Abstract
The symbiotic relationship between legumes and rhizobia is known to be influenced by specific rhizobial type III effectors (T3Es) in certain cases. In this study, we present evidence that the symbiosis between Vigna radiata and Bradyrhizobium elkanii USDA61 is controlled by a T3E called NopP2, and this interaction is highly dependent on the genetic makeup of the host plant. NopP2 plays a crucial role in promoting nodulation in various V. radiata varieties. Additionally, NopP2 is essential for early infection and the formation of nodules in compatible plants. Through evolutionary analysis, we discovered that bradyrhizobial NopPs can be categorized into two distinct clusters: NopP1 and NopP2. Furthermore, both types of bradyrhizobial NopPs were conserved within their respective groups. Our findings suggest that NopP2 serves as a mechanism for optimizing the symbiotic relationship between V. radiata and B. elkanii USDA61 by interacting with the pathogenesis related-10 (PR10) protein and reducing effector-triggered immunity (ETI) responses.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75294-4.
Keywords: T3SS, Effector protein NopP, Bradyrhizobium elkanii USDA61, Vigna radiata, Pathogenesis related-10 (PR10) protein
Subject terms: Microbiology, Plant sciences
Introduction
Symbiosis between legumes and rhizobia in root nodules facilitates the utilization of atmospheric dinitrogen through nitrogen fixation reactions. Rhizobia, which are naturally occurring soil bacteria, encompass a vast array of species that form symbiotic partnerships with various legume plants. In this mutualistic interaction, legume plants produce flavonoids, which serve as crucial signaling molecules recognized by specific rhizobia, thereby triggering the activation of nod genes1. However, numerous rhizobia have evolved diverse strategies to optimize their symbiotic interaction with host plants. Among these strategies, a multitude of rhizobial type III secretion systems (T3SSs) are involved in the nodulation process by delivering type three effectors (T3Es) directly into the cytosol of eukaryotic host cells, which are referred to as nodulation outer proteins (Nops)2. Plant flavonoids activate the transcriptional regulator NodD, which then induces the expression of the T3SS transcription regulator (ttsI)3. Several T3Es can suppress plant defense reactions, thereby promoting nodulation symbiosis4–7, whereas some T3Es also exhibit incompatibility with nodulation8,9.
For Vigna mungo, several T3Es (NopP2, Bel2-5, and InnB) also impact the mutualistic effectiveness depending on the host cultivar10. Although many rhizobial T3Es have been characterized11,12, the specific relationships between V. radiata and T3Es, which are dependent on the genotypic compatibility or incompatibility of the legumes, have remained largely unexplored until now.
Previous research has revealed the involvement of the Bradyrhizobium elkanii USDA61 T3SS in restricting nodulation in V. radiata cv. KPS113. Mutation of the T3SS has been shown to induce distinct symbiotic phenotypes, but the USDA61 wild-type strain exhibited a near-total abolition of nodulation. Interestingly, the InnB effector USDA61 is responsible for host-specific nodulation restriction in V. radiata KPS114; thus, the effector innB-deficient mutant (BEinnB) was compared with the T3SS-deficient strain (BErhcJ). Compared with BErhcJ inoculation, BEinnB drastically increased symbiosis with V. radiata KPS1, suggesting that InnB plays important roles in controlling host-species-specific symbiotic interactions. However, a comparison of nodulation efficiency between BErhcJ and BEinnB implied that USDA61 employs additional T3Es to facilitate symbiosis with V. radiata KPS114. Despite these discoveries, the precise symbiotic implications of the B. elkanii T3SS in V. radiata cultivars have remained uncertain.
V. radiata is an important crop legume in Thailand that is consumed and used in various types of food. However, in organic farming systems, the application of rhizobial inoculants has not been as successful as expected. This may be due to the effectiveness of the rhizobial inoculants and the competition from local strains, which are less effective than commercial strains. This could be attributed to the T3SS system, which can have either positive or negative effects on nodulation in legumes.
Hence, the present study aims to shed further light on the roles of the bradyrhizobial T3SS as a determining factor for symbiosis with V. radiata cultivars. According to previous reports, NopP is a major positive determinant of nodulation in some tropical legumes, such as Flemingia congesta and Tephrosia vogelii6, but it displays negative incapability of V. radiata symbiosis8. NopP is found in various strains of rhizobium genera; however, its role in promoting mutualistic symbiosis in V. radiata has not yet been reported. Therefore, this study focused on investigating the role of NopP in V. radiata symbiosis. Our findings provide compelling evidence that T3SS-triggered symbiosis relies on a combination of T3Es, with NopP2 emerging as a crucial player essential for early infection establishment and nodule organogenesis.
Results
Phylogenetic analyses and gene localization of bradyrhizobial NopP1 and NopP2
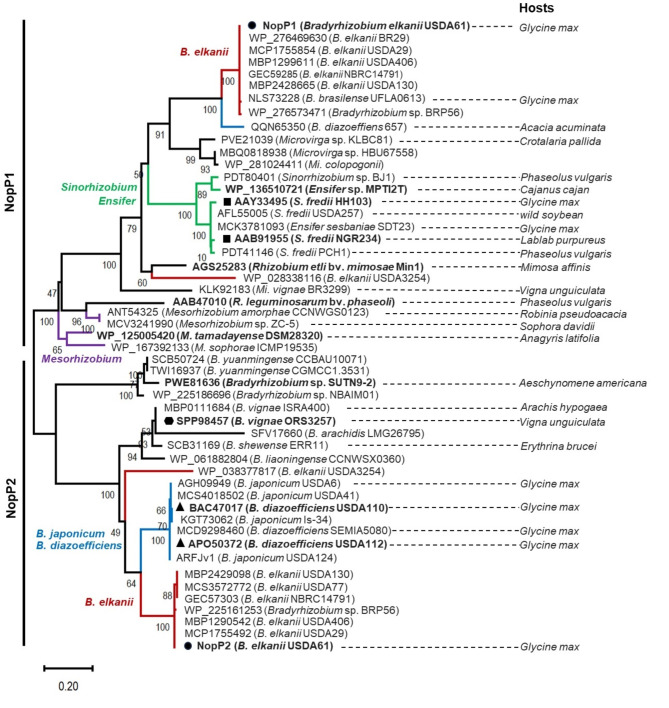
NopP is an effector secreted by the T3SS of various rhizobial species. Its function is not clearly understood, and it has no homology with any avirulence (Avr) effectors in pathogens9. To gain insight into the evolutionary history of bradyrhizobial NopPs, we conducted a phylogenetic analysis to examine the relationships among NopP homologs. The rhizobium genera included in this study were Sinorhizobium, Mesorhizobium, Rhizobium and Ensifer. Additionally, the bradyrhizobium species Bradyrhizobium elkanii, B. diazoefficiens and B. japonicum were also included. Our findings revealed that bradyrhizobial NopPs can be categorized into two distinct clusters: NopP1 and NopP2. Furthermore, both types of bradyrhizobial NopPs were conserved within their respective groups (Fig. 1). Among the various NopP1s, a close phylogenetic relationship between B. elkanii NopP1s and homologs in Microvirga sp. KLBC81, Microvirga sp. HBU67558 and Mi. calopogonii was observed (Fig. 1), whereas the Ensifer clade was slightly separated from the B. elkanii clade. In contrast, NopP1 homologs of the Mesorhizobium group and R. leguminosarum bv. phaseoli were separated from another B. elkanii NopP1.
Fig. 1.
Phylogenetic analysis of Bradyrhizobium elkanii USDA61 NopPs and their homologs among rhizobia was constructed using the Maximum Likelihood method. The analysis used the Jones-Taylor-Thornton (JTT) and Gamma Distributed (G) models, with 1,000 bootstrap replications. NopPs of B. elkanii (red), B. japonicum/B. diazoefficiens (blue), Sinorhizobium/Ensifer (green), and Mesorhizobium/Rhizobium (purple) groups are highlighted in line. The main hosts or hosts from which the rhizobial strains were first isolated are shown. The symbols (●), (■), (▲) and (⬢) indicate the nodulation of rhizobial strains previously tested with V. radiata (B. elkanii, Sinorhizobium sp., B. vignae and B. diazoefficiens, respectively).
