling | ||||||||||||
| None | 20,619 | 16.7 | 2853 | 19.9 | 9312 | 25.1 | 5057 | 11.5 | 1814 | 9.9 | 1583 | 16.9 |
| 1 to 3 years | 26,985 | 21.9 | 3303 | 23.1 | 7896 | 21.2 | 9750 | 22.1 | 4194 | 22.8 | 1842 | 19.7 |
| 4 to 7 years | 26,309 | 21.3 | 3415 | 23.8 | 5957 | 16.0 | 10,074 | 22.9 | 4844 | 26.3 | 2019 | 21.6 |
| 8 to 11 years | 16,836 | 13.7 | 2635 | 18.4 | 3687 | 9.9 | 6284 | 14.3 | 2886 | 15.7 | 1344 | 14.4 |
| 12 years or more | 4985 | 4.0 | 542 | 3.8 | 986 | 2.7 | 2132 | 4.8 | 866 | 4.7 | 459 | 4.9 |
| Missing data | 27,572 | 22.4 | 1579 | 11.0 | 9329 | 25.1 | 10,783 | 24.5 | 3787 | 20.6 | 2094 | 22.4 |
No: Absolute Frequency; %: Relative Frequency.
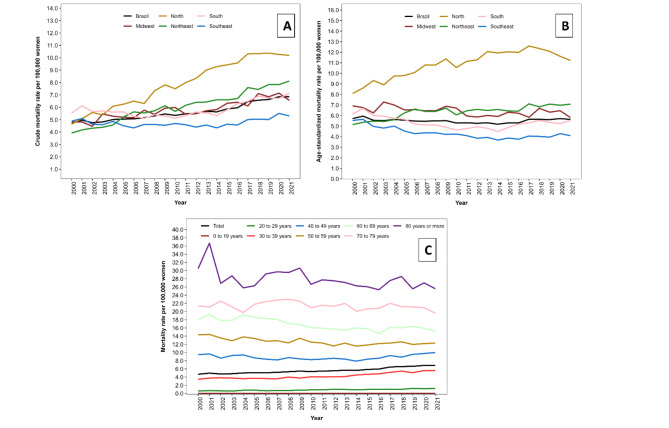
The overall MR was 5.6 deaths per 100,000 women, and the ASMR was 5.5 deaths per 100,000 women. Both the MR and ASMR varied across regions in Brazil over the years, especially in the North and Northeast regions. The MR from 2000 to 2021 increased in the North region from 4.6 to 10.2 deaths per 100,000 women and in the Northeast region from 3.9 to 8.1 deaths per 100,000 women (Fig. 1A). Age standardization weakened these trends that partly were due to aging of the populations, with the ASMR ranged from 8.1 to 11.2 deaths per 100,000 women in the North region and from 5.1 to 7.1 deaths per 100,000 women in the Northeast region (Fig. 1B). Regarding the distribution of rates by age group, higher mortality was observed in the older age groups. However, over the historical series, there was a reduction in mortality from 14.4 to 12.3 deaths per 100,000 women in the age group of 50 to 59 years, from 18.1 to 15.1 in the age group of 60 to 69 years, and from 30.5 to 25.6 in the age group of 80 years or older. In contrast, there was an increase from 0.6 to 1.2 deaths per 100,000 women in the age group of 20 to 29 years and from 3.5 to 5.6 deaths per 100,000 women in the age group of 30 to 39 years (Fig. 1C).
Fig. 1.
Cervical cancer mortality per 100,000 women. Brazil, 2000 to 2021: (A) Crude mortality rate by regions; (B) Age-standardized mortality rate by regions; (C) Mortality rate by age group.
Spatial distribution of cervical cancer-associated mortality: crude and age-standardized
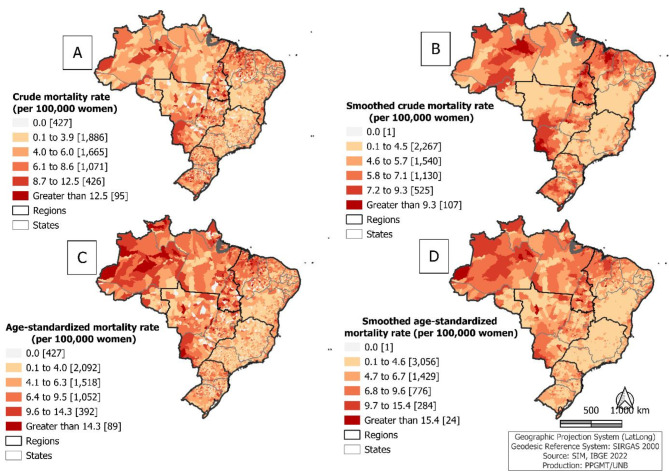
A widespread and heterogeneous spatial distribution of cervical cancer-associated mortality was observed. Figure 2A shows that more than a quarter (1,592 out of 5,570; 28.6%) municipalities had a MR equal to or higher than 6.1 deaths per 100,000 women. This pattern was similar for the ASMR, with 1,533 (27.5%) municipalities recording 6.4 deaths or more per 100,000 women (Fig. 2C). When these rates were smoothed, municipalities with high rates were observed, according to the stratification, in all regions, but especially in the North, Northeast, and Midwest regions of the country, which accounted for 75.9% (480/632) of municipalities with smoothed MR equal to or higher than 7.2 deaths per 100,000 women (higher smoothed mortality rates found) (Fig. 2B) and 87.0% (268/308) of municipalities with smoothed ASMR equal to or higher than 9.7 deaths per 100,000 women (higher smoothed age-standardized mortality rates found) (Fig. 2D).
