Abstract
Purpose
To increase awareness of peri-radiation therapy (RT) intervention that may unduly heighten the risk of toxicity in lung cancer patients and encourage molecular testing and pretreatment consultation with rheumatology for patients with active autoimmune conditions.
Materials and Methods
A 42-year-old male with an autoimmune disease was diagnosed with non–small cell lung cancer. He received 4 cycles of pemetrexed/cisplatin with proton therapy (PT) delivered halfway through for a bronchial stump positive margin. After completing the first cycle of adjuvant chemotherapy, he was given 61.6 Gy in 28 fractionations of PT. Before restarting chemotherapy, he experienced a dry cough and later shortness of breath (SOB), which resolved with an aggressive steroid taper. After completing his third cycle of cisplatin/pemetrexed, his SOB and cough worsened. He was admitted for an urgent bronchoscopy with debridement of the distal trachea and proximal left main bronchus. He received high-dose steroids again and another bronchoscopy, revealing a tracheoesophageal fistula. Rheumatology identified an MDA5+ and PL7-positive dermatomyositis subtype at this time, known to be associated with rare ulcerative symptoms.
Results
A rare MDA5+ and PL7-positive dermatomyositis subtype, discovered post treatment, most likely contributed to SOB and cough following chemotherapy and PT, resulting in bronchoscopy of the irradiated field. A combination of these factors may have contributed to the tracheoesophageal fistula.
Conclusion
Patients with autoimmune disease should be carefully evaluated for rare underlying subtypes that could pose a danger to treatment. Oncologists should continue to be vigilant about underlying genetic predisposing factors that lead to exacerbated toxicity. Immunosuppressive agents given with RT may be considered for patients with autoimmune disease. Avoidance of biopsy, tissue manipulation, debridement, or any form of soft-tissue or hard-tissue violation needs to be discussed across the multidisciplinary spectrum to avoid nonhealing lesions shortly after RT.
Keywords: Autoimmune toxicity, Concurrent chemotherapy, Proton therapy
Introduction
Classically, patients with underlying autoimmune or inflammatory disease have been considered higher risk for radiation therapy (RT).1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 RT, with its proinflammatory effects, has been suggested to aggravate these underlying conditions and lead to toxicity,14 particularly in patients with lupus, inflammatory bowel disease, and scleroderma.1, 3, 4, 5, 9, 10, 11
The risk of heightened short-term or long-term complications in patients with dermatomyositis is less well known due to the rarity of the condition, with fewer than 10 patients in all radiation effects reports. However, 1 center has reported on its experience.12, 15 Of the 4 patients with dermatomyositis treated, none had exacerbated short-term or long-term complications when receiving RT. Likewise, the other patients treated who had differing collagen-vascular disease, such as scleroderma, lupus, polymyositis, and rheumatoid arthritis, had no increased chance of side effects compared to patients who did not have autoimmune disease.12 This lack of an increased risk of radiation toxicity associated with autoimmune disease has appeared more frequently in recent reports.3, 4, 7, 12, 16 This is perhaps a result of more careful attention to RT techniques deployed today, the better control of active autoimmune disease with modern effective drugs and biologics, or selection of patients with more mild autoimmune activity. However, for those with dermatomyositis, the notion that such inflammatory conditions could contraindicate radiation is increasingly being brought into question.3, 4, 7, 12, 16
Here, we present an exceptionally rare case of a Common Terminology Criteria for Adverse Effects Version 5.0 grade 5 tracheoesophageal (TE) fistula in a 42-year-old patient with dermatomyositis who received a relatively modest dose of postoperative proton therapy (PT) to his lung cancer’s positive bronchial margin. This is the first report to our knowledge, and there are numerous unique aspects to this case that could have contributed to or caused the event, not least of which was biopsy and mechanical manipulation of the friable radiated bronchial tissue shortly after treatment. MDA5+ and PL7-positive dermatomyositis have a high propensity to develop interstitial lung disease (ILD),17, 18, 19 requiring computed tomography (CT) ILD screening at the time of molecular diagnosis.20, 21 Not only is ILD a known predictor of radiation toxicity,22, 23 but both PL7-positive and MDA5+ dermatomyositis are known to develop spontaneous tracheal and bronchial ulceration of the cartilage, not seen in other subtypes.24, 25 Moreover, the ILD resultant from his dermatomyositis may have also led to non–small cell lung cancer (NSCLC).26
We discuss this case in detail and offer a brief review and discussion of the literature as it pertains to autoimmune disease and RT, especially particle therapy and dermatomyositis. The goal of this report is to bring attention to treatment complexity and to avoid peri-RT intervention that might unduly heighten the risk of toxicity. We also aim to bring special attention to molecular testing and pretreatment consultation with rheumatology before treating patients with dermatomyositis or other autoimmune conditions and cancer to ensure a more highly sensitive subtype is not present.
