Abstract
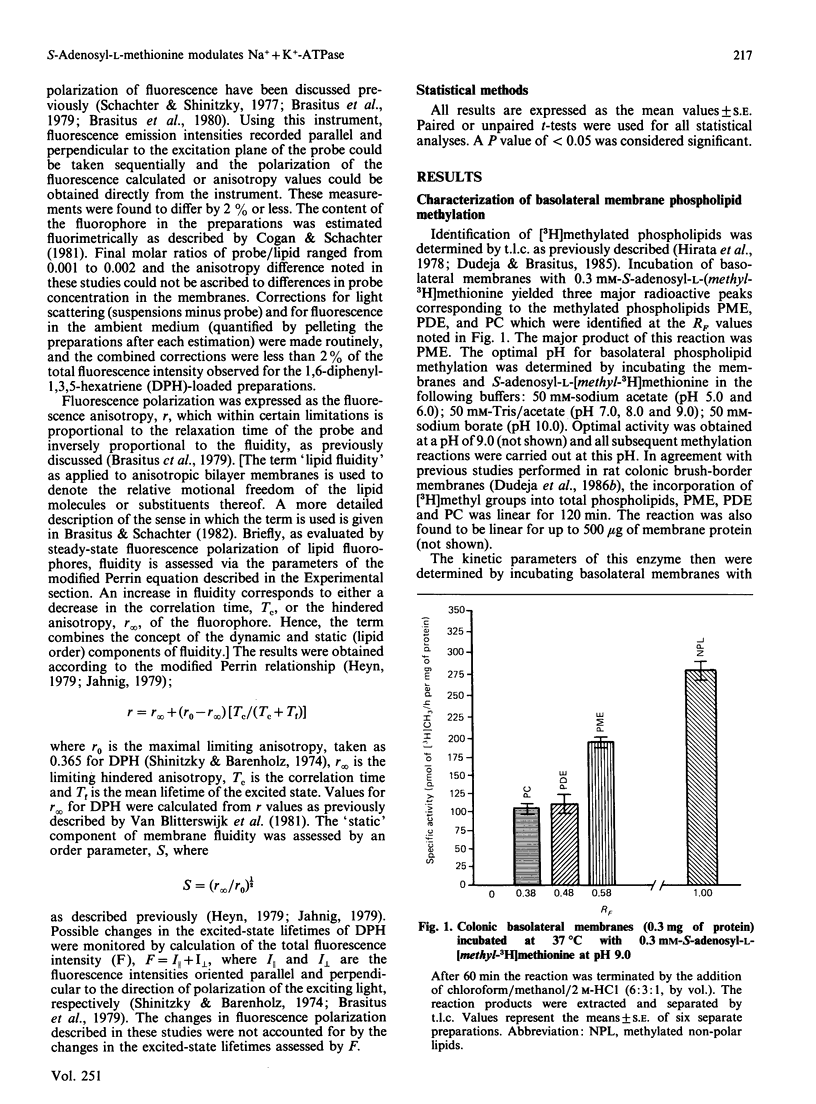
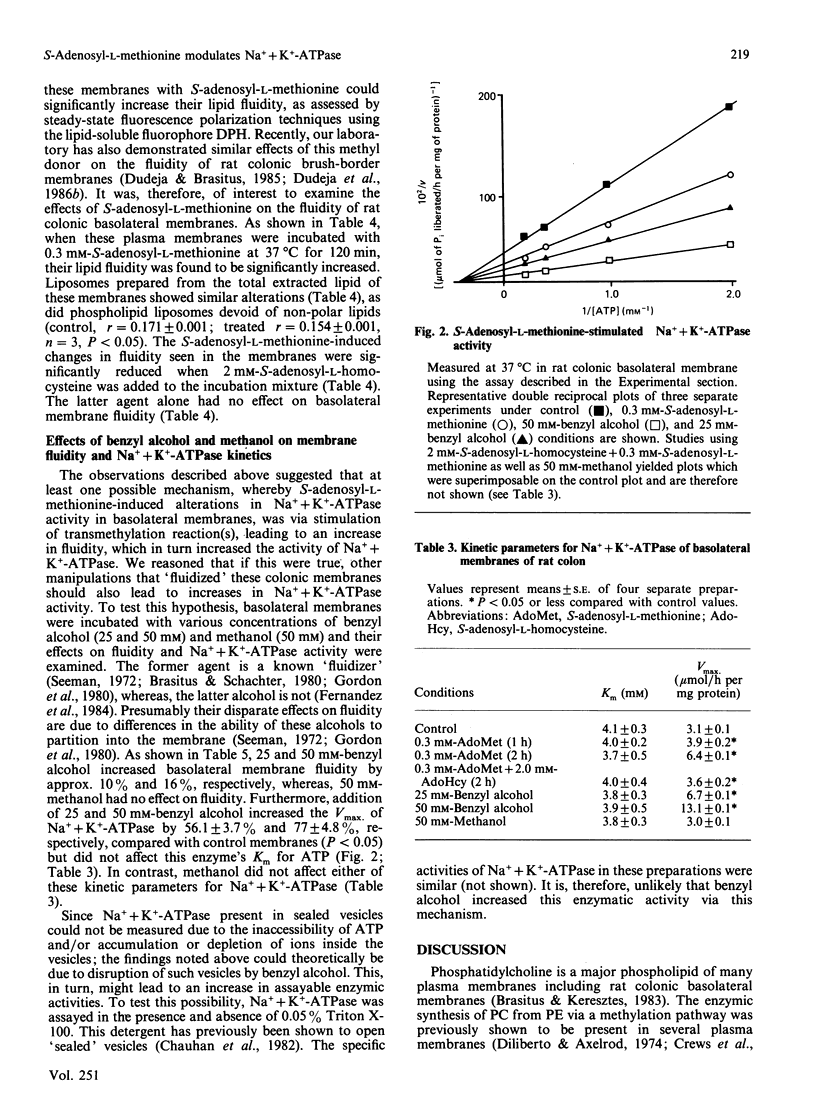
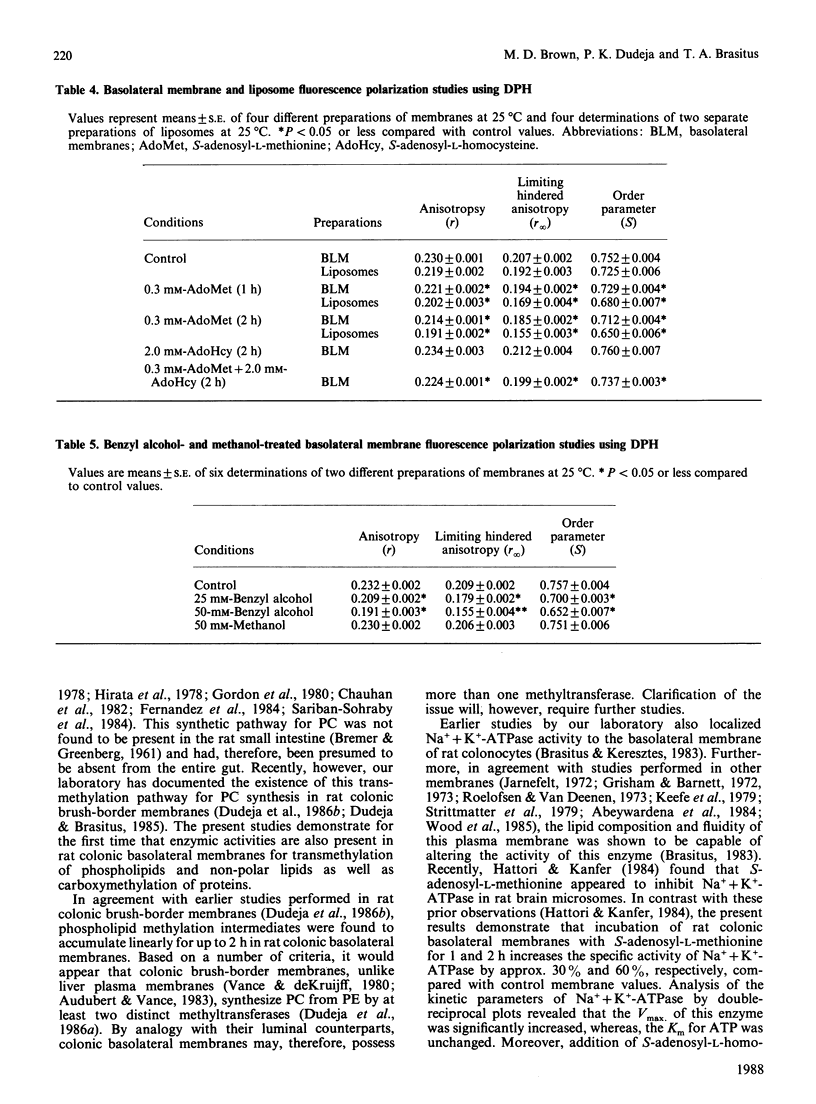
Rat colonic basolateral membranes were incubated with S-adenosyl-L-[methyl-3H]methionine (0.3 mM) at 37 degrees C for 2 h at pH 9.0. This resulted in an increase in the specific activity of Na+ + K+-ATPase by 60%. Kinetic parameter analysis revealed a 2-fold increase in the Vmax. of this enzymatic activity, whereas the Km for ATP was unchanged. The methylation inhibitor S-adenosyl-L-homocysteine (2 mM) significantly reduced these S-adenosyl-L-methionine-stimulated increases in specific activity and the Vmax. of Na+ + K+-ATPase. S-Adenosyl-L-methionine treatment of basolateral membranes was also found to significantly increase the fluidity of these preparations, as assessed by steady-state fluorescence polarization techniques using the fluorophore 1,6-diphenyl-1,3,5-hexatriene; S-adenosyl-L-homocysteine (2 mM) again markedly reduced this S-adenosyl-L-methionine-induced increase in fluidity. While transmethylation reactions involving phospholipids, non-polar lipids and proteins were all found to exist in rat colonic basolateral membranes, based on a number of observations, the results of the present studies suggest that transmethylation of membrane phospholipids, but not membrane non-polar lipids or proteins, influenced the fluidity of basolateral membranes which, in turn, modified Na+ + K+-ATPase activity in these membranes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeywardena M. Y., McMurchie E. J., Russell G. R., Sawyer W. H., Charnock J. S. Response of rat heart membranes and associated ion-transporting ATPases to dietary lipid. Biochim Biophys Acta. 1984 Sep 19;776(1):48–59. doi: 10.1016/0005-2736(84)90249-9. [DOI] [PubMed] [Google Scholar]
- Aloia R. C., Paxton J., Daviau J. S., van Gelb O., Mlekusch W., Truppe W., Meyer J. A., Brauer F. S. Effect of chronic alcohol consumption on rat brain microsome lipid composition, membrane fluidity and Na+-K+-ATPase activity. Life Sci. 1985 Mar 11;36(10):1003–1017. doi: 10.1016/0024-3205(85)90398-4. [DOI] [PubMed] [Google Scholar]
- Audubert F., Vance D. E. Pitfalls and problems in studies on the methylation of phosphatidylethanolamine. J Biol Chem. 1983 Sep 10;258(17):10695–10701. [PubMed] [Google Scholar]