Fig. 2.
Spatial distribution of average cervical cancer mortality per 100,000 women, by municipality of residence: crude mortality rate (A), smoothed crude mortality rate (B), age-standardized mortality rate (C) and smoothed age-standardized mortality rate (D). Brazil, 2000 to 2021.
Spatial and space-time clusters of age-standardized cervical cancer mortality
The Moran I for the average of the ASMR for the entire study period was 0.3294, higher than the expected value of -0.0002, and the p was equal to 0.001, indicating significant positive spatial autocorrelation, indicative of the presence of spatial clusters. As shown in Table 2, significant positive spatial autocorrelation was observed in all groupings for the years studied.
Table 2.
Global spatial autocorrelation analysis of age-standardized cervical cancer mortality per 100,000 women, by municipality of residence. Brazil, 2000 to 2021.
| Year | Moran’s I | Expected index | Variance | Z | P |
|---|---|---|---|---|---|
| 2000 | 0.0205 | -0.0002 | 0.0081 | 2.5748 | 0.014 |
| 2001 | 0.0635 | -0.0002 | 0.0077 | 8.2584 | 0.001 |
| 2002 | 0.0208 | -0.0002 | 0.0079 | 2.6672 | 0.009 |
| 2003 | 0.0199 | -0.0002 | 0.008 | 2.4975 | 0.015 |
| 2004 | 0.0315 | -0.0002 | 0.0082 | 3.8747 | 0.001 |
| 2000 to 2004 | 0.1023 | -0.0001 | 0.0080 | 12.7733 | 0.001 |
| 2005 | 0.0120 | -0.0002 | 0.0078 | 1.5459 | 0.069 |
| 2006 | 0.0256 | -0.0002 | 0.0079 | 3.2445 | 0.003 |
| 2007 | 0.0295 | -0.0001 | 0.0081 | 3.6677 | 0.001 |
| 2008 | 0.0298 | -0.0002 | 0.0085 | 3.5430 | 0.003 |
| 2009 | 0.0401 | -0.0002 | 0.0080 | 5.0429 | 0.001 |
| 2005 to 2009 | 0.1104 | -0.0002 | 0.0080 | 13.7610 | 0.001 |
| 2010 | 0.0202 | -0.0002 | 0.0082 | 2.4969 | 0.015 |
| 2011 | 0.0387 | -0.0002 | 0.0078 | 4.9835 | 0.003 |
| 2012 | 0.0216 | -0.0002 | 0.0077 | 2.8296 | 0.006 |
| 2013 | 0.0543 | -0.0002 | 0.0079 | 6.9270 | 0.001 |
| 2010 to 2013 | 0.1200 | -0.0002 | 0.0078 | 15.3376 | 0.001 |
| 2014 | 0.0505 | -0.0002 | 0.0084 | 6.0284 | 0.001 |
| 2015 | 0.0410 | -0.0002 | 0.0081 | 5.0339 | 0.001 |
| 2016 | 0.0559 | -0.0002 | 0.0084 | 6.6269 | 0.001 |
| 2017 | 0.0419 | -0.0002 | 0.0083 | 5.0621 | 0.001 |
| 2014 to 2017 | 0.1445 | -0.0002 | 0.0082 | 17.6592 | 0.001 |
| 2018 | 0.0584 | -0.0002 | 0.0079 | 7.4650 | 0.001 |
| 2019 | 0.0450 | -0.0002 | 0.0080 | 5.6618 | 0.001 |
| 2020 | 0.0293 | -0.0002 | 0.0082 | 3.6089 | 0.002 |
| 2021 | 0.0458 | -0.0002 | 0.0081 | 5.6858 | 0.001 |
| 2018 to 2021 | 0.1355 | -0.0002 | 0.0082 | 16.5532 | 0.001 |
| All period | 0.3294 | -0.0002 | 0.0082 | 40.0727 | 0.001 |
p: Permutation Test.
