Abstract
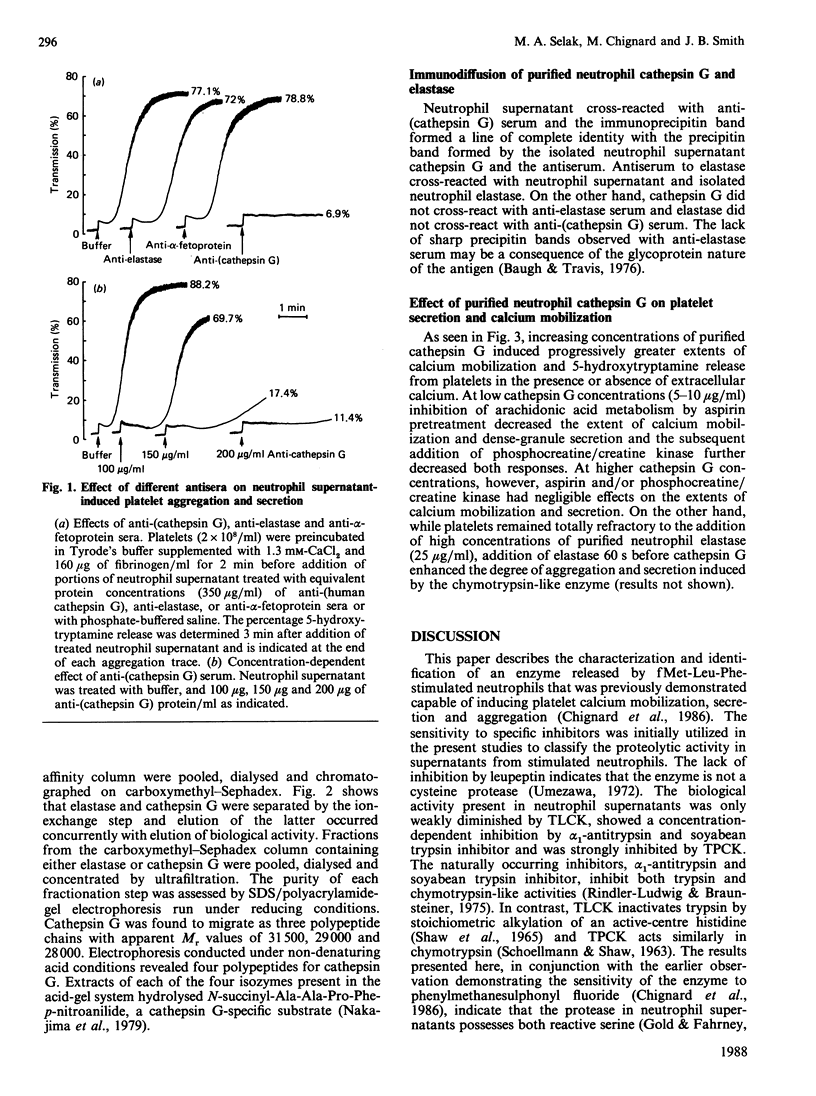
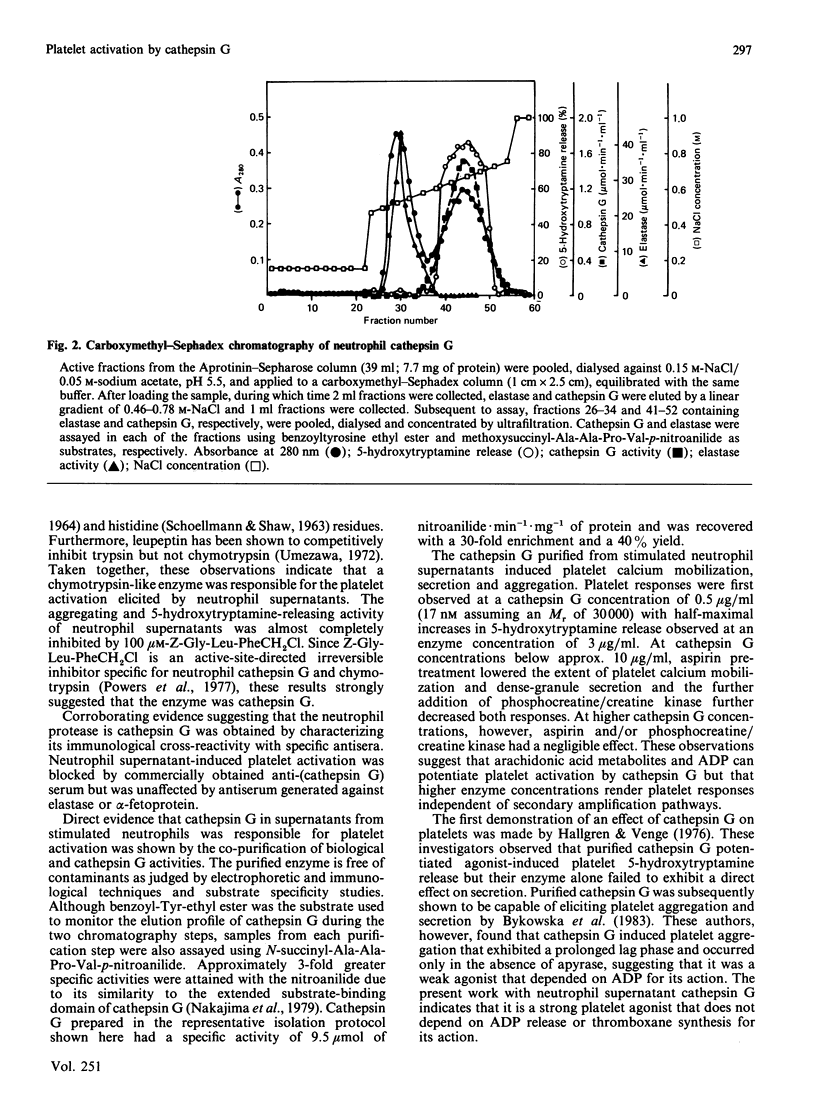
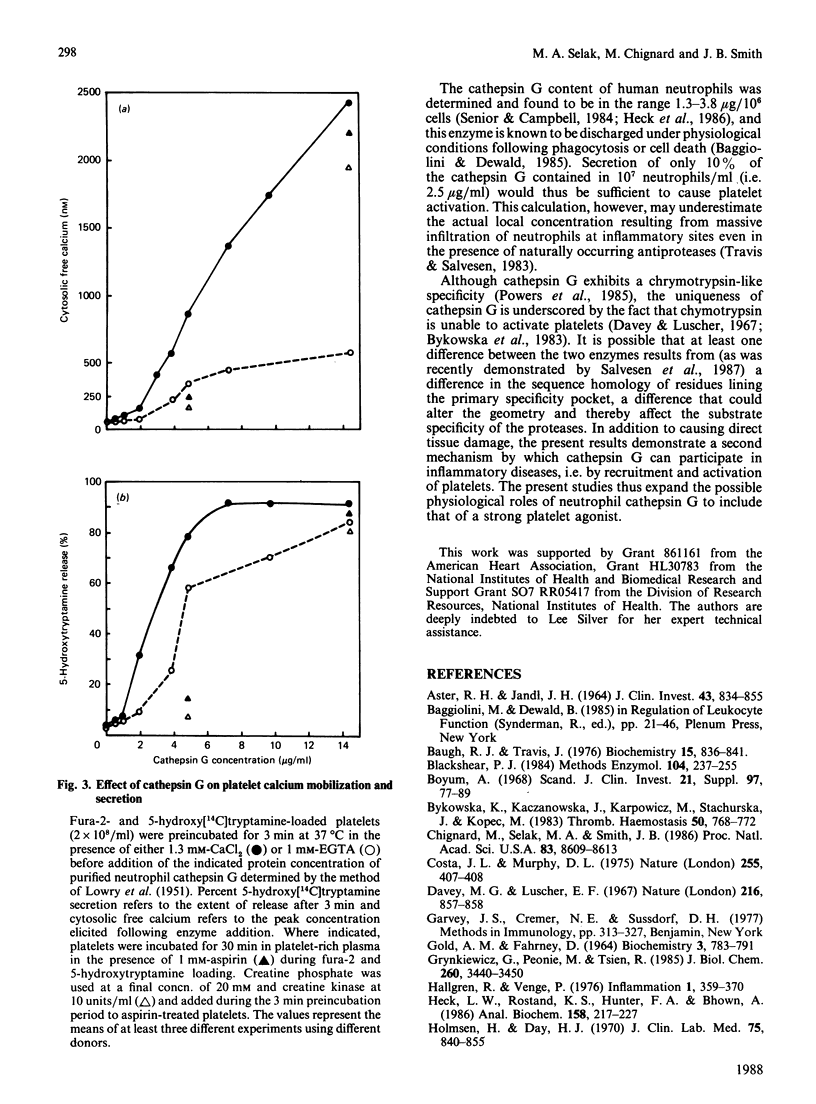
The present studies were undertaken to characterize a serine protease released by N-formyl-L-Met-L-Leu-L-Phe (fMet-Leu-Phe)-stimulated neutrophils that rapidly induces platelet calcium mobilization, secretion and aggregation. The biological activity associated with this protease was unaffected by leupeptin, was only weakly diminished by N-p-tosyl-L-Lys-chloromethane, but was strongly inhibited by alpha 1-antitrypsin, soyabean trypsin inhibitor, N-tosyl-L-Phe-chloromethane and benzoyloxycarbonyl-Gly-Leu-Phe-chloromethane (Z-Gly-Leu-PheCH2Cl). These observations indicated that the biological activity of neutrophil supernatants could be attributed to a chymotrypsin-like enzyme such as cathepsin G. Furthermore, platelet aggregation and 5-hydroxytryptamine release induced by cell-free supernatants from fMet-Leu-Phe-stimulated neutrophils were found to be blocked by antiserum to cathepsin G in a concentration-dependent manner but were unaffected by antiserum to elastase. The biological activity present in neutrophil supernatants co-purified with enzymic activity for cathepsin G during sequential Aprotinin-Sepharose affinity chromatography and carboxymethyl-Sephadex chromatography. SDS/polyacrylamide-gel electrophoresis of the reduced, purified protein, demonstrated three polypeptides with apparent Mr values of 31,500, 29,000 and 28,000 and four polypeptides were resolved on acid-gel electrophoresis. Purified cathepsin G from neutrophils cross-reacted with anti-(cathepsin G) serum in a double immunodiffusion assay and elicited platelet calcium mobilization, 5-hydroxytryptamine secretion and aggregation. Calcium mobilization and secretion induced by low concentrations of cathepsin G were partially dependent on arachidonic acid metabolites and ADP, while stimulation by higher enzyme concentrations was independent of amplification pathways, indicating that cathepsin G is a strong platelet agonist. These results suggest that pathological processes which stimulate neutrophils and release cathepsin G can in turn result in the recruitment and activation of platelets.