- Axelrod J., Daly J. Pituitary gland: enzymic formation of methanol from S-adenosylmethionine. Science. 1965 Nov 12;150(3698):892–893. doi: 10.1126/science.150.3698.892. [DOI] [PubMed] [Google Scholar]
- Barrow D. A., Lentz B. R. Membrane structural domains. Resolution limits using diphenylhexatriene fluorescence decay. Biophys J. 1985 Aug;48(2):221–234. doi: 10.1016/S0006-3495(85)83775-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasitus T. A., Davidson N. O., Schachter D. Variations in dietary triacylglycerol saturation alter the lipid composition and fluidity of rat intestinal plasma membranes. Biochim Biophys Acta. 1985 Jan 25;812(2):460–472. doi: 10.1016/0005-2736(85)90321-9. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Dudeja P. K., Worman H. J., Foster E. S. The lipid fluidity of rat colonic brush-border membrane vesicles modulates Na+-H+ exchange and osmotic water permeability. Biochim Biophys Acta. 1986 Feb 13;855(1):16–24. doi: 10.1016/0005-2736(86)90183-5. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Keresztes R. S. Isolation and partial characterization of basolateral membranes from rat proximal colonic epithelial cells. Biochim Biophys Acta. 1983 Feb 9;728(1):11–19. doi: 10.1016/0005-2736(83)90431-5. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Keresztes R. S. Protein-lipid interactions in antipodal plasma membranes of rat colonocytes. Biochim Biophys Acta. 1984 Jun 27;773(2):290–300. doi: 10.1016/0005-2736(84)90093-2. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A. Lipid dynamics and protein-lipid interactions in rat colonic epithelial cell basolateral membranes. Biochim Biophys Acta. 1983 Feb 9;728(1):20–30. doi: 10.1016/0005-2736(83)90432-7. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Schachter D. Cholesterol biosynthesis and modulation of membrane cholesterol and lipid dynamics in rat intestinal microvillus membranes. Biochemistry. 1982 Apr 27;21(9):2241–2246. doi: 10.1021/bi00538a037. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Schachter D. Lipid composition and fluidity of rat enterocyte basolateral membranes. Regional differences. Biochim Biophys Acta. 1984 Jul 11;774(1):138–146. doi: 10.1016/0005-2736(84)90284-0. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Schachter D. Lipid dynamics and lipid-protein interactions in rat enterocyte basolateral and microvillus membranes. Biochemistry. 1980 Jun 10;19(12):2763–2769. doi: 10.1021/bi00553a035. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Schachter D., Mamouneas T. G. Functional interactions of lipids and proteins in rat intestinal microvillus membranes. Biochemistry. 1979 Sep 18;18(19):4136–4144. doi: 10.1021/bi00586a013. [DOI] [PubMed] [Google Scholar]
- Brasitus T. A., Tall A. R., Schachter D. Thermotropic transitions in rat intestinal plasma membranes studied by differential scanning calorimetry and fluorescence polarization. Biochemistry. 1980 Mar 18;19(6):1256–1261. doi: 10.1021/bi00547a033. [DOI] [PubMed] [Google Scholar]
- Chauhan V. P., Sikka S. C., Kalra V. K. Phospholipid methylation of kidney cortex brush border membranes. Effect on fluidity and transport. Biochim Biophys Acta. 1982 Jun 14;688(2):357–368. doi: 10.1016/0005-2736(82)90347-9. [DOI] [PubMed] [Google Scholar]
- Cogan U., Schachter D. Asymmetry of lipid dynamics in human erythrocyte membranes studied with impermeant fluorophores. Biochemistry. 1981 Oct 27;20(22):6396–6403. doi: 10.1021/bi00525a018. [DOI] [PubMed] [Google Scholar]
- Crews F. T., Hirata F., Axelrod J. Identification and properties of methyltransferases that synthesize phosphatidylcholine in rat brain synaptosomes. J Neurochem. 1980 Jun;34(6):1491–1498. doi: 10.1111/j.1471-4159.1980.tb11229.x. [DOI] [PubMed] [Google Scholar]
- Diliberto E. J., Jr, Axelrod J. Characterization and substrate specificity of a protein carboxymethylase in the pituitary gland. Proc Natl Acad Sci U S A. 1974 May;71(5):1701–1704. doi: 10.1073/pnas.71.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeja P. K., Foster E. S., Brasitus T. A. Regulation of Na+-H+ exchange by transmethylation reactions in rat colonic brush-border membranes. Biochim Biophys Acta. 1986 Jul 10;859(1):61–68. doi: 10.1016/0005-2736(86)90318-4. [DOI] [PubMed] [Google Scholar]