Case presentation
A young 42-year-old male, never-smoker, with a recent history of complex active autoimmune disease was subsequently diagnosed with a cT2aN1M0 NSCLC adenocarcinoma of the left lower lobe (LLL). Preceding this cancer diagnosis, he presented to his primary care physician with joint swelling and tenderness of his fingers and ankles after a recent vacation. He also described times in the past when he had bouts of rash, photosensitivity, and shortness of breath (SOB) consistent with antisynthetase syndrome.
He ultimately underwent referral to rheumatology, where he was found to be ANA+ and dsDNA+ and received the final diagnosis of dermatomyositis. Due to SOB and lung disease that can sometimes be associated with dermatomyositis, he received a CT chest for evaluation. This revealed a moderate 2 to 3 cm cavitary mass in the LLL. This biopsy revealed, unfortunately, revealed NSCLC adenocarcinoma and left hilar adenopathy (N1). Notably, no definite diffuse ILD was seen at that time. Following his diagnosis, he was placed on prednisone 15 mg every day and hydroxychloroquine. He was also offered mycophenolate to help control his dermatomyositis.
Surgery was recommended at the multidisciplinary conference in hopes of avoiding radiation. At the time of surgery, his mediastinal structures were noted to have “dense desmoplastic reactions” and his tissue “stuck like glue” with lung parenchyma “disintegrating like tissue paper” at the time of lobectomy. The video-assisted thoracoscopic surgery was converted to an open procedure for greater mobility due to tissue adhesion. For hemostasis control, a vascularized pedicle soft-tissue flap was bolstered to the bronchial stump following lobectomy (intercostal muscle flap). Despite this, his postoperative course was complicated by hematoma, requiring re-exploration, posterolateral thoracotomy, and subcutaneous flap. His final pathology showed a 2.9 cm LLL moderately differentiated NSCLC adenocarcinoma with positive hilar margins at the bronchial stump. There were positive lymph nodes in stations 4L and 12L (2/35 LNs+; N2). There was 1 intralobar pulmonary metastasis vs satellite lesion and his primary mass adhered to the visceral pleura (T3). Therefore, he was in final Stage IIIB (cT2aN1M9, pT3N2M0) with margin-positive disease. His joint swelling improved after surgery, indicating autoimmune relief either from an anti-immune medication regimen or paraneoplastic syndrome.27
Given the margin status and nodal disease, he was recommended to proceed with concurrent chemoradiation. Before starting concurrent chemoradiation, however, he obtained a second opinion from a major cancer center with PT. His final next generation sequencing/immunohistochemistry profiling identified an anaplastic lymphoma kinase (ALK) rearrangement. Despite this, anti-ALK therapy was not approved or indicated for him as a standard of care option at the time, given that he had nonmetastatic NSCLC.
He received pemetrexed and cisplatin for a total of three cycles with PT between the first and second cycle. Unfortunately, during chemotherapy, he had substantial immune flares and required intravenous immunoglobulin throughout his entire course. JAK2-inhibitor therapy (Xeljanz) was also discussed, but this was ultimately withheld. After completing the first half of adjuvant chemotherapy, he was given 61.6Gy/28 fractinations to the left hilar bronchial stump and station 4 where he had resected adherent N2 nodal disease.
Overall, his PT was well tolerated with only mild grade 2 esophagitis, which resolved 2 weeks after treatment was completed. Before re-starting chemotherapy, he developed a dry cough one month after completion of PT, Alectinib was not considered at that time due to lack of approval and chemotherapy was still considered standard of care. He was placed on high-dose prednisone but his cough continued to symptomatically feel more “wet” and was unable to expectorate sputum well on his own. A chest CT showed consolidation in the prior area of RT and surgery, consistent with post-treatment changes, but also showed scattered airspace opacities throughout both lungs bilaterally and a small volume pneumothorax on the left. He was treated for both pneumonitis from RT vs pneumonia with high-dose steroids and IV antibiotics; antibiotics were eventually stopped due to a lack of culture growth. His SOB and cough eventually improved.