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASTER R. H., JANDL J. H. PLATELET SEQUESTRATION IN MAN. I. METHODS. J Clin Invest. 1964 May;43:843–855. doi: 10.1172/JCI104970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J. Systems for polyacrylamide gel electrophoresis. Methods Enzymol. 1984;104:237–255. doi: 10.1016/s0076-6879(84)04093-3. [DOI] [PubMed] [Google Scholar]
- Bykowska K., Kaczanowska J., Karpowicz M., Stachurska J., Kopeć M. Effect of neutral proteases from blood leukocytes on human platelets. Thromb Haemost. 1983 Dec 30;50(4):768–772. [PubMed] [Google Scholar]
- Chignard M., Selak M. A., Smith J. B. Direct evidence for the existence of a neutrophil-derived platelet activator (neutrophilin). Proc Natl Acad Sci U S A. 1986 Nov;83(22):8609–8613. doi: 10.1073/pnas.83.22.8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa J. L., Murphy D. L. Platelet 5-HT uptake and release stopped rapidly by formaldehyde. Nature. 1975 May 29;255(5507):407–408. doi: 10.1038/255407a0. [DOI] [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- GOLD A. M., FAHRNEY D. SULFONYL FLUORIDES AS INHIBITORS OF ESTERASES. II. FORMATION AND REACTIONS OF PHENYLMETHANESULFONYL ALPHA-CHYMOTRYPSIN. Biochemistry. 1964 Jun;3:783–791. doi: 10.1021/bi00894a009. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heck L. W., Rostand K. S., Hunter F. A., Bhown A. Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil cathepsin G from normal donors. Anal Biochem. 1986 Oct;158(1):217–227. doi: 10.1016/0003-2697(86)90612-3. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Day H. J. The selectivity of the thrombin-induced platelet release reaction: subcellular localization of released and retained constituents. J Lab Clin Med. 1970 May;75(5):840–855. [PubMed] [Google Scholar]