When the evolution of NopP2 was considered, it was distinctly separated from that of NopP1. USDA61 NopP2 was grouped with B. elkanii, with the exception of Bradyrhizobium sp. BRP56, which is also included in this group. Interestingly, both the NopP2 groups of B. japonicum and B. diazoefficiens were evolutionarily related. Among the bradyrhizobium species analyzed, the majority were found to possess at least one copy of NopP. Notably, B. japonicum and B. diazoefficiens commonly had a single copy (NopP2), whereas NopP1 was absent in almost all B. japonicum and B. diazoefficiens, except for B. diazoefficiens 657 (Fig. 1). In the cases of Ensifer and Mesorhizobium, only NopP1 was detected, whereas NopP2 was not detected. Interestingly, B. elkanii contained both NopP1 and NopP2, with the evolution of NopP clearly distinct from that of other Rhizobium species. When the arrangement of the genes nopP1 and nopP2 in the genome of USDA61 was considered, nopP2 was found to be located within the symbiotic island near the nif cluster (Figs. S1A and S1C). In contrast, nopP1 was located further from the symbiotic island and was located near transposases (Figs. S1A and S1B). Moreover, the role of NopP in B. elkanii in promoting mutualistic interactions with legume plants, particularly V. radiata, has not been elucidated before. Therefore, to determine the role of NopP in promoting symbiosis in V. radiata root nodules, we constructed and tested mutants of these genes in subsequent experiments.
Functional analysis of B. elkanii NopP2 in determining the nodulation of V. radiata KPS1
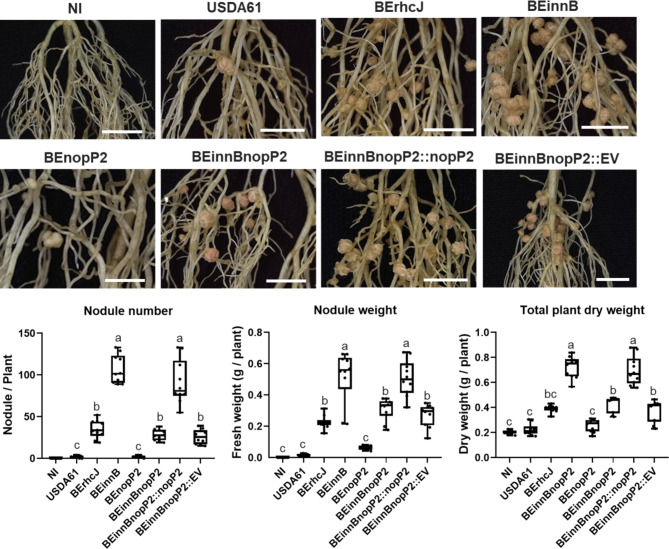
To understand the positive role of other T3Es, the T3E mutants from a previous work10 were preliminarily investigated using single mutations of nopL (BEnopL), nopP1 (BEnopP1), nopP2 (BEnopP2), and bel2-5 (BE5208) and double mutations of both innB/nopP2 (BEinnBnopP2) and innB/bel2-5 (BEinnB5208). The nodulation efficiency of T3E-deficient strains (BEnopL, BEnopP1, BEnopP2 and BE5208) was not significantly different from that of BErhcJ-inoculated strains, whereas BEinnB strongly promoted nodulation in V. radiata KPS1 (Fig. S2). Moreover, the BEinnBnopP2 double mutation strain exhibited significantly lower symbiotic efficiency than BEinnB inoculation did (Fig. 2 and 3), but its BEinnBnopP2 deletion mutant induced V. radiata KPS1 phenotypes similar to those of BErhcJ inoculation. These findings suggest that USDA61-nopP2 plays a crucial beneficial role in V. radiata KPS1 symbiosis (Fig. 2). To confirm the role of NopP2, nopP2 complementation into the BEinnBnopP2 background (BEinnBnopP2::nopP2) was employed. The results revealed that BEinnBnopP2::nopP2 enhanced KPS1 nodulation to the same degree as BEinnB did (Fig. 2). However, BEinnB5208 did not significantly differ from BEinnB in terms of the V. radiata KPS1 phenotype (Fig. S2), suggesting that bel2-5 does not contribute symbiotic properties to V. radiata KPS1 symbiosis.
Fig. 2.
Symbiotic properties of Vigna radiata KPS1 inoculated with B. elkanii USDA61 and its mutant derivatives. Cytological analysis of the nodules induced by strains USDA61, BErhcJ, BEinnB, BEnopP2, BEinnBnopP2, BEinnBnopP2::nopP2 and BEinnBnopP2::EV (empty vector) observed via stereomicroscopy (roots of KPS1; scale bars: 1 cm), nodule number, fresh nodule weight and total plant dry weight of KPS1 plants. The data shown are the means of 10 plant inoculation assays at 30 dpi. The data shown are box and whisker plots. Means followed by different letters are significantly different at the 5% level, “**” P < 0.05 according to Student’s t test.
Nodulation of V. radiata induced by USDA61-nopP2
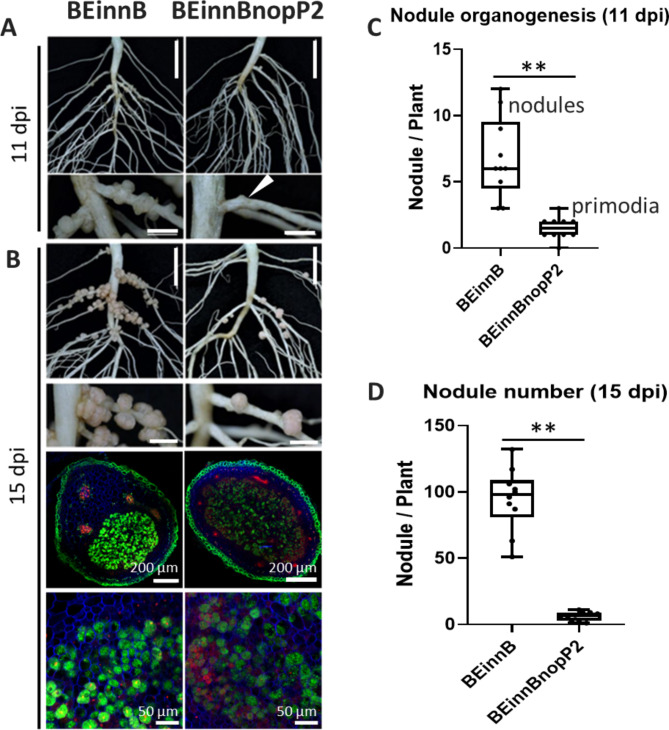
To further investigate the role of NopP2 in promoting symbiosis, we examined nodulation-related organogenesis in V. radiata KPS1 roots inoculated with BEinnB or BEinnBnopP2 at 11 and 15 days post inoculation (dpi) (Fig. 3). At the early stage of nodulation (11 dpi), BEinnBnopP2 inoculation caused a significant reduction in nodule primordia formation in V. radiata KPS1 roots compared with BEinnB, which had already formed young nodules (Fig. 3A and C). At 15 dpi, the number of young nodules established by BEinnBnopP2 inoculation was still drastically lower than that formed by BEinnB inoculation (Fig. 3B and D). Although each single nodule morphology did not seem to differ between BEinnB and BEinnBnopP2, more bacteroid cell death was observed in BEinnBnopP2 than in BEinnB (Fig. 3B).
Fig. 3.
Nodulation properties of V. radiata KPS1 inoculated with B. elkanii strains. The roots were imaged at 11 (A) and 15 (B) dpi. Cytological analysis of the nodules induced by strains BEinnB and BEinnBnopP2 observed by confocal microscopy after staining with SYTO9 (green: live bacteria), calcofluor (blue: plant cell wall), and propidium iodide (red; infected plant nuclei and dead bacteria or bacteria with compromised membranes). The white arrowhead in Figure-A shows the primordia. (C) Nodule organogenesis of root tissue (11 dpi) and (D) numbers of young nodules per plant observed at 15 dpi. The data shown are box and whisker plots of 10 plants. Scale bars: 1 cm, nodule primordia and young nodules; “**” P < 0.01 according to Student’s t test.
