Abstract
We have designed and characterized two new replication-competent avian sarcoma/leukosis virus-based retroviral vectors with amphotropic and ecotropic host ranges. The amphotropic vector RCASBP-M2C(797-8), was obtained by passaging the chimeric retroviral vector RCASBP-M2C(4070A) (6) in chicken embryos. The ecotropic vector, RCASBP(Eco), was created by replacing the env-coding region in the retroviral vector RCASBP(A) with the env region from an ecotropic murine leukemia virus. It replicates efficiently in avian DFJ8 cells that express murine ecotropic receptor. For both vectors, permanent cell lines that produce viral stocks with titers of about 5 × 106 CFU/ml on mammalian cells can be easily established by passaging transfected avian cells. Some chimeric viruses, for example, RCASBP(Eco), replicate efficiently without modifications. For those chimeric viruses that do require modification, adaptation by passage in vitro or in vivo is a general strategy. This strategy has been used to prepare vectors with altered host range and could potentially be used to develop vectors that would be useful for targeted gene delivery.
Retroviral vectors are widely used in studies of gene structure and function in cultured cells and in animal models. Retroviral vectors have also been used for clinical applications, including human somatic cell gene therapy. A number of retroviral vectors have been developed; most are based on avian and mammalian retroviruses. The majority of these vectors are replication-defective derivatives of the murine leukemia virus (MLV). In general, MLV vectors lack all genes for the viral structural proteins that are required for viral replication. The viral genes are usually expressed either by cotransfection or by a packaging cell line that supplies the viral proteins in trans. There are replication-competent MLV vectors; however, the insert size is limited (71, 72). Replication-competent vectors based on avian sarcoma/leukosis viruses (ASLV) can accept larger inserts. Naturally occurring ASLV can have several different envelopes (subgroups A to E). The various ASLV envelopes are distinguished based on host range; none of these envelopes allows the ASLV (or the vectors derived from them) to efficiently infect mammalian cells.
We developed the replication-competent chimeric retroviral vector RCASBP-M2C(4070A) by replacing the subgroup A env gene of the ASLV-based retroviral vector RCASBP(A) with the env-coding sequence of an amphotropic MLV (6). The original amphotropic RCASBP replicated poorly; passage of the virus selected for a variant that has a single change, P242I, in gp70. The adapted vector, RCASBP-M2C(4070A), replicates efficiently in chicken embryo fibroblasts (CEF) or in DF-1 cells (25, 64) and can efficiently transfer genes into cultured mammalian cells; however, the virus is replication defective in mammalian cells. The RCASBP-M2C(4070A) vector has advantages compared with replication-defective MLV-based vectors. Since the RCASBP-M2C(4070A) vector is replication competent in avian cells, it spreads rapidly through an avian cell culture following transfection and rapidly produces a high-titer viral stock. The vector has no sequence homology with endogenous mammalian retroviruses (except in the envelope region), which makes recombination with an endogenous mammalian retrovirus unlikely. This makes the vector safe as well as convenient. Although the RCASBP-M2C(4070A) vector has advantages, there is one problem: the virus is quite toxic to chicken cells. In practical terms, this means that the viral titer increases rapidly as the vector spreads through the culture and then falls as the infected cells die from the cytopathic effect of the virus. The parental vector RCASBP(A) causes no detectable cytopathic effect in cultured chicken cells, which suggests that the murine envelope causes the cytopathic effect.
We took two approaches to developing versions of the RCAS vector that would efficiently infect mammalian cells but not cause such profound cytopathology in avian cells. First, we asked whether an RCAS vector that used another MLV envelope (the ecotropic envelope) would still be cytopathic. Second, we attempted to select a less cytotoxic derivative of RCASBP-M2C(4070A) by passage in chicken embryos. Both of these approaches were successful; we have developed two new vectors.
One vector, RCASBP(Eco), contains the env gene from an ecotropic MLV. The vector replicates in avian (DF-1) cells that express the murine ecotropic receptor. This vector can efficiently infect murine cells and has only modest cytotoxicity in DF-1 cells that express the ecotropic receptor. The second vector, RCASBP-M2C(797-8), was derived by passaging the cytopathic amphotropic vector RCASBP-M2C(4070A) in chicken embryos. The resulting vector is considerably less cytotoxic for DF-1 cells than the 4070A parent. We were able to use both of the new vectors to establish permanent avian producer cell lines that produce high-titer viral stocks.
MATERIALS AND METHODS
Cell culture.