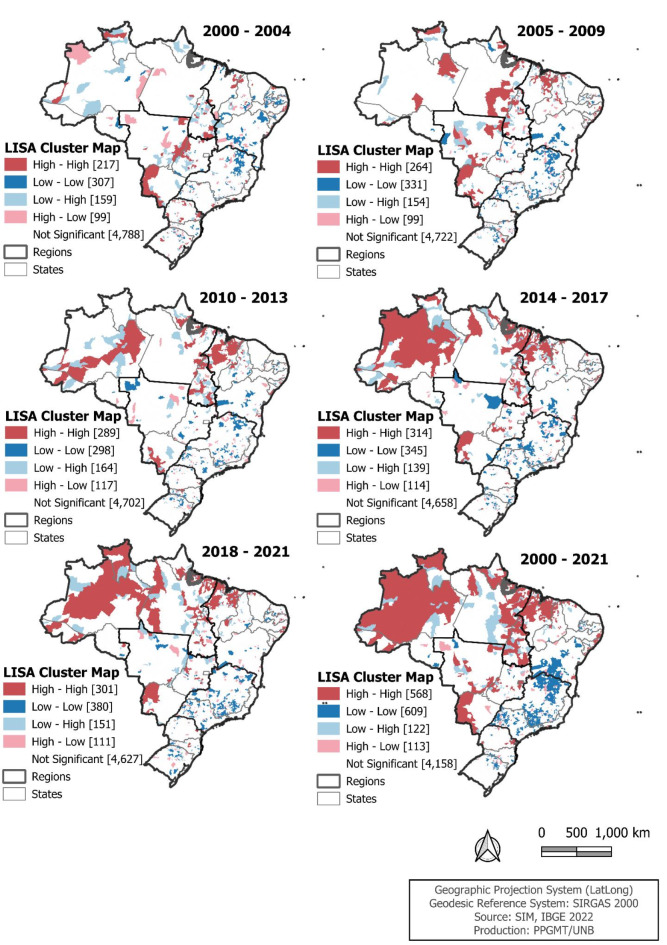
The spatial clustering remained strong over the study period. Figure 3 presents the clusters identified through LISA. We observed a gradual increase in the pattern of spatial clusters, especially those with high ASMR. From 2000 to 2021, it is noteworthy that out of the 568 municipalities classified as high-high, 273 (48.1%) were in the Northeast region, 214 (37.7%) in the North, and 54 (9.5%) in the Midwest. Regarding the 609 municipalities classified as low-low, 407 (66.8%) were in the Southeast region, and 96 (15.8%) in the South.
Fig. 3.
Spatial cluster of age-standardized cervical cancer mortality per 100,000 women, by municipality of residence. Brazil, 2000 to 2021.
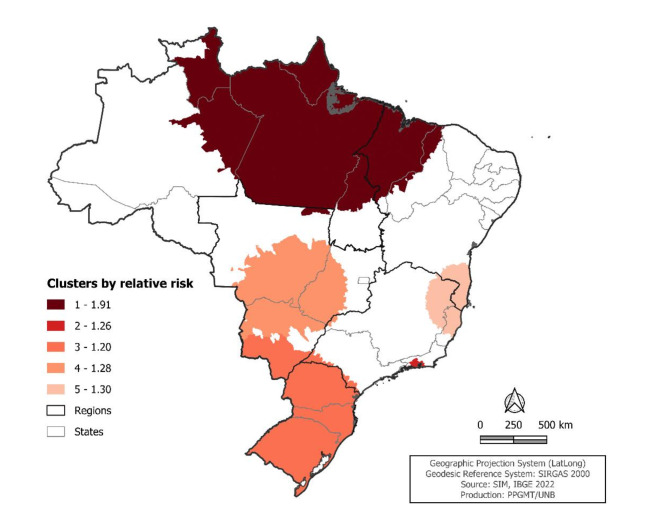
The estimated relative risk through space-time scanning analysis detected five statistically significant risk clusters (p < 0.05), whose locations are shown in Fig. 4.
Fig. 4.
Spatiotemporal cluster of relative risk of cervical cancer mortality per 100,000 women, comparing the municipalities and periods belonging to the clusters with the other municipalities and periods. Brazil, 2000 to 2021.
The primary and highest-risk cluster of mortality occurred from 2011 to 2021, involving 555 municipalities across nine states located in the North, Northeast, and Midwest regions, described in Table 3. Within this cluster, there were 12,562 deaths, and the mortality rate was 121.3 deaths per 100,000 women (LLR = 1,994.7; p < 0.001).
Table 3.
Spatiotemporal clusters of cervical cancer mortality per 100,000 women, by municipality of residence. Brazil, 2000 to 2021.
| Cluster | Period | Number of municipalities | States | Radius (Km) | Number of deaths | Expected number of deaths | Annual mortality per 100,000 women within the cluster | LLR | P |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2011 to 2021 | 555 | Amazonas, Roraima, Pará, Amapá, Tocantins, Maranhão, Piauí, Ceará and Mato Grosso | 1,239.7 | 12,562 | 6,911.5 | 121.3 | 1,994.7 | < 0.001 |
| 2 | 2011 to 2021 | 25 | Rio de Janeiro | 64.9 | 5,141 | 4,107.0 | 80.4 | 124.9 | < 0.001 |
| 3 | 2016 to 2021 | 1,279 | São Paulo, Santa Catarina, Rio Grande do Sul and Mato Grosso do Sul | 1,072.5 | 6,684 | 5,582.3 | 43.3 | 107.4 | < 0.001 |
| 4 | 2014 to 2021 | 215 | Minas Gerais, Mato Grosso, Mato Grosso do Sul and Goiás | 3,460.6 | 1,683 | 1,313.6 | 63.0 | 48.2 | < 0.001 |
| 5 | 2017 to 2020 | 225 | Bahia, Minas Gerais and Espírito Santo | 345.9 | 1,058 | 809.6 | 31.1 | 35.0 | < 0.001 |
LLR: log likelihood ratio; p: Poisson Test.