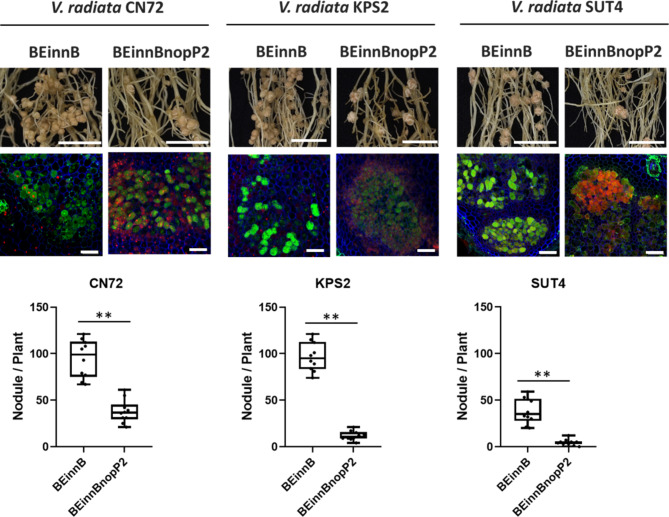
To evaluate the impact of USDA61-NopP2 on various genetic interactions in V. radiata, we inoculated various V. radiata cultivars with the BEinnB and BEinnBnopP2 bradyrhizobial strains (Fig. 4 and Table S1). Compared to BEinnB, the growth of V. radiata CN72, KPS2, and SUT4 was significantly lower with BEinnBnopP2 inoculation. BEinnBnopP2 resulted in the formation of fewer nodules (approximately 4–15 nodules per plant, depending on the cultivar) than did BEinnB, which produced significantly more symbiotic nodules (approximately 100 nodules per plant) on the CN72 and KPS2 cultivars. However, the nodule morphology in each cultivar was similar when BEinnB and BEinnBnopP2 were inoculated. The bacteroid cells derived from BEinnB were still alive inside V. radiata nodules, whereas bacteroid cell deaths were more common in nodules from BEinnBnopP2-inoculated plants (Fig. 4). Among the 12 V. radiata cultivars tested, BEinnB promoted V. radiata symbiosis better than that of BEinnBnopP2. (Table S1).
Fig. 4.
Nodulation properties of V. radiata CN72, KPS2 and SUT4 inoculated with B. elkanii strains. Roots were imaged at 30 dpi, and cytological analysis of the nodules induced by strains BEinnB and BEinnBnopP2 was performed via confocal microscopy after staining with SYTO9, calcofluor, and propidium iodide. Nodule numbers of CN72, KPS2 and SUT4 observed at 30 dpi. The data shown are box and whisker plots of 10 plants. Scale bars: 1 cm, nodule primordia and young nodules; “**” P < 0.01 according to Student’s t test.
Phylogenetic tree analysis revealed that USDA61-nopP2 clusters with B. diazoefficiens USDA122-nopP2, B. diazoefficiens USDA110-nopP2, and B. vignae ORS3257-nopP2. However, ORS3257-nopP2 clearly inhibits nodule formation in V. radiata8, and USDA122-nopP2 also has a negative effect on nodule formation in KPS19. In contrast, USDA110-nopP2 has positive effects on KPS1 nodulation9, similar to USDA61-nopP2. To clarify the positive effects of NopP2 on KPS1 symbiosis, we tested the impact of USDA61-nopP2 compared with USDA110-nopP2 at 11 and 15 dpi (Fig. S3). The results revealed that the USDA110-nopP2-deficient (110nopP) strain had a similar effect on USDA110 wild-type (USDA110) inoculation (producing approximately 40–50 nodules per plant). In contrast, BEinnBnopP2 resulted in the least amount of nodule formation (approximately 3–5 nodules per plant), but the BEinnB strain led to the greatest amount of nodule formation (approximately 100 nodules per plant). These findings suggest that USDA61-nopP2 clearly has a positive effect on KPS1 nodulation compared with USDA110-nopP2.
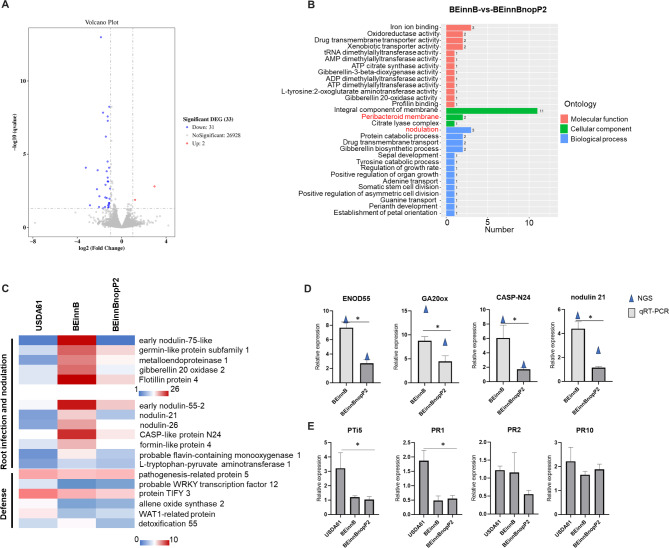
V. radiata transcriptomic analysis in response to the USDA61-T3Es (innB and nopP2) effector
The aim of the transcriptomic analysis was to elucidate the mechanisms involved in the early stage of nodulation (4 dpi) influenced by NopP2 effectors in V. radiata KPS1. A total of nine libraries generated approximately 40–50 million total reads. Among them, more than 97% of the reads from Q20 were clean (Table S5). A total of 33 differentially expressed genes (DEGs) were discerned on the basis of filtering data at an FDR < 0.05 and log2 (fold change) > 1, which were compared between genes in the BEinnB- and BEinnBnopP2-inoculated groups. The total number of upregulated genes and downregulated genes was 2 and 31, respectively (Fig. 5A). Differentially expressed genes (DEGs) were analyzed using Gene Ontology (GO) functional enrichment analysis (Fig. 5B). The number of DEGs significantly changed (P value ≤ 0.05) in each bradyrhizobial inoculation were analyzed. To better understand the impact of NopP2 effectors on the response of KPS1, a comparison of the genes differentially expressed between the BEinnB and BEinnBnopP2 treatments was performed (Fig. 5C). The upregulated DEGs were associated with protein sieve element occlusion B and protein FEZ, both of which are related to cell division. Interestingly, the downregulated genes were involved in root infection and nodule formation, including early nodulin 55 (ENOD55), early nodulin 75 (ENOD75), nodulin 21, nodulin 26, germin-like protein subfamily 1 (GLP subfamily 1), metalloendoproteinase 1 (MMPL1), gibberellin 20 oxidase (GA20ox), CASP-like protein N24 (CASP-N24), formin protein 4, flavin-containing monooxygenase 1 (FMO1), fotillin-like gene 4 (FLOT4) and L-tryptophan-pyruvate aminotransferase 1 (Fig. 5C). We also observed similar levels of genes involved in the defense pathway, such as WRKY transcription factor 12, WAT1-related protein, aspartic protease At2g35615, and allene oxide synthase 2, which are known to play roles in plant immune responses against pathogenic invasion. These results suggest that BEinnBnopP2 has fewer symbiotic interactions with V. radiata KPS1 than does BEinnB by impeding root infection and nodule organogenesis. To confirm the transcriptome data, the expression levels of 4 plant symbiosis-related genes were verified by qRT‒PCR analysis. qRT‒PCR was performed for genes associated with GO terms such as early nodulin 55 (ENOD55), gibberellin 20 oxidase (GA20ox), CASP-like protein N24 (CASP-N24), and nodulin 21 in V. radiata KPS1. The expression of these genes significantly differed between the BEinnB and BEinnBnopP2 inoculation groups, the results of which were similar to those of the transcriptome analyses (Fig. 5D). These results revealed that genes related to infection and nodule organogenesis were more highly expressed in BEinnB than in BEinnBnopP2. This finding is consistent with a previous finding that BEinnBnopP2 delays primordia formation in the early stages of nodule organogenesis compared with BEinnB. The genes related to the nodulation process of V. radiata were highly expressed at 4 days of BEinnB infection. Therefore, to understand the process more clearly at the early stage, we investigated the expression of KPS1 genes related to the plant immune system at 1 dpi. These genes included the pathogenesis-related genes transcriptional activator (PTi5), pathogenesis-related protein 1 (PR1), PR2, and PR10 (Fig. 5E). The results revealed that the PTi5 and PR1 genes were highly expressed after USDA61 inoculation, whereas BEinnB and BEinnBnopP2 presented significantly lower expression of these genes than USDA61 did. Additionally, PR2 and PR10 gene expression did not significantly differ across all of the experiments. These results indicate that USDA61-InnB inhibits the nodulation of KPS1 roots by activating the plant immune system. However, the mechanism by which USDA61-NopP2 promotes mutualistic symbiosis remains unclear. Therefore, we conducted experiments to test the interaction of USDA61-NopP2 with biomolecules in plant cells, as shown below.