The avian cell line DF-1 (25, 64) was kindly provided by Douglas Foster (University of Minnesota, Minneapolis). Construction of the avian cell line DFJ8, which expresses the murine ecotropic receptor, is described below. DF-1 cells were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum, 5% newborn calf serum, 10% tryptose-phosphate broth (GIBCO BRL, Gaithersburg, Md.), 100 U of penicillin per ml, and 100 μg streptomycin (Quality Biological, Inc., Gaithersburg, Md.) per ml. DFJ8 cells were grown in the same medium supplemented with G418 (200 μg/ml; GIBCO BRL). The murine packaging cell line PA317 and the BAG2 retroviral vector-producing cell line CRE-BAG2 (57) were obtained from the American Type Culture Collection (Manassas, Va.). These cells were maintained in Dulbecco's modified Eagle's medium with 10% calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
Recombinant DNAs.
Plasmids were constructed using standard techniques (63). The recombinant retroviral vector RCASBP(Eco) was prepared as follows. The env coding region of an ecotropic Moloney MLV was PCR amplified from plasmid pRR88 (kind gift from Alan Rein, HIV Drug Resistance Program, National Cancer Institute), using primers RSV-ECOF2E (GCTTCGCCCGGCTCCAGT) and RSV-ECOR2 (ACACACGCGGCCGCCTATGGCTCGTACTCTATAGGC). The fragment spanning the unique KpnI site, the env splice acceptor site, and the coding region of the signal peptide was PCR amplified from the retroviral vector RCASBP(A) (55), using primers RSV-ECOF1 (GAGTGGGAAAAAGGATGGAACG) and RSV-ECORIE (AGTGGACCCGGGCGAAGCAGCTCTTACCCCCGTAACCTCA). The resulting PCR fragments were fused and amplified by overlap extension PCR (32) with primers RSV-ECOF1 and RSV-ECOR2. The PCR product contained a gene for a chimeric Env consisting of the signal peptide from RCASBP(A) and the surface and transmembrane proteins from ecotropic MLV. To construct the retroviral vector RCASBP (Eco), an aliquot of the PCR product was cleaved with KpnI and NcoI. A second aliquot of the PCR product was cleaved with NcoI and NotI in a separate reaction. The env region was removed from the retroviral vector RCASBP-M2C(4070A) (6) by cleavage with KpnI and NotI, and the chimeric ecotropic env gene was inserted by three way-ligation of this fragment with the KpnI-NcoI and NcoI-NotI PCR fragments, generating the plasmid RCASBP(Eco).
To construct the recombinant murine retroviral vector LRNL-J8, the coding region for the ecotropic receptor MCAT-1 was isolated from plasmid pJET (3) by cleavage with BamHI. The BamHI ends were filled using T4 DNA polymerase. SalI linkers (New England Biolabs, Beverly, Mass.) were ligated to the fragment. Subsequently, the fragment was cleaved with SalI and inserted into the SalI site of a murine retroviral vector, LRNL (79), generating LRNL-J8.
Transfection and preparation of viral particles.
The DF-1 and DFJ8 cells were transfected by a modified CaPO4 precipitation technique (22). Briefly, a CaPO4 precipitate containing 10 μg of plasmid DNA was added to the culture medium, and the cultures were incubated at 37°C for 4 h. The cells were then incubated in the medium containing 15% glycerol for 5 min at 37°C, washed two times in phosphate-buffered saline (PBS), and grown in culture medium. The transfected cell cultures were passaged to allow the virus to spread through the culture.
To transfect PA317 cells, a CaPO4 precipitate containing 10 μg of plasmid DNA was added to the culture medium, and the cultures were incubated at 37°C overnight. Twenty-four hours posttransfection, the culture medium was replaced.
To prepare viral proteins for Western blot analysis, culture medium from infected cells was clarified by centrifugation at 3,000 rpm for 10 min, and the viral particles were pelleted through a 15% sucrose cushion by centrifugation at 35,000 rpm for 1 h at 4°C in an SW41 rotor (Beckman, Fullerton, Calif.). The pellet was resuspended in protein gel sample buffer, heated at 100°C for 4 min, and loaded onto a gel as described below.
Western blot analysis.