Temporal trend of cervical cancer-associated mortality
Table 4 presents the temporal trend of the ASMR in Brazil as a whole, by region and state, and the crude mortality rate associated with cervical cancer according to age groups. Considering the entire period, Brazil showed a stable trend (AAPC = 1.4). Regarding age groups, increasing trends were observed in the 20 to 29 years age group (AAPC = 3.3) and the 30 to 39 years age group (AAPC = 2.3), while the others remained stable. Regarding regions and states, there was an increasing trend in the North (AAPC = 1.5) and Northeast (AAPC = 1.5) regions, and in the following states: Rondônia (AAPC = 5.7), Acre (AAPC = 4.4), Amazonas (AAPC = 1.8), Pará (AAPC = 1.1), Maranhão (AAPC = 4.1), Ceará (AAPC = 0.9), Paraíba (AAPC = 6.5), Alagoas (AAPC = 1.6), and Bahia (AAPC = 0.9). In contrast, a decreasing trend was observed in the Southeast (AAPC=-1.2), South (AAPC=-0.8), and Midwest (AAPC=-0.6) regions, and in the states of Minas Gerais (AAPC=-1.0), Rio de Janeiro (AAPC=-1.1), São Paulo (AAPC=-2.0), Paraná (AAPC=-1.0), and Rio Grande do Sul (AAPC=-0.8).
Table 4.
Joinpoint regression analysis of cervical cancer mortality per 100,000 women. Brazil, 2000 to 2021.
| Variables | ASMR | Segmented period | Entire period | |||
|---|---|---|---|---|---|---|
| Period | APC (95% CI) | Trend | AAPC (95% CI) | Trend | ||
| Brazil | 5.5 | 2000 to 2014 | -0.7 (-1.0 to -0.4) | Decreasing | 1.4 (-0.2 to 2.0) | Stable |
| 2014 to 2021 | 1.4 (0.7 to 2.8) | Increasing | ||||
| Age group in years (MR) | ||||||
| 20 to 29 | 0.9 | 2000 to 2021 | 3.3 (1.9 to 5.0) | Increasing | 3.3 (1.9 to 5.0) | Increasing |
| 30 to 39 | 4.3 | 2000 to 2011 | 0.7 (-2.5 to 1.8) | Stable | 2.3 (1.8 to 2.8) | Increasing |
| 2011 to 2021 | 4.1 (3.1 to 7.0) | Increasing | ||||
| 40 to 49 | 8.9 | 2000 to 2011 | -1.9 (-3.7 to -0.8) | Decreasing | 0.1 (-0.3 to 0.5) | Stable |
| 2011 to 2021 | 2.4 (1.3 to 4.4) | Increasing | ||||
| 50 to 59 | 12.7 | 2000 to 2011 | -2.1 (-8.6 to 0.5) | Stable | -0.7 (-1.4 to 0.0) | Stable |
| 2011 to 2021 | 0.7 (-0.7 to 7.6) | Stable | ||||
| 60 to 69 | 16.8 | 2000 to 2021 | -1.0 (-2.1 to 0.3) | Stable | -1.0 (-2.1 to 0.3) | Stable |
| 70 to 79 | 21.4 | 2000 to 2021 | -0.2 (-1.5 to 1.1) | Stable | -0.2 (-1.5 to 1.1) | Stable |
| 80 or more | 27.9 | 2000 to 2021 | -0.6 (-2.2 to 1.2) | Stable | -0.6 (-2.2 to 1.2) | Stable |
| Region/State | ||||||
| North | 10.8 | 2000 to 2007 | 3.6 (2.8 to 7.1) | Increasing | 1.5 (1.1 to 1.8) | Increasing |
| 2007 to 2018 | 1.5 (0.8 to 2.1) | Increasing | ||||
| 2018 to 2021 | -3.7 (-8.1 to -1.2) | Decreasing | ||||
| Rondônia | 6.9 | 2000 to 2002 | 71.0 (10.8 to 142.1) | Increasing | 5.7 (2.3 to 9.2) | Increasing |
| 2002 to 2021 | 0.5 (-1.6 to 1.9) | Stable | ||||
| Acre | 8.9 | 2000 to 2021 | 4.4 (2.2 to 7.5) | Increasing | 4.4 (2.2 to 7.5) | Increasing |
| Amazonas | 16.8 | 2000 to 2013 | 5.0 (3.8 to 6.9) | Increasing | 1.8 (1.0 to 2.6) | Increasing |
| 2013 to 2021 | -3.3 (-6.7 to -1.1) | Decreasing | ||||
| Roraima | 10.7 | 2000 to 2021 | 1.3 (-1.9 to 4.8) | Stable | 1.3 (-1.9 to 4.8) | Stable |
| Pará | 9.5 | 2000 to 2021 | 1.1 (0.4 to 1.8) | Increasing | 1.1 (0.4 to 1.8) | Increasing |
| Amapá | 12.7 | 2000 to 2021 | 1.5 (-1.0 to 4.4) | Stable | 1.5 (-1.0 to 4.4) | Stable |
| Tocantins | 9.1 | 2000 to 2002 | 33.8 (1.0 to 81.1) | Increasing | 3.3 (-0.1 to 6.2) | Stable |
| 2002 to 2021 | 0.5 (-10.1 to 2.0) | Stable | ||||
| Northeast | 6.3 | 2000 to 2006 | 3.6 (2.2 to 7.7) | Increasing | 1.5 (1.2 to 2.0) | Increasing |