Fig. 5.
Differentially expressed genes (DEGs) in V. radiata KPS1 inoculated with USDA61, BEinnB or BEinnBnopP2. (A) The total number of upregulated genes and downregulated genes was 2 and 31, respectively (B) GO functional analysis of DEGs in the BEinnB vs. BEinnBnopP2 treatment groups. (C) The expression patterns of DEGs of USDA61, BEinnB and BEinnBnopP2 are focused on and displayed as the log2-fold change (Padj < 0.05) of the DEGs. The color scale bars indicate normalized expression levels of DEGs from USDA61, BEinnB, and BEinnBnopP2. The heatmap was constructed via Microsoft Excel 365. (D) qRT‒PCR verification from RNA‒seq analysis data for V. radiata KPS1 between BEinnB and BEinnBnopP2 inoculation at and 4 dpi. (E) qRT‒PCR of pathogenetic-related data at 1 dpi. Significance is indicated by the mean ± standard deviation (n = 3), “*”, P < 0.05 according to Student’s t test.
USDA61-NopP2 and V. radiata KPS1 interacting proteins targeted
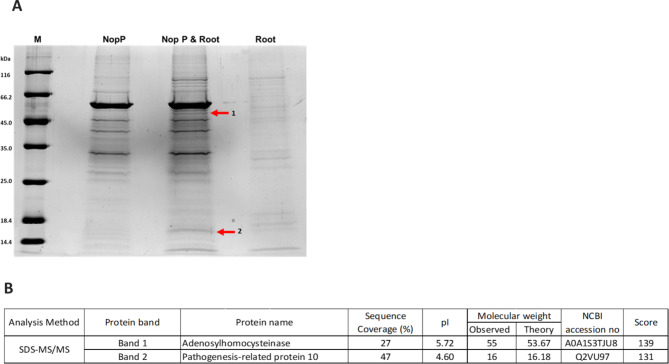
To gain more insight into the role of NopP2 in B. elkanii USDA61 in promoting symbiotic interactions with V. radiata KPS1, we determined the interactions between the USDA61-NopP2 effector and V. radiata KPS1 root proteins using an in vitro pull-down assay. The purified NopP2-GST was used as bait to capture total root proteins from V. radiata KPS1 after USDA61 inoculation (Fig. 6). The candidate plant-interacting proteins revealed two significantly intense bands in the rNopP2-GST versus root protein combination treatment (Fig. 6A), whereas these bands were not detected in the treatments with either rNopP2-GST or plant root protein alone. These candidate bands were selected for MS/MS analysis, which identified the top amino acid sequence matches in the database: band 1 was adenosylhomocysteinase, and band 2 was pathogenesis-related protein 10 (Fig. 6B).
Fig. 6.
SDS‒PAGE of the pull-down assay. (A) SDS‒PAGE; lane NopP, elution fraction from the pull-down assay of rNopP2-GST; lane NopP & Root, elution fraction from the pull-down assay of rNopP2-GST and root protein; lane Root, elution fraction from the pull-down assay of root protein. The numbers on the left of the panels indicate the positions of the molecular mass markers (Lane M) in kDa. (B) Candidate proteins derived from the pull-down assay, which were analyzed via SDS‒MS/MS.
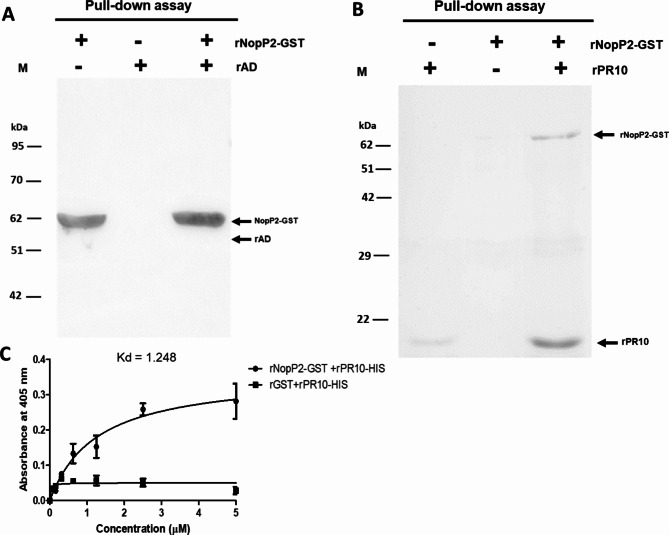
To confirm the interaction between the NopP2 effector and plant proteins, an in vitro pull-down assay was performed using individually purified proteins. Both candidate plant proteins were tagged with 6×-His, and the full-length sequence of each plant protein was also overexpressed in the pET system, while NopP2 was tagged with GST. The results demonstrated that recombinant adenosylhomocysteinase (rAD) protein was not detected in the rNopP2-GST versus rAD combination treatment (Fig. 7A), indicating that there was no interaction between adenosylhomocysteinase and USDA61-NopP2. Conversely, recombinant rNopP2-GST was found interaction in the rNopP2-GST versus rPR10 combination treatment (with a protein molecular weight of approximately 17 kDa) (Fig. 7B). Therefore, the interaction between rNopP2-GST and rPR10 was further confirmed via ELISA (Fig. 7C). Purified rPR10-His was immobilized, and different concentrations of rNopP2-GST (0–5 µM) were added to the rPR10-coated plate. The absorbance of anti-GST increased with increasing concentration of rNopP2-GST added, whereas the absorbance of anti-GST did not change with increasing rGST. These results indicate that PR10 can directly bind to USDA61-NopP2.
Fig. 7.
Western blot analysis of the in vitro pull-down assay. Lane M, protein molecular weight marker. Glutathione Sepharose was used to perform the pull-down assay. Fifteen microliters were loaded into each lane. In vitro pull-down assay of protein‒protein interactions between the recombinant root proteins and rNopP2. The binding of rAD (A) or rPR10 (B) to rNopP2 was assayed. The protein‒protein complexes were separated via 12% SDS‒PAGE and analyzed via Western blotting with an anti-His antibody and an anti-GST antibody. (C) Binding affinity between rNopP2-GST and rPR10-HIS determined by ELISA. The purified rPR10-HIS was immobilized. Recombinant NopP2-GST or rGST (0–5 µM) was added to a purified recombinant pathogen-related protein 10-coated plate, followed by probing with mouse anti-GST as the specific primary antibody and a goat anti-mouse-conjugated HRP secondary antibody. Finally, after the addition of the 2,2’-azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS) substrate, the absorbance at 405 nm (A 405) was measured. The solid lines represent the fitted curves.