Viral proteins were fractionated by electrophoresis in a sodium dodecyl sulfate (SDS)–4 to 20% gradient polyacrylamide gel and electroblotted onto a polyvinylidene difluoride membrane (Immobilon P; Millipore, Bedford, Mass.). ASLV capsid protein was detected by incubation of the membrane with rabbit antiserum that recognized p27 (generated by immunization of rabbits with virus particles). MLV envelope proteins were detected by incubation with goat antiserum against gp70 or with rabbit antiserum against p15E (kindly provided by Alan Rein). Protein bands were visualized by enhanced chemiluminescence detection with the alkaline phosphatase substrate CDP-Star (Boehringer Mannheim, Indianapolis, Ind.).
p27 antigen capture enzyme-linked immunosorbent assay (ELISA).
Rabbit anti-p27 antibodies conjugated with horseradish peroxidase (anti-p27-HRP) were obtained from SPAFAS, Inc. (North Franklin, Conn.). Ninety-six-well plates were coated with anti-p27 antibodies (generated by immunization of rabbits with ASLV particles) in 100 mM sodium carbonate-sodium hydrocarbonate buffer (pH 9) overnight at 4°C. The wells were washed with PBS containing 0.1% Tween 20 and were incubated in a solution of 5% nonfat dried milk in PBS at 37°C for 1 h to block nonspecific binding. Samples were prepared by adding Tween 20 to the cell culture supernatants (to a final concentration of 0.5%), followed by three cycles of freezing at −70°C and thawing at 37°C. The anti-p27 antibody-coated wells were incubated with the supernatants at 37°C for 1 h. The wells were then washed with PBS containing 0.1% Tween 20 and incubated with anti-p27-HRP in 5% milk–PBS. The antigen-antibody complexes were detected using the trimethylbenzidine-hydrogen peroxide substrate reagent (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). The color reaction was measured using a Dynatech plate reader with a 405-nm filter.
Avian cell line DFJ8.
The avian cell line DFJ8 was prepared by stably transferring the MCAT-1 cDNA, which encodes the receptor for ecotropic MLV, into DF-1 cells using a retroviral vector. PA317 cells were transfected with the retroviral vector LRNL-J8 (described above). Forty-eight hours posttransfection, culture medium containing the virus was harvested and used to infect DF-1 cells. Polybrene (final concentration, 8 μg per ml) was added to the culture medium obtained from the transfected PA317 cells. The mixture was added to 5 × 105 DF-1 cells in a 60-mm-diameter tissue culture dish. The cells were incubated for 4 h at 37°C, and 3.5 ml of culture medium was added. Forty-eight hours postinfection, the cells were trypsinized and plated in culture medium that contained G418 (400 μg/ml; GIBCO BRL). Two days later, the culture medium was replaced with the fresh medium containing G418 (200 μg/ml). Colonies of G418-resistant cells appeared 15 days postinfection. Ten clones were isolated by using cloning cylinders, and cell lines were developed. To test these cell lines for expression of the receptor, 5 × 105 cells were infected with the ecotropic retroviral vector BAG2 produced by CRE-BAG2 murine cells (57); 48 h postinfection, the cells were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (57).
Adaptation of RCASBP-M2C(4070A) in chicken embryos.
Embryonated eggs (line EV-0) received 0.1 to 0.2 ml of the RCASBP-M2C(4070A) virus stock at day 6 of incubation. A 21-gauge, 1-in. needle was used to inject the virus into embryos through the air cell of the egg. At day 12 of incubation, the live embryos were processed. Under sterile conditions, the large end of the egg was opened, and the shell membrane was removed. To prepare the embryo extract, the embryos were placed into a 10-ml syringe fitted with an 18-gauge needle and then pushed through the needle into 10 ml of complete LM medium (8.75 g of Leibowitz L-15 medium, 5.0 g of McCoy 5A medium, 1.5 g of NaHCO3 per liter) supplemented with 10% fetal bovine serum, amphotericin B (Fungizone; 3 μg/ml), and gentamicin (50 μg/ml). The suspension was vortexed vigorously. Embryo extract samples were centrifuged at 400 × g to remove cells and debris; 0.5 ml of each sample was used to infect DF-1 cell cultures that were approximately 60% confluent. The infected DF-1 cells were passaged five times to prepare virus stocks.
Confocal microscopy.
Cells infected with RCASBP(Eco) or with RCASBP-M2C(4070A) were grown in plastic petri dishes. Confocal microscopy was performed with an inverted laser confocal microscope (Zeiss, Jena, Germany).
Titration of retroviral vectors expressing the puromycin resistance gene on mammalian cells.