| 2006 to 2021 | 0.7 (0.1 to 1.1) | Increasing | ||||
| Maranhão | 10.2 | 2000 to 2006 | 12.4 (8.5 to 20.5) | Increasing | 4.1 (3.4 to 5.2) | Increasing |
| 2006 to 2021 | 1.0 (0.0 to 1.8) | Stable | ||||
| Piauí | 7.7 | 2000 to 2009 | 3.5 (1.5 to 11.1) | Increasing | 0.8 (-0.2 to 2.0) | Stable |
| 2009 to 2021 | -1.1 (-4.7 to 0.1) | Stable | ||||
| Ceará | 6.3 | 2000 to 2021 | 0.9 (0.2 to 1.7) | Increasing | 0.9 (0.2 to 1.7) | Increasing |
| Rio Grande do Norte | 5.4 | 2000 to 2021 | 0.7 (-0.4 to 1.9) | Stable | 0.7 (-0.4 to 1.9) | Stable |
| Paraíba | 5.1 | 2000 to 2006 | 21.3 (15.7 to 29.9) | Increasing | 6.5 (5.5 to 7.9) | Increasing |
| 2006 to 2010 | -9.3 (-17.7 to -1.2) | Decreasing | ||||
| 2010 to 2013 | 15.6 (4.9 to 23.4) | Increasing | ||||
| 2013 to 2021 | 1.5 (-3.3 to 3.6) | Stable | ||||
| Pernambuco | 6.4 | 2000 to 2013 | -0.8 (-4.8 to 0.0) | Stable | 0.3 (-0.4 to 0.8) | Stable |
| 2013 to 2021 | 2.0 (0.4 to 8.6) | Increasing | ||||
| Alagoas | 6.6 | 2000 to 2021 | 1.6 (0.5 to 2.9) | Increasing | 1.6 (0.5 to 2.9) | Increasing |
| Sergipe | 7.4 | 2000 to 2021 | -1.0 (-2.1 to 0.0) | Stable | -1.0 (-2.1 to 0.0) | Stable |
| Bahia | 4.9 | 2000 to 2021 | 0.9 (0.4 to 1.5) | Increasing | 0.9 (0.4 to 1.5) | Increasing |
| Southeast | 4.4 | 2000 to 2014 | -2.7 (-3.7 to -2.1) | Decreasing | -1.2 (-1.6 to -0.8) | Decreasing |
| 2014 to 2021 | 1.8 (0.0 to 7.0) | Increasing | ||||
| Minas Gerais | 3.8 | 2000 to 2021 | -1.0 (-1.4 to -0.5) | Decreasing | -1.0 (-1.4 to -0.5) | Decreasing |
| Espírito Santo | 5.9 | 2000 to 2012 | -2.8 (-15.1 to 12.8) | Stable | -0.6 (-2.4 to 1.0) | Stable |
| 2012 to 2021 | 2.4 (-9.1 to 20.9) | Stable | ||||
| Rio de Janeiro | 5.6 | 2000 to 2021 | -1.1 (-1.7 to -0.5) | Decreasing | -1.1 (-1.7 to -0.5) | Decreasing |
| São Paulo | 4.0 | 2000 to 2002 | -10.1 (-13.2 to -4.2) | Decreasing | -2.0 (-2.4 to -1.5) | Decreasing |
| 2002 to 2014 | -3.2 (-3.9 to -0.4) | Decreasing | ||||
| 2014 to 2021 | 2.6 (1.1 to 5.4) | Increasing | ||||
| South | 5.3 | 2000 to 2010 | -3.3 (-4.4 to -2.5) | Decreasing | -0.8 (-1.1 to -0.5) | Decreasing |
| 2010 to 2021 | 1.5 (0.8 to 2.5) | Increasing | ||||
| Paraná | 5.5 | 2000 to 2012 | -3.0 (-6.0 to -1.8) | Decreasing | -1.0 (-1.7 to -0.3) | Decreasing |
| 2012 to 2021 | 1.7 (-0.3 to 7.6) | Stable | ||||
| Santa Catarina | 4.9 | 2000 to 2004 | -7.9 (-19.9 to -0.3) | Decreasing | -0.5 (-1.4 to 0.8) | Stable |
| 2004 to 2021 | 1.3 (0.1 to 6.7) | Increasing | ||||
| Rio Grande do Sul | 5.4 | 2000 to 2011 | -3.4 (-6.0 to -2.1) | Decreasing | -0.8 (-1.4 to -0.2) | Decreasing |
| 2011 to 2021 | 2.2 (0.6 to 5.9) | Increasing | ||||
| Midwest | 6.5 | 2000 to 2021 | -0.6 (-1.1 to -0.1) | Decreasing | -0.6 (-1.1 to -0.1) | Decreasing |
| Mato Grosso do Sul | 7.5 | 2000 to 2021 | -1.2 (-2.4 to 0.0) | Stable | -1.2 (-2.4 to 0.0) | Stable |
| Mato Grosso | 6.7 | 2000 to 2007 | 4.9 (2.4 to 9.9) | Increasing | 0.2 (-0.5 to 1.2) | Stable |
| 2007 to 2012 | -7.6 (-14.8 to -3.1) | Decreasing | ||||
| 2012 to 2021 | 1.2 (-0.8 to 8.9) | Stable | ||||
| Goiás | 6.1 | 2000 to 2006 | -3.2 (-12.2 to 0.3) | Stable | -0.6 (-1.4 to 0.5) | Stable |
| 2006 to 2021 | 0.5 (-4,8 to 7.5) | Stable | ||||
| Distrito Federal | 6.0 | 2000 to 2021 | -0.4 (-1.5 to 0.7) | Stable | -0.4 (-1.5 to 0.7) | Stable |
ASMR: age-standardized mortality rate; MR: crude mortality rate; APC: annual percentage change; AAPC: average annual percentage change; CI 95%: confidence interval 95%.