- Dudeja P. K., Foster E. S., Brasitus T. A. Synthesis of phosphatidylcholine by two distinct methyltransferases in rat colonic brush-border membranes: evidence for extrinsic and intrinsic membrane activities. Biochim Biophys Acta. 1986 Feb 28;875(3):493–500. doi: 10.1016/0005-2760(86)90069-x. [DOI] [PubMed] [Google Scholar]
- Fernandez Y. J., Boigegrain R. A., Cambon-Gros C. D., Mitjavila S. E. Sensitivity of Na+-coupled D-glucose uptake, Mg2+-ATPase and sucrase to perturbations of the fluidity of brush-border membrane vesicles induced by n-aliphatic alcohols. Biochim Biophys Acta. 1984 Mar 14;770(2):171–177. doi: 10.1016/0005-2736(84)90127-5. [DOI] [PubMed] [Google Scholar]
- Gordon L. M., Sauerheber R. D., Esgate J. A., Dipple I., Marchmont R. J., Houslay M. D. The increase in bilayer fluidity of rat liver plasma membranes achieved by the local anesthetic benzyl alcohol affects the activity of intrinsic membrane enzymes. J Biol Chem. 1980 May 25;255(10):4519–4527. [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The interrelationship of membrane and protein structure in the functioning of the (Na + = K + )-activated ATPase. Biochim Biophys Acta. 1972 Jun 20;266(3):613–624. doi: 10.1016/0006-3002(72)90005-4. [DOI] [PubMed] [Google Scholar]
- Grisham C. M., Barnett R. E. The role of lipid-phase transitions in the regulation of the (sodium + potassium) adenosine triphosphatase. Biochemistry. 1973 Jul 3;12(14):2635–2637. doi: 10.1021/bi00738a013. [DOI] [PubMed] [Google Scholar]
- Hattori H., Kanfer J. N. Inhibition of rat brain microsomal Na+,K+-ATPase by S-adenosylmethionine. J Neurochem. 1984 Jan;42(1):204–208. doi: 10.1111/j.1471-4159.1984.tb09718.x. [DOI] [PubMed] [Google Scholar]
- Heyn M. P. Determination of lipid order parameters and rotational correlation times from fluorescence depolarization experiments. FEBS Lett. 1979 Dec 15;108(2):359–364. doi: 10.1016/0014-5793(79)80564-5. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic methylation of phosphatidylethanolamine increases erythrocyte membrane fluidity. Nature. 1978 Sep 21;275(5677):219–220. doi: 10.1038/275219a0. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Phospholipid methylation and biological signal transmission. Science. 1980 Sep 5;209(4461):1082–1090. doi: 10.1126/science.6157192. [DOI] [PubMed] [Google Scholar]