Virus titers were determined on mammalian cells as described previously (6). Briefly, DF-1 cells were transfected with retroviral vectors that the expressed puromycin resistance gene. At each cell passage, serial dilutions of the culture medium from the transfected cells were prepared and used to infect D17 (dog) cells. Forty-eight hours postinfection, the cells were trypsinized and plated in culture medium that contained puromycin. Fifteen days postinfection, colonies of puromycin-resistant cells were Giemsa stained and counted.
Cloning of adapted RCASBP(Eco) and RCASBP-M2C(4070A) genomes.
The cloning of genomes of adapted viruses and the reconstruction of corresponding replication-competent retroviral vectors were performed as described (6). Briefly, Hirt DNA was extracted from infected DFJ8 or DF-1 cells and used to construct a library in the λZAPExpress cloning vector (Stratagene, La Jolla, Calif.). The library was probed with radioactively labeled fragments of the ecotropic or the amphotropic env gene. Positive phage clones were purified and converted into plasmid clones. The resulting plasmid DNAs were cut with SacI and ClaI. The SacI-ClaI fragments that contained the gag, pol, and env genes of the adapted retroviruses were used to replace the SacI-ClaI fragments of nonadapted retroviral vectors, generating adapted replication-competent retroviral vectors.
Sequencing of the env gene.
The cloned env genes were sequenced by the dideoxy-chain termination method with primers specific for the ecotropic and amphotropic env genes (6).
RESULTS
The avian cell line DFJ8 expresses a functional ecotropic receptor.
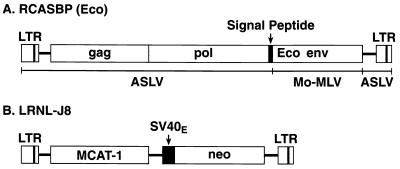
Ecotropic Moloney MLV use the murine cationic amino acid transporter MCAT-1 as a cellular receptor. Most nonrodent cells are not permissive for ecotropic MLV infection; however, these cells become permissive if they express MCAT-1 (5, 8, 28, 51, 58, 67, 68). Since avian cells lack a functional receptor for ecotropic murine retroviruses, they are not efficiently infected by ecotropic murine retroviruses. We constructed an avian cell line, DFJ8, which stably expresses MCAT-1; this line is efficiently infected by ecotropic retroviruses. A replication-defective murine retroviral vector, LRNL (79), was used to transfer the MCAT-1 cDNA into the avian cell line DF-1. The vector LRNL-J8 expresses MCAT-1 from the long terminal repeat (Fig. 1B); this vector also expresses the G418 resistance gene (neo).
FIG. 1.
Schematic of structures of chimeric retroviral vector RCASBP(Eco) (A) and murine retroviral vector LRNL-J8 (B) LTR, long terminal repeat; Mo-MLV, Moloney MLV; SV40E, simian virus 40 early promoter.
Ten G418-resistant DF-1 clones were isolated and tested for expression of a functional MCAT-1 by infection with the ecotropic retroviral vector BAG2 (57). All of the cell lines that we isolated could be infected with the murine vector; the parental DF-1 cell line could not (data not shown). The cell line that was most susceptible to infection by the BAG2 vector was designated DFJ8 and was used for experiments with the vector RCASBP-M(Eco) and its derivatives.
The chimeric retroviral vector RCASBP(Eco) replicates in DFJ8 cells but not in DF-1 cells.
A chimeric ecotropic env gene was prepared in which the region encoding the ecotropic gp70 surface glycoprotein was fused to a sequence encoding the Env signal peptide of an ASLV (see Materials and Methods). The amphotropic env gene was removed from the vector RCASBP-M2C(4070A) (6) and replaced with chimeric ecotropic env gene (Fig. 1A), creating the vector RCASBP(Eco).
One of the RCASBP(Eco) clones turned out to be aberrant. It carried the fragment of the pBR322 plasmid DNA between the 3′ terminus of the ecotropic env gene and the 3′ long terminal repeat. In this clone, the sequence encoding the two C-terminal amino acid residues of p15E and the stop codon was replaced with a segment of the pBR322 sequence. This aberrant construct was designated RCASBP(Eco1) and was used in the experiments in parallel with the normal RCASBP(Eco) vector.