Discussions
Efficient nodulation of V. radiata by Bradyrhizobium species, including B. elkanii USDA61, B. diazoefficiens USDA110, B. diazoefficiens USDA122 and B. vignae ORS3257, has been reported. Previous findings demonstrated that the NopP effectors from B. diazoefficiens USDA110 slightly promoted nodulation in V. radiata KPS19, whereas NopP from B. diazoefficiens USDA122 hindered symbiotic nodulation9. Additionally, the NopP2 protein from B. vignae ORS3257 is also responsible for incompatibility with V. radiata symbionts8, highlighting the critical role of NopPs in regulating V. radiata-specific symbiotic interactions; thus, NopP2 is not always successful or efficient for V. radiata symbiosis. Therefore, to further understand the evolutionary history of NopPs, phylogenetic analysis using NopP amino acid sequence was performed among rhizobial genera (Fig. 1). NopP rhizobia were predominantly found in Bradyrhizobium, although several were also identified in Mesorhizobium, Microvirga, and Sinorhizobium/Ensifer. Notably, many of these rhizobia can efficiently nodulate or are the main symbionts of Vigna spp. (Fig. 1). Unlike other rhizobium-specific T3Es, the NopP2 homolog was not identified in Mesorhizobium sp., Rhizobium sp., or Sinorhizobium sp./Ensifer sp. Surprisingly, B. japonicum and B. diazoefficiens were found to have only NopP2, except for B. diazoefficiens 657. The B. elkanii-NopP2 group was phylogenetically related to the NopPs of the B. japonicum/B. diazoefficiens clade, which are primarily soybean (Glycine max) symbionts (Fig. 1). The phylogenetic relationship of NopP2 suggests that the B. elkanii clade and the B. japonicum/B. diazoefficiens clade might initially recruit the nopP2 gene in a similar manner; subsequently, the evolution of nopP2 began to diverge. This finding implied that Bradyrhizobium species continuously experience changes in nopP2, with even minor genetic changes significantly impacting their ability to form nodules in soybean, as observed for strains USDA110 and USDA122. The inability of USDA122 to nodulate Rj2-soybeans is mediated by the Bradyrhizobium-specific effector NopP2, with three amino acid residues (R60, R67, and H173) playing a role9, Additionally, NopP2 of B. diazoefficiens Is-1 is incompatible with Rj2-soybean plants15, whereas USDA110-NopP2 is more compatible with Rj2-soybeans. Therefore, rhizobia may use NopPs and/or other T3Es as needed to promote symbiosis with their hosts. When comparing the amino acid sequences of NopP2 between USDA61 and USDA110 in relation to mutualistic symbiosis, and between USDA122 and ORS3257 in their negative interaction with the plant KPS1, the amino acid alignment revealed low similarity across all four strains (286 overlapping amino acid residues) (Fig. S4). The differences in amino acid sequences may cause USDA110-NopP2 to play a different role in mutualistic symbiosis with KPS1 than USDA61-NopP2 does. Perhaps, expressing USDA110-NopP2 directly in the B. elkanii USDA61 BEinnBnopP2 background could partially help demonstrate whether the protein itself has a positive regulatory effect on symbiosis, independent of its genomic background. Additionally, Bradyrhizobium species can generally nodulate V. radiata either efficiently or inefficiently, but the functions of NopPs from USDA61 remain unknown. Furthermore, nopP1 and nopP2 in the USDA61 genome were located within the symbiotic island near the nif-cluster, whereas nopP1 was situated further from the symbiotic island and was located between transposases (Fig. S1). These findings implied that nopP1 may have undergone horizontal gene transfer within the B. elkanii group. In addition, the features of NopP1 and NopP2 of USDA61, which have different numbers of amino acids (273 residues and 383 residues, respectively), revealed a putative secretion signal at the N-terminus (Fig. S5A). Research has shown that USDA61-NopP2 can be delivered into plant cells via the T3SS16. Furthermore, the three-dimensional structures of the NopP1 and NopP2 proteins were significantly different (Fig. S5B). These results suggest that the features and 3D structural models of NopPs indicate that USDA61-NopP1 and USDA61-NopP2 likely play different roles in mutualistic symbiosis with V. radiata. Therefore, it is worth studying the roles of USDA61-NopP in mutualistic interactions with KPS1 (Fig. 2).
We further functionally characterized the T3Es of USDA61, which control symbiotic interactions with the KPS1 cultivar. KPS1 established few nodules following inoculation with the wild-type strain (USDA61), whereas the nodulation efficiency drastically increased when the plants were inoculated with BEinnB. These findings indicate that USDA61-InnB is the key factor obstructing symbiosis with KPS1. However, the nodulation efficiency of BEinnB was significantly greater than that of the T3SS-deficient (BErhcJ) and InnB/NopP2 (BEinnBnopP2) double mutants, with nodule formation by BEinnBnopP2 not significantly different from that by BErhcJ. Therefore, NopP2 from USDA61 may be a crucial factor controlling KPS1 mutualism. In the case of the double mutation of InnB/Bel2-5 (BEinnB5208), the nodulation efficiency was similar to that of the single mutation of BEinnB, whereas the double mutation of InnB/nopP2 (BEinnBnopP2) drastically reduced the nodulation efficiency compared with that of BEinnB (Fig. S2). These results indicate that USDA61-Bel2-5 does not affect nodulation on KPS1, which is consistent with previous reports that Bel2-5 is not the main T3E controlling V. mungo symbiosis10. Therefore, we can conclude that USDA61-NopP2 is the key T3E that increases the efficiency of KPS1 symbiosis.
Indeed, the BEinnBnopP2 double mutation of USDA61 impaired both nodule organogenesis and young root nodule formation in KPS1 (Fig. 3A and C). Although the nodule morphology did not seem different between BEinnB and BEinnBnopP2, bacteroid cell death was more common in BEinnBnopP2 (Figs. 3B and 4). It seems that USDA61-NopP2 promotes nodulation and enhances bacteroid health. Similarly, a similar phenomenon was observed in the symbiotic interaction between B. diazoefficiens USDA110 and V. radiata, where a high number of nodules occasionally formed in inoculated KPS1 plants. In contrast, the USDA110-NopE1/E2-deficient strain nearly completely prevented nodulation4. These findings suggest that rhizobia have evolved different T3Es to inhabit the root nodules of V. radiata. Additionally, legumes may have developed multiple strategies to respond to different T3Es, thereby increasing the efficiency of their mutualistic relationships. In addition, in almost all Thai V. radiata cultivars, USDA61-NopP2 promoted symbiotic nodulation (Fig. 4 and Table S1), and nodule formation by BEinnBnopP2 was not significantly different from that by BErhcj. Perhaps USDA61-NopP2 is the key factor that promotes Thai V. radiata-B. elkanii USDA61 symbiosis. (Table S1). Our results suggest that USDA61-NopP2 might be directly recognized or indirectly recognized by an unknown specific V. radiata protein, consequently promoting nodulation. These results suggest that such symbiosis in V. radiata is likely induced through NFs in the absence of USDA61-NopP2, albeit weakly. Thus, USDA61-NopP2 may play complementary functions in modulating symbiosis and/or reducing V. radiata defense responses via unknown pathways to increase nodulation.
High-throughput sequencing and qRT‒PCR are generally used to confirm the expression of transcripts (Fig. 5). Transcriptomes were analyzed in KPS1 to decipher how KPS1 responds to USDA61-NopP2. In this study, transcriptomic analysis was useful for identifying plant genes associated with BEinnB and BEinnBnopP2 inoculation (Fig. 5). The GO term analysis indicated that the number of downregulated genes was greater than the number of upregulated genes related to biological processes such as nodulation, gibberellin biosynthetic processes, cellular components, and the peribacteriod membrane after BinnBnopP2 inoculation. It is related to the network of metabolic pathways, such as their growth, development, and nodule formation. It seems that USDA61-NopP2 facilitate KPS1 symbiosis. The expression of some intermediates of GA signaling-related genes (GA20ox), fotillin-like gene 4 (FLOT4), ENOD55, nodulin 26 and CASP-24 was suppressed by BEinnBnopP2 compared with BEinnB. Because nodule organogenesis is systemically controlled by legume plants, several phytohormones are regulated in response to rhizobia NFs and USDA61-NopP2. In addition, the biosynthesis of gibberellic acid (GA) in legumes requires enzymes such as GA20 oxidase (GA20ox), which is a rate-limiting factor that involves intermediate steps in GA production17. Our results revealed that GA20ox is more highly expressed in BEinnB roots than in BEinnBnopP2 roots at 4 dpi. This finding is consistent with previous reports in Sesbania rostrata and V. radiata, where GA20ox is primarily upregulated in plant tissues involved in infection and nodule primordia formation4,18, indicating that USDA61-NopP2 is a factor related to legume GA production. Moreover, FLOT4 was also significantly expressed in BEinnB compared with BEinnBnopP2 inoculation, similar to FLOT4 of S. meliloti, which is localized to the infection thread membrane of Medicago truncatula, and it plays an important role in symbiotic bacterial infection19. CASP-24, nodulin 26, and ENOD55/ENOD75 are also factors expressed very early in the development of the root nodules of legumes20–22. In KPS1, USDA61-NopP2 appears to play a role in enhancing the expression of genes related to infection and nodule organogenesis, which are important for the nodulation process. Additionally, this is a novel finding regarding the mechanism of USDA61-NopP2 and its interaction with plant biomolecules, as USDA61-NopP2 can interact with pathogenesis-related protein 10 (PR10) (Fig. 7). The PR10 protein is part of the plant’s defense against biotic stresses. PR10 proteins can collaborate with other factors to participate in pathogen resistance23,24. Research has shown that the expression of the PR10 gene increases in the roots and nodules of M. truncatula when it is invaded by microorganisms25. Therefore, USDA61-NopP2 may bind to KPS1-PR10, resulting in the inactivation of the role of PR10. This, in turn, may enhance the mutualistic relationship between BEinnB and KPS1 compared with BEinnBnopP2. However, PR10 performs various functions, including binding to hormone molecules, interacting with secondary metabolites, and defending against biotic and abiotic stressors23. The role of PR10 in the development of root nodules in KPS1 is not clearly understood and may be a subject for future research. We propose a model modulated by USDA61-NopP2 in Fig. 8. The USDA61-NopP2 effector is required to optimize nodulation efficiency by interacting with the plant PR10 protein, which is deleterious to rhizobial infection. Therefore, USDA61-NopP2 can trigger nodulation by modulating the expression of genes related to infection and nodule organogenesis in KPS1.