Weeks later, he finished his third cycle of cisplatin/pemetrexed but developed worsening SOB and a dry cough for the second time. Chest CT showed progressive consolidation in the left upper lung (LUL) (nondissected) and soft tissue density at the hilum with narrowing of the major bronchus on that side. There was concern for stump anastomosis microleak given air bubbles in the inferior LUL. He has resumed on empiric antibiotics outpatient and a higher dose steroid taper.
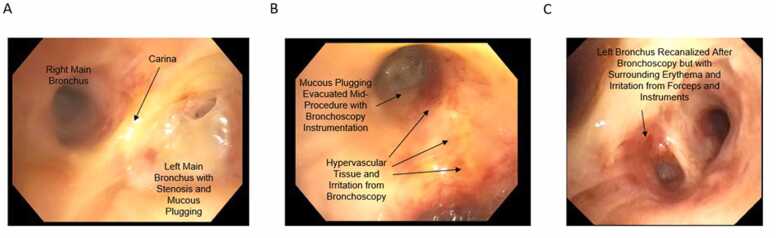
Due to concern of a microleak, he was planned for bronchoscopy with an evaluation of the stump. Before the outpatient evaluation could occur, he was admitted back to the hospital with worsening SOB and cough. He was taken for an urgent bronchoscopy, which showed ischemic changes to the left mainstem bronchus and upper lobe airways. There was no sign of a fistula at that time (Figure 1). There was membranous stenosis with pale yellow obstruction to the LUL anterior segment with postobstruction mucoid impactions. He had therapeutic aspiration with the large bronchoscope and biopsy forceps used with thin bronchoscopy to recanalize the LUL stenosis in the irradiated field. There was no sign of an anastomotic leak at the stump, and the distal airway appeared healthier and patent compared to the proximal airway.
Figure 1.
Endoscopic findings of airway after proton therapy (PT). Bronchoscopy done 4 to 6 weeks after PT showed (A) substantial mucous plugging around the left main bronchus. Mid-way through the procedure, (B) inflammation from instruments but early clearance of the airway. (C) Recanalization of the procedure but resultant swelling, erythema, and inflammation from the scope as well as residual PT changes.
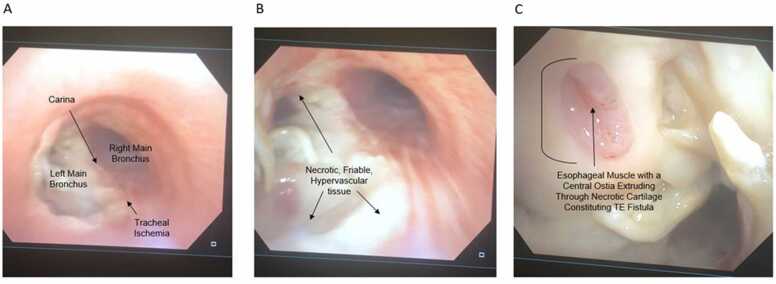
In the following weeks, he developed a cough when consuming liquid and solids, leading to readmission for suspected pneumonia. As his SOB and cough worsened, he was placed on high-dose steroids again, and a subsequent bronchoscopy was performed. At this time, rheumatology discovered that he had the MDA5+ and PL7-positive dermatomyositis subtype, associated with rare ulcerative symptoms of airway cartilage on its own as well as ILD.17 At the time of the second bronchoscopy, he was found to have mildly stenosed but patent airways, but now there was a very friable necrotic yellow-white membrane throughout his distal trachea at the carina as well as the proximal left main bronchus. In addition, there was now mucosal erosion of the esophagus through a small subcentimeter portion of the distal trachea consistent with TE fistula, the likely cause of his cough (Figure 2). The fistula and aspiration were confirmed on subsequent barium swallows.
Figure 2.