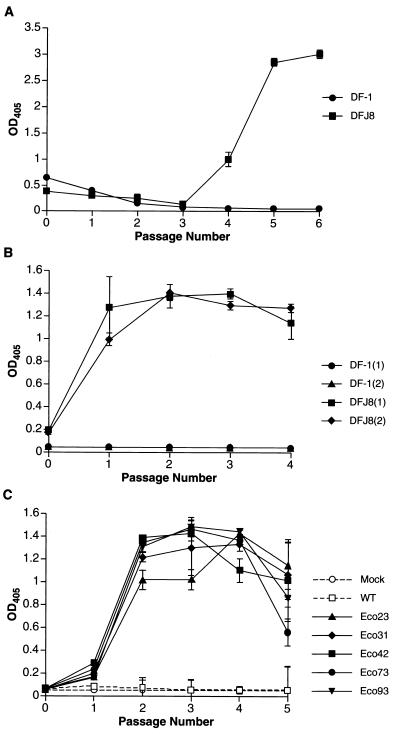
To generate virus stock, DFJ8 cells were transfected separately with RCASBP(Eco) and RCASBP(Eco1) plasmid DNAs, and the cells were passaged to allow the virus to replicate. Two plates of DFJ8 cells were independently transfected with each plasmid. To test the ability of the viruses to replicate specifically in DFJ8 cells, two plates of unmodified DF-1 cells were transfected in parallel with RCASBP(Eco) and RCASBP(Eco1), and the cells were passaged. Samples of culture media were collected at each passage, and then the samples were analyzed for the presence of viral particles using a p27 antigen capture ELISA (see Materials and Methods). The RCASBP(Eco) virus replicated rapidly in transfected DFJ8 cells. Virus production reached peak level by passage 2 (data not shown). However, the aberrant RCASBP(Eco1) virus initially replicated slowly. As shown in Fig. 2A, 24 h posttransfection, the cells transfected with RCASBP(Eco1) released virus particles that could be detected by ELISA. This initial burst of virus production was followed by a lag period, during which production of the virus dropped to a level that was practically undetectable by ELISA. Virus production rose again at passage 4 and reached a peak at passage 6, suggesting that the virus had spread through the culture (Fig. 2A). No virus production was observed in transfected DF-1 cells, showing that both RCASBP(Eco) and RCASBP(Eco1) replicated only in cells expressing the ecotropic receptor.
FIG. 2.
Replication and adaptation of the ecotropic vector RCASBP(Eco1) in avian cells. DF-1 and DFJ8 cells were transfected with RCASBP(Eco1) plasmid DNA or infected with RCASBP(Eco1) virus and passaged every other day. At each passage of the transfected cells, samples of the culture medium were harvested and the level of virus production was quantified by p27 capture ELISA (see Materials and Methods). (A) Replication of unadapted virus. Cells were transfected with plasmid DNA. (B) Replication of the adapted virus. DF-1 and DFJ8 cells (two plates of each) were infected with cell culture medium harvested at passage 6 of the unadapted virus (A). (C) Replication of molecular clones of adapted RCASBP(Eco1). DFJ8 cells were transfected with plasmid DNAs of adapted RCASBP(Eco1) and, separately, with plasmid DNA of unadapted RCASBP(Eco1). Mock, uninfected cells; WT, wild-type (unadapted) vector. Values are averages of two independent determinations. OD405, optical density at 405 nm.
To show that the virus stocks were infectious for DFJ8 cells, aliquots of the cell culture medium obtained at passage 6 were used to infect fresh DFJ8 cells. DF-1 cells were also treated with the same culture medium. As in the previous experiment, we collected samples of cell culture medium at each passage and measured the production of virus by p27 ELISA. Both viruses were detected in the culture medium from DFJ8 cells at passage 1 and reached the peak level by passage 2, suggesting that both viruses replicated efficiently and rapidly infected all cells in the culture. No lag period was seen in the second infection by RCASBP(Eco1) (compare graphs in Fig. 2A and B). DF-1 cells did not produce detectable viral particles (Fig. 2B). This result suggests that the chimeric retroviral vectors RCASBP(Eco) and RCASBP(Eco1) have an ecotropic host range and that the RCASBP(Eco1) virus generated by transfection replicated slowly at first and adapted in the course of passaging on DFJ8 cells.
Sequence changes in the ecotropic env gene of adapted RCASBP(Eco1).
To detect possible sequence changes in the rapidly replicating RCASBP(Eco) virus, the entire env region was PCR amplified from the total genomic DNA of infected DFJ8 cells and sequenced. As expected, no sequence changes were found, showing that RCASBP(Eco) did not undergo adaptation upon passaging in DFJ8 cells.