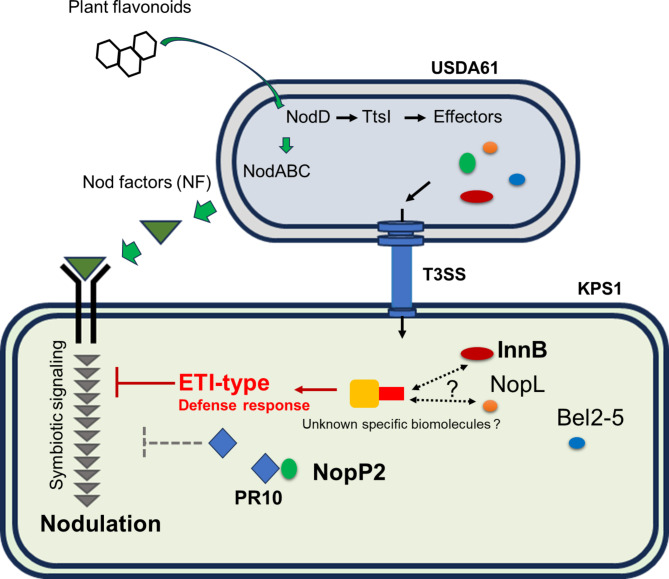
Fig. 8.
Putative models of host genotype-specific symbiotic interactions between B. elkanii USDA61 and V. radiata KPS1 may be controlled mainly by the two type III effectors InnB and NopP2. Hypothetically, InnB might be recognized by unknown biomolecule(s) or a specific resistance (R) protein in legumes, consequently activating an R protein-mediated ETI-type defense. In contrast, NopP2 is a nodulation determinant that enables the nodulation of V. radiata KPS1 by interacting with the pathogenesis-related 10 (PR10) protein, thereby reducing the defense response (ETI-type) to inhibit nodulation. The dotted line indicates unclear symbiotic mechanisms.
Materials and methods
Microbiological and molecular techniques
Bradyrhizobium elkanii USDA61 and its mutant strains were grown at 28 °C in arabinose-gluconate (AG) medium26, and Escherichia coli was grown on LB medium at 37 °C27. The bacteria and plasmids used are summarized in Table S2, and the antibiotic concentrations that were added to the media as follows (µg/ml): ampicillin, 100; kanamycin, 200; and polymyxin, 50. For USDA61-nopP2 complementation, the USDA61-nopP2 gene was amplified (including the tts box promoter region) by PCR were cloned and inserted into the plasmids pMG103 sm/sp and km-gfp. The primers used are listed in Table S3. The electrocompetent cells of BEinnBnopP2 were prepared by growing bacterial cells in AG medium until the OD600 of the cells reached 0.4–0.6. The bacterial cells were subsequently harvested by centrifugation at 4,000 rpm for 15 min, carefully washed twice with cold sterilized water and finally resuspended in 10% cold glycerol (stored at − 80 °C before use). The plasmid transformation was carried out using electroporation with capacitor settings of 25 µF, 100 Ω, and a voltage of 1.75 kv/cm for 0.2 cm cuvettes, and the complement strain was selected on AG agar plates with appropriate antibiotics.
Plant materials and nodulation assays
The 12 genotypes of Vigna radiata that were tested are provided by Professor Piyada Alisha Tantasawat, all Vigna radiata genotypes were Thai published varieties that are normally cultivated by Thai farmers (KPS1, KPS2, SUT1, SUT2, SUT3, SUT4, SUT5, M4-2, M5-1, CN36, CN72 and CN84-1)4 (Table S4). This study complies with local and national regulations in Thailand. The V. radiata seeds used were sterilized and subsequently germinated as described previously4. Five days after transplantation, the seedlings were inoculated with USDA61 and its derivative mutants (1 ml of 107 cells ml− 1 per seedling). The plants were watered with BNM solution28 and grown under the following controlled environmental conditions: 28 ± 2 °C and 70% relative humidity under a 16 h light/8 h dark photoperiod and a light intensity of 300 µE/m2/s. The V. radiata symbiotic phenotypes were analyzed at 11, 15, and 30 dpi by evaluating the nodule image, nodule number, nodule fresh weight, whole-plant dry weight, and nodule primordia formation.
Cytological analysis of nodules
A nodule image was examined using a Leica Microsystems EZ4 Stereo Microscope (Leica, Nanterre, France). Fresh nodules were embedded in 5% agar and sectioned into 40–50 μm thick slices with a VT1000S vibratome (Leica, Nanterre, France). The nodule sections were immersed in a live/dead staining solution (5 µM SYTO 9 and 30 µM propidium iodide in 50 mM Tris buffer, pH 7.0) for 15 min. To stain the plant cell wall, the nodule sections were additionally incubated for 20 min with calcofluor white M2R (0.01% (wt/vol) calcofluor white M2R in 10 mM phosphate saline buffer). The stained nodules were then observed under an inverted confocal microscope using a Nikon A1Rsi (Nikon, USA). Lasers were used to detect Calcofluor, SYTO-9, and propidium iodide (PI) as follows: Calcofluor white was excited at 405 nm and detected using a 460–500 nm emission filter. For SYTO-9 and PI, excitation wavelengths of 488 nm and 555 nm, respectively, were used to collect emission signals at 490–522 nm and 555–700 nm.
V. radiata KPS1 mRNA transcriptome
For RNA-seq, KPS1 seeds were surface sterilized and germinated at 25 °C for one day. They were then transplanted into Leonard’s jars and watered with BMN solution. After five days of growth, the KPS1 seedlings were inoculated with 1 ml of bacterial cell culture containing 107 cells ml−1. At 4 dpi, the KPS1 roots were washed and immediately transferred to liquid nitrogen. The frozen roots were then ground into a fine powder. Total RNA was subsequently extracted from 100 mg of root powder using the RNeasy Plant Mini Kit (Qiagen) with DNase I treatment, following the manufacturer’s instructions. Reverse transcription of 4 µg of total RNA was performed according to the manufacturer’s protocol for the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina). Each library was sequenced using the Illumina platform, and the sequences were analyzed using a bioinformatics approach via GENEWIZ in Suzhou, China. Differentially expressed genes (DEGs) were selected for further analysis on the basis of a Padj value of less than 0.05 and a fold change greater than 1.5. Data is provided within supplementary information files.
qRT‒PCR of DEGs
The selected DEGs of KPS1 were further analyzed via qRT‒PCR using the same protocol described above. The primers used for qRT‒PCR are listed in Table S4. The PCR amplification program included an initial denaturation step at 95 °C for 2 min, followed by 40 cycles at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s, with a final extension step at 72 °C for 10 min. Relative gene expression was analyzed using the comparative Ct (−ΔΔCT) method29, and the transcript levels of selected V. radiata DEGs were normalized to the expression of the housekeeping gene β-actin30–35. The data for each sample were calculated from three biological replicates.