- ISHIZAKA T., ISHIZAKA K. Biological activities of aggregated gamma-globulin. V. Agglutination of erythrocytes and platelets. J Immunol. 1962 Nov;89:709–716. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakajima K., Powers J. C., Ashe B. M., Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979 May 25;254(10):4027–4032. [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- Powers J. C., Tanaka T., Harper J. W., Minematsu Y., Barker L., Lincoln D., Crumley K. V., Fraki J. E., Schechter N. M., Lazarus G. G. Mammalian chymotrypsin-like enzymes. Comparative reactivities of rat mast cell proteases, human and dog skin chymases, and human cathepsin G with peptide 4-nitroanilide substrates and with peptide chloromethyl ketone and sulfonyl fluoride inhibitors. Biochemistry. 1985 Apr 9;24(8):2048–2058. doi: 10.1021/bi00329a037. [DOI] [PubMed] [Google Scholar]
- Rindler-Ludwig R., Braunsteiner H. Cationic proteins from human neutrophil granulocytes. Evidence for their chymotrypsin-like properties. Biochim Biophys Acta. 1975 Feb 27;379(2):606–617. doi: 10.1016/0005-2795(75)90167-1. [DOI] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Salvesen G., Farley D., Shuman J., Przybyla A., Reilly C., Travis J. Molecular cloning of human cathepsin G: structural similarity to mast cell and cytotoxic T lymphocyte proteinases. Biochemistry. 1987 Apr 21;26(8):2289–2293. doi: 10.1021/bi00382a032. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J. Cathepsin G in human mononuclear phagocytes: comparisons between monocytes and U937 monocyte-like cells. J Immunol. 1984 May;132(5):2547–2551. [PubMed] [Google Scholar]
- Stibbe J., Holmsen H. Effects of sodium azide on platelet function. Thromb Haemost. 1977 Dec 15;38(4):1042–1053. [PubMed] [Google Scholar]
- Travis J., Bowen J., Baugh R. Human alpha-1-antichymotrypsin: interaction with chymotrypsin-like proteinases. Biochemistry. 1978 Dec 26;17(26):5651–5656. doi: 10.1021/bi00619a011. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]