- Hirata F., Viveros O. H., Diliberto E. J., Jr, Axelrod J. Identification and properties of two methyltransferases in conversion of phosphatidylethanolamine to phosphatidylcholine. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1718–1721. doi: 10.1073/pnas.75.4.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järnefelt J. Lipid requirements of functional membrane structures as indicated by the reversible inactivation of (Na + -K + )-ATPase. Biochim Biophys Acta. 1972 Apr 14;266(1):91–96. doi: 10.1016/0005-2736(72)90123-x. [DOI] [PubMed] [Google Scholar]
- Katyal S. L., Lombardi B. Quantitation of phosphatidyl N-methyl and N,N-dimethyl aminoethanol in liver and lung of N-methylaminoethanol fed rats. Lipids. 1974 Feb;9(2):81–85. doi: 10.1007/BF02532130. [DOI] [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefee E. B., Scharschmidt B. F., Blankenship N. M., Ockner R. K. Studies of relationship among bile flow, liver plasma membrane NaK-ATPase, and membrane microviscosity in the rat. J Clin Invest. 1979 Dec;64(6):1590–1598. doi: 10.1172/JCI109620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog Y., Zatz M., Rivnay B., Dudley P. A., Markey S. P. Nonpolar lipid methylation-identification of nonpolar methylated products synthesized by rat basophilic leukemia cells, retina and parotid. Biochem Pharmacol. 1982 Mar 1;31(5):753–759. doi: 10.1016/0006-2952(82)90459-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore J. P., Johannsson A., Hesketh T. R., Smith G. A., Metcalfe J. C. Calcium signals and phospholipid methylation in eukaryotic cells. Biochem J. 1984 Aug 1;221(3):675–684. doi: 10.1042/bj2210675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Jun 25;779(2):217–251. doi: 10.1016/0304-4157(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Roelofsen B., van Deenen L. L. Lipid requirement of membrane-bound ATPase. Studies on human erythrocyte ghosts. Eur J Biochem. 1973 Dec 3;40(1):245–257. doi: 10.1111/j.1432-1033.1973.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Sariban-Sohraby S., Burg M., Wiesmann W. P., Chiang P. K., Johnson J. P. Methylation increases sodium transport into A6 apical membrane vesicles: possible mode of aldosterone action. Science. 1984 Aug 17;225(4663):745–746. doi: 10.1126/science.6463652. [DOI] [PubMed] [Google Scholar]