KPS1 root protein extraction
KPS1 was used for root protein extraction. Five days after transplantation, the seedlings were inoculated with USDA61 or its derivative mutant BEinnB (1 ml of 107 cells ml− 1 per seedling). The KPS1 roots were collected at 1 dpi and 2 dpi and then immediately frozen in liquid nitrogen. Three grams of roots were ground into a fine powder under liquid nitrogen and mixed with 10 ml of extraction buffer (0.5 M Tris-HCl, 10% sucrose, 3 mM PMSF, 10 mM ascorbic acid, 0.2% Triton X-100 and 1% β-mercaptoethanol; pH 8.0). The root extraction buffer was centrifuged at 12,000 × g at 4 °C for 20 min, and the organic phase was transferred into a new tube. Then, 7.5 ml of Tris-EDTA-saturated phenol and 1 ml of 1 M dithiothreitol (DTT) were added. The solution was mixed thoroughly and centrifuged again at 12,000 × g at 4 °C for 20 min. The phenol phase was transferred into a new tube containing 20 ml of methanol, 300 µl of 8 M ammonium acetate, and 400 µl of 1 M DTT. The solution was mixed well and stored at -20 °C overnight. The mixture was subsequently centrifuged at 4 °C and 10,000 × g for 10 min, followed by resuspension with 1 ml of 70% ethanol for washing. After centrifugation, the ethanol was removed, and the pellet was air dried. The root proteins were resuspended to saturation in Tris-HCl (20 mM, pH 7.4) and quantified according to the Bradford method36.
Recombinant protein production of NopP2, adenosyl homocysteinase and pathogen-related protein 10
To construct expression vectors for recombinant protein production, the NopP2 and adenosyl homocysteinase genes were cloned and inserted into the pGEX4T-3 vector, and the coding sequence for pathogen-related protein 10 was cloned and inserted into the pET22b vector. The recombinant plasmid was subsequently transformed into E. coli BL21 for expression. E. coli BL21 harboring the recombinant plasmid was grown at 37 °C in 5 ml of LB medium supplemented with 100 µg/ml ampicillin with shaking at 200 rpm and 37 °C overnight. 1% v/v of each overnight culture was inoculated into LB medium supplemented with 100 µg/ml ampicillin. The culture was subsequently incubated with shaking at 37 °C until the OD 600 reached 0.4–0.6. The induction was performed by adding 1 M isopropyl-βd-thiogalactopyranoside (IPTG) to achieve a final concentration of 1 mM. After the cells were grown for 4 h at 30 °C with shaking, they were collected by centrifugation at 16 °C and 8,000 rpm for 15 min and resuspended in buffer (20 mM Tris-HCl, 150 mM NaCl, and 20 mM imidazole; pH 7.4). Sonication was then performed before the purification of the recombinant protein. The GST/hexahistidine tag was used for detection and purification. The protein concentration was quantified by the Bradford method, and the purity of the purified protein was determined via SDS‒PAGE and Western blotting with mouse anti-GST (Abbkine, USA) or mouse anti-HSI (Abbkine, USA) and goat anti-mouse conjugated HRP.
Pull-down assay
To identify novel interacting partners the predicted protein–protein interaction, a pull-down assay was performed to investigate protein–protein interactions between NopP2 and the root protein. The purified rNopP2-GST fusion protein was attached to glutathione beads. The extracted root protein was added and incubated at 4 °C overnight. The protein mixture was then washed twice with phosphate-buffered saline (PBS). Elution of the GST-tagged proteins was performed by adding 50 mM reduced glutathione in 100 mM Tris, pH 7.4. The total amount of eluted protein was subsequently analyzed using polyacrylamide gel electrophoresis. The distinct bands of the rNopP2-GST/root, compared with those of root protein and rNopP2-GST, were identified and excised for SDS‒MS/MS analysis. The LC‒MS/MS system consists of a liquid chromatograph (Dionex Ultimate 3000, RSLCnano System, Thermo Fisher Scientific, Waltham, MA, USA) in combination with a captivespray ionization/mass spectrometer (Model Q-ToF Compact, Bruker, Germany) at the Proteomics Services, Faculty of Medical Technology, Mahidol University (Salaya Campus, Mahidol University, Nakhon Pathom, Thailand). Mass spectral data from 300 to 1500 m/z were collected in positive ionization mode. In order to confirm the interaction of candidate root proteins (rAD and rPR10), rNopP2-GST fusion protein or recombinant root proteins were attached to glutathione beads or Ni-NTA agarose bead. The protein-protein interaction was again carried out by pull-down assay and detected by western blot analysis as describe below.
Western blot analysis
The recombinant proteins were blotted onto nitrocellulose membranes (Bio-Rad, USA) from the SDS polyacrylamide gel using a membrane transfer machine. The membrane was blocked with PBS containing skim milk (5% MPBS) for 1 h, followed by washing with PBS twice. After that, mouse anti-GST (Abbkine, USA) and/or mouse anti-HIS (Abbkine, USA) were used to detect the GST tag/HIS tag. The membrane was incubated with primary antibody at a dilution of 1:5000 in PBS for 1 h, followed by 3 washes with PBS supplemented with 0.05% Tween 20 detergent (PBST) and 2 washes with PBS. After that, goat anti-mouse conjugated HRP (Abbkine, USA) diluted 1:5000 in PBS was added, and the membrane was incubated for 1 h before development. The enhanced chemiluminescence (ECL) substrate (Cytiva, USA) was used according to the manufacturer’s protocol for developing the signals on the membrane.
ELISA
The binding affinity of NopP2 for PR10 was determined by ELISA according to the protocol of Eble37 with some modifications. An Immuno96 microwell plate (Nunc, Denmark) was used to immobilize 5 µg of rPR10-HIS in 100 µl of 100 mM NaHCO3, pH 9.0, at 4 °C overnight. The wells were rinsed 3 times with PBS to remove any non-specific binding, followed by stabilization with 100 µl of stabilizer (5% sucrose, 0.3% BSA, and 50 mM NaHCO3) for 45 min. The wells were rinsed with PBS twice and blocked with 0.5% (w/v) BSA at room temperature for 1 h. After that, the wells were rinsed 4 times with PBST and 2 times with PBS. Then, 0–5 µM rNopP2-GST or rGST was added to the wells and incubated at room temperature for 1 h. The wells were washed 4 times with PBST, followed by 2 washes with PBS before the addition of the antibody against the GST tag mouse anti-GST (1:5000). The wells were washed 4 times with PBST, followed by 2 washes with PBS. After the plates were incubated at room temperature for 1 h, a 1:5000 dilution of goat anti-mouse conjugated HRP in 100 µl of PBS was added to each well, and the mixture was incubated at room temperature for 1 h. The wells were washed 4 times with PBST followed by 2 washes with PBS. The color of the reaction was developed by adding 200 µl of ABTS substrate (Wako, Japan), and the plates were incubated at room temperature for 5 min before the absorbance was measured at 405 nm with an ELISA plate reader.
Phylogenetic construction and statistical analysis
For phylogenetic and evolutionary analyses, NopP homologs were queried via BLASTx against genomic databases of rhizobial species. A phylogenetic tree was constructed using the Maximum Likelihood method from the MEGA 11.0 package. The analysis used the Jones-Taylor-Thornton (JTT) and Gamma Distributed (G) models, with 1,000 bootstrap replications. For statistical analyses, one-way analysis of variance (ANOVA) followed by post hoc tests (Tukey’s tests at P ≤ 0.05) was performed using Minitab version 16.0 for multiple test sample comparisons. Two-tailed Student’s t tests were also performed for pairwise comparisons when needed. P values < 0.05 were considered statistically significant. The sample size and replications are detailed in the figure and table legends.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by (i) Suranaree University of Technology (SUT), (ii) Thailand Science Research and Innovation (TSRI), and (iii) National Science, Research, and Innovation Fund (NSRF) (grant number 195582), (iv) NSRF via the Program Management Unit for Human Resources & Institutional Development, Research, and Innovation (grant number B13F660055), (v) JSPS-NRCT by National Research Council of Thailand (grant number N11A670769). (vi) The Office of the Permanent Secretary of the Ministry of Higher Education, Science, Research and Innovation.