Discussion
The study identified strong spatial and temporal patterns spanning various age groups, all regions, and states of Brazil. We detected clusters of high and low mortality, as well as transition areas. These findings are very informative for the implementation of cost-effective measures in specific regions where the reduction of cervical cancer-associated mortality might be achieved with greatest and most rapid impact.
Identification of high-risk regions/cluster permits selective strategic allocation of resources in populations at higher risk for cervical cancer allowing for highly-efficient risk-based cervical screening programs as suggested by Perkins et al.25. In addition, for a screening program to be more effective requires the use of an efficient risk-stratification method, such as HPV testing with viral genotyping25.
The results of this study revealed an overall age-adjusted mortality rate for Brazil higher than the expected for a high HDI country according to GLOBOCAN1. Additionally, we noted a significant change in the temporal trend of cervical cancer mortality. Until 2014, a significant reduction in the mortality rate was observed; however, from this year onward, an increase was observed. As previously reported in another study26, various factors may have contributed to the initial observed reduction, including the implementation of the Unified Health System and Family Health Programs, the expansion of diagnostic resources and screening programs, as well as government incentives to strengthen available therapeutic actions26.
In contrast to the reduction, the subsequent increase in mortality may be partially attributed to the ongoing improvement in the quality of cause-of-death data in Brazil. Enhanced accuracy in the national records within the Mortality Information System has resulted in a clearer understanding of the disease’s epidemiological situation in recent years27. However, other factors may also have contributed to this trend reversal, such as changes in the population’s lifestyle habits, unequal access to healthcare services, and even possible flaws in cervical cancer screening programs. The low coverage of screening tests in certain regions, combined with barriers to accessing diagnostic and treatment services, may have negatively impacted disease control, leading to an increase in mortality from 2014 onwards.
In addition to the increasing trend in mortality at the national level from 2014 onwards, significant differences were observed between regions. While the North and Northeast regions showed an increase and a concentration of spatial clusters characterized by high mortality rates, the South, Southeast, and, to a lesser extent, Midwest regions showed decreasing trends, with a concentration of spatial clusters of low mortality risk.
It has been previously reported that the North and Northeast regions of the country present higher social vulnerability compared to the Midwest, South and Southeast, which may indicate difficulties accessing screening and/or access to prompt and adequate diagnosis and treatment of precancerous lesions and/or cancer and thus explaining the observed discrepancies in temporal and spatial patterns28.
Despite Brazil having a universal healthcare system with infrastructure, human resources, and equipment designated for cervical cancer prevention, the highlighted regional disparities indicate an uneven distribution of these resources29. It becomes crucial, especially in those regions with higher-risk to expand health education, increase HPV vaccination coverage, invest in screening programs and in more efficient treatment of precancerous lesions, accompanied by adequate access to treatment for the population diagnosed with cancer and affordable if not free treatment30,31. The implementation of other strategies, such as screening with HPV genotyping in areas identified as higher risk in this study, would contribute to targeting pre-cancer cases more efficiently, permitting concentrated follow-up to prevent loss to follow-up29. Actually, earlier this year a single-dose scheme of the HPV vaccine has been adopted in the National Immunization Program (PNI) and HPV detection tests with partial or extended genotyping were incorporated in Brazil32,33.
Despite the decentralization of primary care actions in Brazil, it is relevant to note that treatment of precancerous lesion and cervical cancer is predominantly concentrated in referral centers, mostly located in states capital cities34. This geographical concentration, combined with social barriers, results in unequal access to preventive and curative treatments, especially in the North and Northeast regions35.
Although access to healthcare services is an important factor in understanding regional disparities in cervical cancer-associated mortality, the creation of a map showing the distance to treatment centers was not possible due to difficulties in consolidating geospatial data from a single source. While geographic data are available, the lack of a unified repository that integrates them hindered the analysis. Considering the location of these centers and the transportation infrastructure in different regions of Brazil is crucial, as it may reveal how accessibility influences health outcomes. The inclusion of such a map could enrich the discussion, providing a clearer perspective on the barriers faced by populations in remote areas. This approach underscores the importance of future research that seeks to integrate geographic and socioeconomic data for a more comprehensive understanding of inequalities in access to cancer treatment.
Moreover, it is crucial to consider the influence of social vulnerability on regional disparities in quality and advancements in the surveillance of deaths from cervical cancer, which show significant inequalities31. The improvement was observed nationally but particularly in the South, Southeast, and Midwest regions with more consistent advances; however, the North and Northeast regions did not show uniform improvements, and in some cases, there were reduction in data quality31. This scenario suggests that the increase in mortality rate for the North and Northeast regions may be directly related to social inequalities and limited access to health services27.