Endoscopic findings of airway and esophageal ischemia in the setting of TE fistula prior to bleeding event. These series of images were taken after the patient began violent coughing and choking following prior pulmonary intervention to denude, biopsy, and suction friable tissue to “clean” the airway plugging following radiation therapy. (A) Endobronchoscope mild ischemic changes along the posterior tracheal wall with the right and left main bronchi branching at the carina below; (B) necrotic white-yellow mucosa and cartilaginous tissue along the right proximal major bronchus at a closer view with hypervascularity; (C) erosion of the posterior tracheal cartilage near the right major bronchus branchpoint with esophageal pink mucosa jutting through. An ostia within the friable esophageal wall is appreciable in the middle of the tissue consistent with a communicating TE fistula. Abbreviation: TE, tracheoesophageal.
His radiation oncologist recommended that no bronchoscopic intervention be performed as this could further disintegrate tissue and lead to worsening fistula(es). Hyperbaric oxygen was recommended with pentoxifylline, vitamin E, continued steroids with mucolytics, and chest therapy to break up secretions, cough suppressants at night (to prevent worsening of the TE fistula with intense coughing and intrathoracic pressure), and other conservative management. Unfortunately, his tissue was too delicate and friable to support clipping, a silicone or metal stent, or surgery with resection and re-anastomosis of the airway and esophagus to fix the fistula, nor was aggressive intervention recommended given the morbidity and his current performance status.28
Although conservative management had an initial good response, he was readmitted 2 to 3 weeks later with worsening cough, SOB, and aspiration. He eventually succumbed to his TE with infection and pulmonary artery hemorrhage distal to the TE, likely resulting from friable tissues along the stump.
Discussion
While it is frequently promulgated that toxicity is a major concern in patients with autoimmune disease, the data in support are increasingly sparse and refuted.1, 2, 6 Aside from this case, there is only one other report on toxicity in patients with autoimmune disease who received proton or particle therapy. In this study, out of a group of 38 patients with different autoimmune conditions, none had grade 4 to 5 events following proton or particle treatment. Although short-term acute toxicity was higher in this case-matched report with autoimmune disease, there was notably no increase in late grade 3 or higher toxicity in patients with autoimmune conditions.29 To our knowledge, this report represents the first documenting a highgrade event in a patient with underlying dermatomyositis receiving particle therapy. Although data are limited,7 active autoimmune disease at the time of treatment may increase the risk of toxicity in the autoimmune setting.1, 9
In addition, violation of irradiated tissue with instrumentation shortly after radiotherapy may cause non-healing lesions, including fistula.25, 30
Certain autoimmune diseases have a greater association with radiation toxicity during RT.3, 9 MDA5+ and PL7-positive dermatomyositis is prone to lung parenchymal as well as cartilaginous airway disease or ulceration31, 32 which may predispose to radiation toxicity. We recommend that patients dermatomyositis and other rare autoimmune subtypes be tested for MDA5+ and PL7-positivity before RT. Biomarkers such as ferritin, Krebs von den Lungen-6 (KL-6), MDA5 antibody titers, and baseline forced vital capacity (FVC) are important in evaluating disease activity and prognosis in this rare subtype.33 Ultimately, this subtype may be considered a contraindication to RT while dermatomyositis generally is not.
The sites and number of RT treatments given to patients with autoimmune disease have also been suggested as a risk factor for toxicity. For example, late life-threatening severe toxicity has been suggested in pelvis treatments as compared to breast. Hypofractionation has also been espoused as being worse than conventional fx (1.8-2 Gy/fx) for late toxicity risk in the autoimmune setting, and the total dose delivered is implicated as a risk factor for escalated toxicity.2, 6, 7
Although the prescription was modest, <66 Gy relative biological equivalent (RBE), the variable RBE of PT, including the highest linear energy transfer (LET) and energy deposition occurring at the end of beams’ range (Bragg peak), may have accounted for some moderately increased tissue changes at the stump, carina, esophagus, and associated pulmonary vessels. The LET analysis for this case showed, however, that the LET distribution was not clustered at the site of toxicity and did not warrant concern for such effects.34 Yet, given the patient’s exquisitely sensitive tissue, attributed to his underlying autoimmune disease, even low or moderate amounts of elevated linear energy transfer (LETd) in this case could have had substantial effects with protons not commonly considered or encountered photon therapy.