Author contributions
ContributionsP.P., H.P.N., P.S., P.T., N.B., K.T., T.G., P.N., J.W., S.S. and N.T. designed the experiments. P.P., H.P.N., N.P., S.O., P.B. and N.T. performed the experiments and analysed the data. P.P., S.O., and N.T. wrote the paper.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). The RNA sequences generated in this study were deposited in NCBI under accession numbers PRJNA1167634.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors equally contributed as co-first authors: Pongdet Piromyou, Natcha Pruksametanan and Hien P. Nguyen.
Contributor Information
Pakpoom Boonchuen, Email: pakpoom.b@sut.ac.th.
Shin Okazaki, Email: sokazaki@cc.tuat.ac.jp.
Neung Teaumroong, Email: neung@sut.ac.th.
References
- 1.Oldroyd, G. E. D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol.11, 252–263 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Krause, A., Doerfel, A. & Göttfert, M. Mutational and transcriptional analysis of the type III secretion system of Bradyrhizobium Japonicum. Mol. Plant-Microbe Interact.15, 1228–1235 (2002). [DOI] [PubMed] [Google Scholar]
- 3.Wassem, R. et al. TtsI regulates symbiotic genes in Rhizobium species NGR234 by binding to tts boxes. Mol. Microbiol.68, 736–748 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piromyou, P. et al. The Bradyrhizobium diazoefficiens type III effector NopE modulates the regulation of plant hormones towards nodulation in Vigna radiata. Sci. Rep.11, 1–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai, W. J., Zeng, Y., Xie, Z. P. & Staehelin, C. Symbiosis-promoting and deleterious effects of NopT, a novel type 3 effector of Rhizobium sp. strain NGR234. J. Bacteriol.190, 5101–5110 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skorpil, P. et al. NopP, a phosphorylated effector of Rhizobium sp. strain NGR234, is a major determinant of nodulation of the tropical legumes Flemingia congesta and Tephrosia vogelii. Mol. Microbiol.57, 1304–1317 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Jiménez-Guerrero, I. et al. The Sinorhizobium (Ensifer) fredii HH103 nodulation outer protein NopI is a determinant for efficient nodulation of soybean and cowpea plants. Appl. Environ. Microbiol.83, 1–13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Songwattana, P. et al. Identification of type III effectors modulating the symbiotic properties of Bradyrhizobium vignae strain ORS3257 with various Vigna species. Sci. Rep.11, 4874. 10.1038/s41598-021-84205-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugawara, M. et al. Variation in bradyrhizobial NopP effector determines symbiotic incompatibility with Rj2-soybeans via effector-triggered immunity. Nat. Commun.9, 3139. 10.1038/s41467-018-05663-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen, H. P., Ratu, S. T. N., Yasuda, M., Teaumroong, N. & Okazaki, S. Identification of Bradyrhizobium elkanii USDA61 type III effectors determining symbiosis with Vigna mungo. Genes11, 474. 10.3390/genes11050474 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miwa, H. & Okazaki, S. How effectors promote beneficial interactions. Curr. Opin. Plant. Biol.38, 148–154 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Staehelin, C. & Krishnan, H. B. Nodulation outer proteins: Double-edged swords of symbiotic Rhizobia. Biochem. J.470, 263–274 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Nguyen, H. P., Miwa, H., Kaneko, T., Sato, S. & Okazaki, S. Identification of Bradyrhizobium elkanii genes involved in incompatibility with Vigna radiate. Genes8, 374. 10.3390/genes8120374 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen, H. P., Ratu, S. T. N., Yasuda, M., Göttfert, M. & Okazaki, S. InnB, a novel type III effector of Bradyrhizobium elkanii USDA61, controls symbiosis with Vigna species. Front. Microbiol.9, 3155. 10.3389/fmicb.2018.03155 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizuka, J., Yokoyama, A. & Suemasu, Y. Relationship between serotypes of Bradyrhizobium japonicum and their compatibility with Rj-cultivars for nodulation. Soil. Sci. Plant. Nutr.37, 23–30 (1991). [Google Scholar]
- 16.Okazaki, S., Zehner, S., Hempel, J., Lang, K. & Göttfert, M. Genetic organization and functional analysis of the type III secretion system of Bradyrhizobium elkanii. FEMS Microbiol. Lett.295, 88–95 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Hedden, P. The current status of research on gibberellin biosynthesis. Plant. Cell. Physiol.61, 1832–1849 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lievens, S. et al. Gibberellins are involved in nodulation of Sesbania rostrata. Plant. Physiol.139, 1366–1379 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haney, C. H. & Long, S. R. Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc. Natl. Acad. Sci. U.S.A.107, 478–483 (2010). [DOI] [PMC free article] [PubMed]
- 20.Okazaki, S., Kaneko, T., Sato, S. & Saeki, K. Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system. Proc. Natl. Acad. Sci. U S A. 110, 17131–17136 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivers, R. L. et al. Functional Analysis of Nodulin 26, an aquaporin in soybean root nodule symbiosomes. J. Biol. Chem.272, 16256–16261 (1997). [DOI] [PubMed] [Google Scholar]
- 22.Franssen, H. J. et al. Characterization of cDNA for nodulin-75 of soybean: A gene product involved in early stages of root nodule development. Proc. Natl. Acad. Sci. U S A. 84, 4495–4499 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes, N. et al. Pathogenesis-related protein 10 in resistance to biotic stress: progress in elucidating functions, regulation and modes of action. Front. Plant. Sci.14, 1193873. 10.3389/fpls.2023.1193873 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha, R. K., Verma, S. S. & Rastogi, A. Role of pathogen-related protein 10 (PR 10) under abiotic and biotic stresses in plants. Phyton89, 167–182 (2020). [Google Scholar]
- 25.Gamas, P., De Billy, F. & Truchet, G. Symbiosis-specific expression of two Medicago Nodulin genes, MtN1 and MtN13, encoding homologous to Plant Defense proteins. Mol. Plant-Microbe Interact.11, 393–403 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Sadowsky, M. J., Tully, R. E., Cregan, P. B. & Keyser, H. H. Genetic diversity in Bradyrhizobium japonicum Serogroup 123 and its relation to genotype-specific nodulation of soybean. Appl. Environ. Microbiol.53, 2624–2630 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood, E. Molecular cloning A: Laboratory manual. Biochem. Educ.11, 82 (1983). [Google Scholar]
- 28.Ehrhardt, D. W., Atkinson, M., Long, S. R. & E. & Depolarization of alfalfa root hair membrane potential by Rhizobium meliloti nod factors. Science256, 998–1000 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Narancio, R., John, U., Mason, J. & Spangenberg, G. Selection of optimal reference genes for quantitative RT-PCR transcript abundance analysis in white clover (Trifolium repens L). Funct. Plant. Biol.45, 737–744 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Chi, C. et al. Selection and validation of reference genes for gene expression analysis in Vigna angularis using quantitative real-time RT-PCR. PLoS One11, e0168479. 10.1371/journal.pone.0168479 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ke, X. W. et al. Reference genes for quantitative real-time PCR analysis of gene expression in mung bean under abiotic stress and Cercospora canescens infection. Legume Res.44, 646–651 (2021). [Google Scholar]
- 33.Kundu, A., Patel, A. & Pal, A. Defining reference genes for qPCR normalization to study biotic and abiotic stress responses in Vigna mungo. Plant. Cell. Rep.32, 1647–1658 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Dasgupta, U. et al. Comparative RNA-Seq analysis unfolds a complex regulatory network imparting yellow mosaic disease resistance in mungbean [Vigna radiata (L.) R. Wilczek]. PLoS One16, e0244593. 10.1371/journal.pone.0244593 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjarnadottir, H. & Jonsson, J. J. A rapid real-time qRT-PCR assay for ovine β-actin mRNA. J. Biotechnol.117, 173–182 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- 37.Eble, J. A. Titration elisa as a method to determine the dissociation constant of receptor ligand interaction. J. Vis. Exp. e57334 (2018). 10.3791/57334 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files). The RNA sequences generated in this study were deposited in NCBI under accession numbers PRJNA1167634.