Thus, for these prevention and control strategies to be truly effective, it is necessary to discuss the logistical challenges involved, such as the proper training of healthcare professionals and the logistics of distributing self-collection kits. Furthermore, it is crucial to establish mechanisms that ensure follow-up for women with positive results, minimizing losses in monitoring and ensuring that all have access to the necessary treatment. Considering the existing healthcare infrastructure and the socioeconomic barriers faced by these populations is essential for developing a more equitable and effective approach to combating cervical cancer.
The overall mortality rate has decreased; however, 5.8 deaths per 100,000 women shows that the country is far from eliminating cervical cancer as a public health problem. It exceeds the target global incidence of less than 4 cases per 100,000 women for the elimination of cervical cancer36. Underscoring the imperative need to urgently implement comprehensive cervical cancer control strategies across the entire country1.
As expected, the results of this study showed higher mortality among older women. However, in line with previous findings regarding risk trends, a temporal trend of increasing mortality rate associated with cervical cancer was observed among younger women, in contrast to older ones. This reflects the need for the development of policies aimed at reducing cervical cancer incidence and mortality across all age groups37.
Furthermore, it is important to consider the demographic transition, aging population, that have occurred in Brazil10,38. Since the demographic transition can impact the health needs of the population, it consequently affects the provision of health services for women and influences mortality patterns throughout the country10,38.
It is important to consider some limitations of this research when interpreting the results. Since this investigation falls within the domain of ecological studies, it is not possible to draw conclusions about the characteristics of the population at the individual level. Some inferences, established for larger territorial scales, may, in part, derive from the unique behavior of smaller spatial units that were not assessed. Additionally, it is plausible to argue that the use of secondary data sources may have resulted in information loss, especially due to diagnostic challenges, which may have led to an underestimation of the results. Furthermore, regional variation in the quality and effectiveness of mortality surveillance may have contributed partially to the observed rate differences.
Conclusion
The results indicated an increasing mortality trend from 2014 onwards, after 14 years of decline. Regarding specific groups, an increase trend in mortality was observed among younger age groups, and also, regardless of age, in the North and Northeast regions, while the South, Southeast and Midwest regions showed a decrease. The heterogeneity in the spatial distribution of cervical cancer mortality was evident, with the formation of clusters of high mortality in all regions of the country, mostly concentrated in the North and Northeast. Similarly, space-temporal clusters emerged in all regions of Brazil, covering the most recent years of the study.
In light of this scenario, Brazil could adopt a more effective approach in health surveillance routines by identifying high-risk areas for particular diseases, as conducted in this study with great precision and detail for cervical cancer, that will in turn allow for the design of more efficient targeted interventions. The visual representation of geographic location, as demonstrated, can be an efficient method for identifying these areas. It is crucial to recognize that remote areas, far from major urban centers, could significantly benefit from a fairer strategy that gives visibility to populations with greater socioeconomic vulnerability, and therefore, at a higher risk of developing cervical cancer.
Acknowledgements
The authors wish to thank Oswaldo Cruz Foundation (Fiocruz) and National Council for Scientific and Technological Development (CNPq) for the support provided for this study.
Author contributions
Conception and design: M.S.M. and T.R.; analysis and interpretation: M.S.M. and T.R.; drafting of the article: M.S.M., S.V.M.A.L., C.J.N.R., A.D.S., P.A.B.J., T.K.S.S., F.C., A.M., L.T.R., T.R.; critical revision of the article: M.S., A.C.R., A.R., T.R.; final approval of the article: all authors approved the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available in the Department of Health Informatics of the Unified Health System (DATASUS) repository, https://datasus.saude.gov.br/transferencia-de-arquivos/ and Brazilian Institute of Geography and Statistics (IBGE) https://sidra.ibge.gov.br/.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Das, M. WHO launches strategy to accelerate elimination of cervical cancer. Lancet Oncol.22, 20–21 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Instituto Nacional do Câncer (INCA). Dados e número sobre o câncer do colo do útero. Relatório anual—022. INCA 1–31. (2022).
- 4.Choi, S., Ismail, A., Pappas-Gogos, G. & Boussios, S. HPV and cervical cancer: A review of epidemiology and screening uptake in the UK. Pathogens12, 298 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burd, E. M. Human papillomavirus and cervical Cancer. Clin. Microbiol. Rev.16, 1–17 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, X. Y. et al. Non-genetic factors and risk of cervical cancer: An umbrella review of systematic reviews and meta-analyses of observational studies. Int. J. Public Health68, 1605198. 10.3389/ijph.2023.1605198 (2023). [DOI] [PMC free article] [PubMed]
- 7.Okunade, K. S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol.40, 602–608 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corina Gomes, L., Conceição Pinto, M. & De Jesus Reis, B. & Santos Silva, D. Epidemiologia do câncer cervical no Brasil: Uma revisão integrativa. J. Nurs. Health12 (2022).