The prescription was also slightly hypofractionated regimen (2.2 Gy/fx). Yet, this aspect has been explored and has not been vetted to lead to abnormal toxicity in the autoimmune population. Indeed, a recent report in JAMA, with a median follow-up of 2 years and over 100 patients with collagen-vascular disease, showed no increased chance of acute toxicity or late toxicity with hypofractionated treatment.16
Taken together, attributing this rare event to any one aspect of the patient’s prescribed treatment is difficult. Rather, the combination of surgical resection with potentially impaired stump healing, preoperative active autoimmune disease, proton particle therapy, LET distribution, fractionation, and, notably, the airway biopsy and instrumentation with manipulation shortly after RT in sum acted as contributing or catalyzing events in the setting of MDA5+ and PL7-positive dermatomyositis.
As such, while there are some unique aspects of the treatment, the data thus far for dermatomyositis and other autoimmune conditions do not substantiate that surgery, systemic therapy, or particle therapy alone was likely the resultant factor that led to the bleed. A recent meta-analysis including 18 studies of photon patients showed a <5% risk of late grade 4 toxicity and <1% chance of grade 5 toxicity, concluding that RT is generally safe and not contraindicated in the autoimmune population.7 This highlights the rarity of this case.
To this end, oncologists should continue to be vigilant about underlying genetic predisposing factors that lead to exacerbated toxicity (here, MDA5+ and PL7-positivity). To uncover such allelic predisposition, sophisticated genetic studies on large numbers of patients, such as genome-wide association studies, would need to be employed to uncover which alterations predispose certain patients to heightened risks of toxicity. Such continued scientific research is very much needed to understand the biology and risk profile of patients undergoing treatment, especially those who have unanticipated toxicities. Biomarkers from genome-panel-based investigations of toxicity risk to RT have shown a correlation with toxicity related to connective tissue modulation and the immune response.35, 36 With more study, it is likely to be found that patients with heightened sensitivity harbor unique genetic profiles that explain an exaggerated response to RT treatment—perhaps even beyond their assigned autoimmune diagnosis.
Data are sparse supporting the use of immunosuppressive agents to abate the pro-inflammatory effects. The impact of immunosuppressive/modulatory agents, such as corticosteroids, tyrosine kinase inhibitors, cyto/immunotoxic agents, or anti-immune antibodies during RT is also unknown and represents another area for further investigation.
In conclusion, this case highlights the nuances of treating in the autoimmune setting and the importance of multidisplinary discussion and care. Patients in active autoimmune states with cancer should be approached cautiously and immunosuppressive agents given with RT for patients with prior aggressive autoimmune flares or signs of autoimmune activity around the time of treatment. It may be prudent to withhold RT if active autoimmunity cannot be quelled. If RT must be given in the setting of active autoimmune disease, consideration should be given to fractionation regimens and delivery techniques that maximize protection of normal tissues and avoidance if possible of invasive procedure and tissue manipulation. LETd effects should be considered, and in the presence of vital organ proximity to end-ranging, a technique such as LET-optimization could mitigate high RBE. The use of any immune-promoting agents, given concurrent or adjuvant to RT, needs to be discussed in a multidisciplinary setting as the chance for overactive immune response resulting in heighted toxicity. Lastly, continued discussion about the avoidance of biopsy, tissue manipulation, debridement, or any form of soft-tissue or hard-tissue violation needs to be had across the multidisciplinary spectrum to avoid nonhealing lesions shortly after RT.
Ethics
Patient consent was obtained for this case report.
Funding
This study received no funding.
Author Contributions
Mark Artz: Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing- Original draft, Writing- Review and Editing, Project administration. Eric Brooks: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing- Original draft, Writing- Review and Editing, Visualization, Supervision, Project administration.
Declaration of Conflicts of Interest
The authors declare no conflicts of interest.
Data Availability Statement
The authors agree to share anonymized data upon reasonable request by researchers.