- Schachter D., Shinitzky M. Fluorescence polarization studies of rat intestinal microvillus membranes. J Clin Invest. 1977 Mar;59(3):536–548. doi: 10.1172/JCI108669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. J., Vance D. E. Conversion of phosphatidylethanolamine to phosphatidylcholine in rat liver. Partial purification and characterization of the enzymatic activities. J Biol Chem. 1979 May 25;254(10):3886–3891. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Siegel G. J., Goodwin B. Sodium-potassium-activated adenosine triphosphatase: potassium regulation of enzyme phosphorylation. Sodium-stimulated, potassium-inhibited uridine triphosphate hydrolysis. J Biol Chem. 1972 Jun 10;247(11):3630–3637. [PubMed] [Google Scholar]
- Strittmatter W. J., Hirata F., Axelrod J. Increased Ca2+ -ATPase activity associated with methylation of phospholipids in human erythrocytes. Biochem Biophys Res Commun. 1979 May 14;88(1):147–153. doi: 10.1016/0006-291x(79)91709-1. [DOI] [PubMed] [Google Scholar]
- Van Blitterswijk W. J., Van Hoeven R. P., Van der Meer B. W. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim Biophys Acta. 1981 Jun 22;644(2):323–332. doi: 10.1016/0005-2736(81)90390-4. [DOI] [PubMed] [Google Scholar]
- Vance D. E., de Kruijff B. The possible functional significance of phosphatidylethanolamine methylation. Nature. 1980 Nov 20;288(5788):277–279. doi: 10.1038/288277a0. [DOI] [PubMed] [Google Scholar]
- Wiesmann W. P., Johnson J. P., Miura G. A., Chaing P. K. Aldosterone-stimulated transmethylations are linked to sodium transport. Am J Physiol. 1985 Jan;248(1 Pt 2):F43–F47. doi: 10.1152/ajprenal.1985.248.1.F43. [DOI] [PubMed] [Google Scholar]
- Wood P. A., McBride M. R., Baker H. J., Christian S. T. Fluorescence polarization analysis, lipid composition, and Na+, K+-ATPase kinetics of synaptosomal membranes in feline GM1 and GM2 gangliosidosis. J Neurochem. 1985 Mar;44(3):947–956. doi: 10.1111/j.1471-4159.1985.tb12909.x. [DOI] [PubMed] [Google Scholar]
- Zatz M., Dudley P. A., Kloog Y., Markey S. P. Nonpolar lipid methylation. Biosynthesis of fatty acid methyl esters by rat lung membranes using S-adenosylmethionine. J Biol Chem. 1981 Oct 10;256(19):10028–10032. [PubMed] [Google Scholar]