- 9.Singh, G. K., Miller, B. A., Hankey, B. F. & Edwards, B. K. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer101, 1051–1057 (2004). [DOI] [PubMed] [Google Scholar]
- 10.IBGE. Instituto Brasileiro de Geografia e Estatística. https://www.ibge.gov.br/ (2023)
- 11.Viacava, F., Porto, S. M., de Carvalho, C. & Bellido, J. G. Desigualdades regionais e sociais em saúde segundo inquéritos domiciliares (Brasil, 1998–2013). Cien Saude Colet24, 2745–2760 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Galvão, J.R., Almeida, P.F., Santos, A.M. & Bousquat, A. Percursos e obstáculos na Rede de Atenção à Saúde: Trajetórias assistenciais de mulheres em região de saúde do Nordeste brasileiro. Cad Saude Publica35 (2019). [DOI] [PubMed]
- 13.Szwarcwald, C. L., Souza Júnior, P. R., Marques, A. P., Almeida, W. D. & Montilla, D. E. Inequalities in healthy life expectancy by Brazilian geographic regions: Findings from the National Health Survey, 2013. Int. J. Equity Health15, 141 (2016). [DOI] [PMC free article] [PubMed]
- 14.Ministério da Saúde. Funasa. Manual de Procedimentos Do Sistema de Informações Sobre Mortalidade (2001).
- 15.Ministério da Saúde. DATASUS. Transferência de arquivos.https://datasus.saude.gov.br/transferencia-de-arquivos/ (2023).
- 16.IBGE. Instituto Brasileiro de Geografia e Estatística (2023).
- 17.Ahmad, O., Ben & Pinto, C. B. Age Standardization of Rates: A New WHO Standard Obesity Project View Project Medical Migration View Project. https://www.researchgate.net/publication/284696312 (2001).
- 18.Assunção, R. M., Barreto, S. M., Guerra, H. L. & Sakurai, E. Mapas De Taxas epidemiológicas: Ima abordagem Bayesiana. Cad Saude Publica14, 713–723 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Jenks, G. F. The data model concept in statistical mapping. Int. Yearb. Cartogr. 186–190 (1967).
- 20.Moran, P. A. P. The interpretation of statistical maps. J. R. Stat. Soc. Ser. B Methodol.10, 243–251 (1948). [Google Scholar]
- 21.Anselin, L. Local indicators of spatial association—LISA. Geogr. Anal.27, 93–115 (1995). [Google Scholar]
- 22.Kulldorff, M. A spatial scan statistic. Commun. Stat. Theory Methods26, 1481–1496 (1997). [Google Scholar]
- 23.Kim, H. J., Fay, M. P., Feuer, E. J. & Midthune, D. N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med.19, 335–351 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Kim, H., Chen, H., Byrne, J., Wheeler, B. & Feuer, E. J. Twenty years since Joinpoint 1.0: Two major enhancements, their justification, and impact. Stat. Med.41, 3102–3130 (2022). [DOI] [PubMed] [Google Scholar]
- 25.Perkins, R. B. et al. Use of risk-based cervical screening programs in resource-limited settings. Cancer Epidemiol.84, 102369 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Lima, M. S. et al. Temporal trend of cancer mortality in a Brazilian state with a medium Human Development Index (1980–2018). Sci. Rep.10, 21384 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.França, E. et al. Changes in the quality of cause-of-death statistics in Brazil: Garbage codes among registered deaths in 1996–2016. Popul. Health Metr.18, 20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Instituto de Pesquisa Econômica Aplicada. Atlas Da Vulnerabilidade Social Nos Municípios Brasileiros (2015).
- 29.Ribeiro, A. et al. Rethinking Cervical Cancer Screening in Brazil Post COVID-19: A global opportunity to adopt higher impact strategies. Cancer Prev. Res.14, 919–926 (2021). [DOI] [PubMed] [Google Scholar]
- 30.Zygmunt, E. et al. Contemporary methods of prevention and early detection of cervical cancer—a review of the literature. J. Educ. Health Sport10, 173–179 (2020). [Google Scholar]
- 31.Costantino, C., Alba, D., Cimino, L., Conforto, A. & Mazzucco, W. The role of vaccination and screening in limiting the worldwide disease burden of preventable female cancers: A review. Women1, 16–28 (2020). [Google Scholar]
- 32.Ministério da Saúde do Brasil. Portaria SECTICS/MS no 3, de 7 de março de 2024 (2024).
- 33.Ministério da Saúde do Brasil. Nota Técnica no 41/2024-CGICI/DPNI/SVSA/MS (2024).
- 34.Corrêa, F. M., Migowski, A., de Almeida, L. M. & Soares, M. A. Cervical cancer screening, treatment and prophylaxis in Brazil: Current and future perspectives for cervical cancer elimination. Front. Med.9 (2022). [DOI] [PMC free article] [PubMed]
- 35.Dantas, M. N. P. et al. Fatores associados ao acesso precário aos serviços de saúde no Brasil. Rev. Bras. Epidemiol.24 (2021). [DOI] [PubMed]
- 36.World Health Organization. Cerv. Cancer Elimination Initiative. https://www.who.int/initiatives/cervical-cancer-elimination-initiative (2020).
- 37.Luizaga, C. T., de Jardim, M., Wünsch-Filho, B. C., Eluf-Neto, V & Silva, G. A. Mudanças recentes Nas tendências da mortalidade por câncer de colo do útero no Sudeste do Brasil. Rev. Saude Publica57, 25 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castiglioni, A. H. Transição Urbana E demográfica no Brasil: Características, percursos e tendências. Ateliê Geogr.14, 6–26 (2020). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available in the Department of Health Informatics of the Unified Health System (DATASUS) repository, https://datasus.saude.gov.br/transferencia-de-arquivos/ and Brazilian Institute of Geography and Statistics (IBGE) https://sidra.ibge.gov.br/.