References
- 1.Chon B.H., Loeffler J.S. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist. 2002;7:136–143. doi: 10.1634/theoncologist.7-2-136. [DOI] [PubMed] [Google Scholar]
- 2.Gold D.G., Miller R.C., Pinn M.E., Osborn T.G., Petersen I.A., Brown P.D. Chronic toxicity risk after radiotherapy for patients with systemic sclerosis (systemic scleroderma) or systemic lupus erythematosus: association with connective tissue disorder severity. Radiother Oncol. 2008;87:127–131. doi: 10.1016/j.radonc.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Chen A.M., Obedian E., Haffty B.G. Breast-conserving therapy in the setting of collagen vascular disease. Cancer J. 2001;7:480–491. [PubMed] [Google Scholar]
- 4.Phan C., Mindrum M., Silverman C., Paris K., Spanos W. Matched-control retrospective study of the acute and late complications in patients with collagen vascular diseases treated with radiation therapy. Cancer J. 2003;9:461–466. doi: 10.1097/00130404-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lin A., Abu-Isa E., Griffith K.A., Ben-Josef E. Toxicity of radiotherapy in patients with collagen vascular disease. Cancer. 2008;113:648–653. doi: 10.1002/cncr.23591. [DOI] [PubMed] [Google Scholar]
- 6.Morris M.M., Powell S.N. Irradiation in the setting of collagen vascular disease: acute and late complications. J Clin Oncol. 1997;15:2728–2735. doi: 10.1200/JCO.1997.15.7.2728. [DOI] [PubMed] [Google Scholar]
- 7.Lin D., Lehrer E.J., Rosenberg J., Trifiletti D.M., Zaorsky N.G. Toxicity after radiotherapy in patients with historically accepted contraindications to treatment (CONTRAD): an international systematic review and meta-analysis. Radiother Oncol. 2019;135:147–152. doi: 10.1016/j.radonc.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Hareyama M., Nagakura H., Tamakawa M., et al. Severe reaction after chemoradiotherapy of nasopharyngeal carcinoma with collagen disease. Int J Radiat Oncol Biol Phys. 1995;33:971. doi: 10.1016/S0360-3016(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 9.Fleck R., McNeese M.D., Ellerbroek N.A., Hunter T.A., Holmes F.A. Consequences of breast irradiation in patients with pre-existing collagen vascular diseases. Int J Radiat Oncol Biol Phys. 1989;17:829–833. doi: 10.1016/0360-3016(89)90074-6. [DOI] [PubMed] [Google Scholar]
- 10.Robertson J.M., Clarke D.H., Pevzner M.M., Matter R.C. Breast conservation therapy. Severe breast fibrosis after radiation therapy in patients with collagen vascular disease. Cancer. 1991;68:502–508. doi: 10.1002/1097-0142(19910801)68:3<502::aid-cncr2820680310>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Varga J., Haustein U.F., Creech R.H., Dwyer J.P., Jimenez S.A. Exaggerated radiation-induced fibrosis in patients with systemic sclerosis. JAMA. 1991;265:3292–3295. [PubMed] [Google Scholar]
- 12.Ross J.G., Hussey D.H., Mayr N.A., Davis C.S. Acute and late reactions to radiation therapy in patients with collagen vascular diseases. Cancer. 1993;71:3744–3752. doi: 10.1002/1097-0142(19930601)71:11<3744::aid-cncr2820711144>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 13.De Naeyer B., De Meerleer G., Braems S., Vakaet L., Huys J. Collagen vascular diseases and radiation therapy: a critical review. Int J Radiat Oncol Biol Phys. 1999;44:975–980. doi: 10.1016/s0360-3016(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 14.Giaj-Levra N., Sciascia S., Fiorentino A., et al. Radiotherapy in patients with connective tissue diseases. Lancet Oncol. 2016;17:e109–e117. doi: 10.1016/S1470-2045(15)00417-9. [DOI] [PubMed] [Google Scholar]
- 15.Bellon J.R., Recht A. Radiation therapy in a patient with dermatomyositis and delayed wound healing. Int J Radiat Oncol Biol Phys. 2021;110:942. doi: 10.1016/j.ijrobp.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 16.Yoon S.M., Chu F.I., Ruan D., Steinberg M.L., Raldow A., Lee P. Assessment of toxic effects associated with dose-fractionated radiotherapy among patients with cancer and comorbid collagen vascular disease. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.34074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu W., Guo L., Fu Y., et al. Interstitial lung disease in anti-MDA5 positive dermatomyositis. Clin Rev Allergy Immunol. 2021;60:293–304. doi: 10.1007/s12016-020-08822-5. [DOI] [PubMed] [Google Scholar]
- 18.Zuo Y., Ye L., Chen F., et al. Different multivariable risk factors for rapid progressive interstitial lung disease in anti-MDA5 positive dermatomyositis and anti-synthetase syndrome. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.845988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marie I., Josse S., Decaux O., et al. Clinical manifestations and outcome of anti-PL7 positive patients with antisynthetase syndrome. Eur J Intern Med. 2013;24:474–479. doi: 10.1016/j.ejim.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Cao H., Liang J., Xu D., et al. Radiological characteristics of patients with anti-MDA5-antibody-positive dermatomyositis in (18)F-FDG PET/CT: a pilot study. Front Med. 2021;8 doi: 10.3389/fmed.2021.779272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo Y., Ye L., Liu M., et al. Clinical significance of radiological patterns of HRCT and their association with macrophage activation in dermatomyositis. Rheumatology. 2020;59:2829–2837. doi: 10.1093/rheumatology/keaa034. [DOI] [PubMed] [Google Scholar]
- 22.Goodman C.D., Nijman S.F.M., Senan S., et al. A primer on interstitial lung disease and thoracic radiation. J Thorac Oncol. 2020;15:902–913. doi: 10.1016/j.jtho.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Bahig H., Filion E., Vu T., et al. Severe radiation pneumonitis after lung stereotactic ablative radiation therapy in patients with interstitial lung disease. Pract Radiat Oncol. 2016;6:367–374. doi: 10.1016/j.prro.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Sato S., Yamakawa H., Takemura T., et al. Anti-PL-7 antibody-positive dermatomyositis with progressive interstitial pneumonia complicated with tracheal ulcer. Respir Med Case Rep. 2021;33 doi: 10.1016/j.rmcr.2021.101449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsumiyama E., Yamakawa H., Sato S., et al. A case of anti-melanoma differentiation-associated gene 5 antibody-positive interstitial lung disease complicated with tracheobronchial ulcers. Respir Med Case Rep. 2018;25:189–191. doi: 10.1016/j.rmcr.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naccache J.M., Gibiot Q., Monnet I., et al. Lung cancer and interstitial lung disease: a literature review. J Thorac Dis. 2018;10:3829–3844. doi: 10.21037/jtd.2018.05.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh N., Baby D., Rajguru J.P., Patil P.B., Thakkannavar S.S., Pujari V.B. Inflammation and cancer. Ann Afr Med. 2019;18:121–126. doi: 10.4103/aam.aam_56_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C., Hu Y., Xiao Y., Yin W. Current treatment of tracheoesophageal fistula. Ther Adv Respir Dis. 2017;11:173–180. doi: 10.1177/1753465816687518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riva G., Vischioni B., Gandini S., et al. Particle beam therapy tolerance and outcome on patients with autoimmune diseases: a single institution matched case-control study. Cancers. 2021;13:5183. doi: 10.3390/cancers13205183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C.Y., Tseng L.M., Chen H.H., Hsieh C.H., Hsiao S.M. Fatal rectovaginal fistula in post-radiotherapy locally advanced cervical cancer patients. Taiwan J Obstet Gynecol. 2022;61:1069–1072. doi: 10.1016/j.tjog.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Kabuto M., Fujimoto N., Teramura K., et al. A case of dermatomyositis with esophageal fistula in whom blind mucosal biopsy detected occult oropharyngeal carcinoma. Case Rep Dermatol. 2014;6:268–273. doi: 10.1159/000368274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ju T.R., Lee C.C., Lin Y.C. Aortoesophageal fistula causing massive gastrointestinal bleeding and death in a patient with dermatomyositis: a case report. Am J Case Rep. 2018;19:1025–1029. doi: 10.12659/AJCR.910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motegi S.I., Sekiguchi A., Toki S., et al. Clinical features and poor prognostic factors of anti-melanoma differentiation-associated gene 5 antibody-positive dermatomyositis with rapid progressive interstitial lung disease. Eur J Dermatol. 2019;29:511–517. doi: 10.1684/ejd.2019.3634. [DOI] [PubMed] [Google Scholar]
- 34.Engeseth G.M., He R., Mirkovic D., et al. Mixed effect modeling of dose and linear energy transfer correlations with brain image changes after intensity modulated proton therapy for skull base head and neck cancer. Int J Radiat Oncol Biol Phys. 2021;111:684–692. doi: 10.1016/j.ijrobp.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grossberg A.J., Lei X., Xu T., et al. Association of transforming growth factor beta polymorphism C-509T with radiation-induced fibrosis among patients with early-stage breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:1751–1757. doi: 10.1001/jamaoncol.2018.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.West C.M., Barnett G.C. Genetics and genomics of radiotherapy toxicity: towards prediction. Genome Med. 2011;3:52. doi: 10.1186/gm268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors agree to share anonymized data upon reasonable request by researchers.