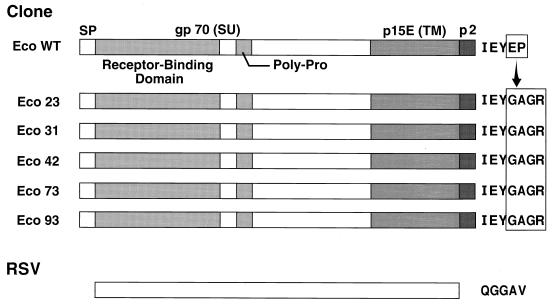
Given the delay in the appearance of rapidly replicating viruses after transfection of RCASBP(Eco1) in DFJ8 cells, we considered the possibility that the rapidly replicating virus was a genetic variant of the original vector RCASBP(Eco1). Molecular clones were prepared from the adapted viral stock, and the sequence of the env gene in each clone was determined. In each of the five clones sequenced, the aberrant amino acid sequence at the C terminus of the transmembrane protein p15E was changed to IEYGAGR, followed by a stop codon (Fig. 3). Comparison of the sequences at the 3′ end of the env gene and the downstream pBR322 fragment in the adapted and unadapted RCASBP(Eco1) showed that in the adapted virus, a portion of the pBR322 sequence was deleted. This deletion resulted in a fusion of the C-terminal p15E sequence with the downstream segment of pBR322 encoding the amino acid sequence GAGR and a stop codon. These changes were identical in all five ecotropic env genes. No other mutation was found in any of the five ecotropic env genes.
FIG. 3.
Changes in the amino acid sequence of the Env protein in molecular clones of adapted RCASBP(Eco1) viruses. Eco WT, wild-type ecotropic Env protein; Eco 23 to Eco 93, Env proteins in molecular clones of the adapted virus. RSV (Rous sarcoma virus), the envelope of the avian virus used to construct RCASBP(A), showing the sequence at the end of the transmembrane protein (TM). SP, signal peptide; SU, surface protein.
DFJ8 cells were transfected with each of the adapted RCASBP(Eco1) clones that we obtained. Replication was measured by testing culture medium for the presence of p27 by ELISA. As shown in Fig. 2C, all of the cloned viruses spread rapidly through DFJ8 cultures. In contrast, the cells transfected with nonadapted RCASBP(Eco1) began to produce virus only at passage 6 (data not shown).
Cytopathic effect of RCASBP(Eco) and the adapted RCASBP(Eco1) viruses in DFJ8 cells.
The amphotropic vector RCASBP-M2C(4070A) (6) caused pronounced cytopathic effects (vacuolization of cytoplasm and a formation of syncytia) when the virus replicated in CEF. Similar morphological changes were seen in DF-1 cells infected by RCASBP-M2C(4070A). The peak of the cytopathic effect in DF-1 cells was seen three to four passages after transfection (or infection) with RCASBP-M2C(4070A) and was preceded by a peak of viral production measured by ELISA (data not shown). The infected DF-1 cultures went through a crisis, and most of the cells died by passage 5 or 6. A small number of cells survived, replicated, and formed a monolayer 2 to 3 weeks later. These cells released substantially less virus into the culture medium than the acutely infected cells. This suggested that only those cells that expressed relatively low amounts of viral proteins (and produced fewer viral particles) survived.
To determine whether the adapted ecotropic vector RCASBP(Eco1) would cause cytopathic changes in DFJ8 cells, the cells were transfected with a cloned adapted RCASBP(Eco1), RCASBP(Eco73) (clone 73; Fig. 2C and 3). Separate cultures of DFJ8 cells were transfected with RCASBP-M2C(4070A). At passage 4, RCASBP(Eco73) caused formation of only a few syncytia. A similar effect was seen in cells infected with the RCASBP(Eco) vector (data not shown). In contrast, there were profound cytotoxic effects in the cells infected with RCASBP-M2C(4070A); vacuoles were seen in the cytoplasm, and there were numerous syncytia (data not shown).
We investigated whether it was possible to establish a permanent cell line that would produce high-titer stocks of either the ecotropic or the amphotropic RCASBP vector. A gene that confers resistance for hygromycin B (24) was inserted into the ClaI site of the adapted retroviral vectors RCASBP(Eco73) and RCASBP-M2C(4070A), generating vectors RCASBP(Eco73)Hyg and RCASBP-M2C(4070A)Hyg. These vectors were introduced into DFJ8 or DF-1 cells by transfection; the cells were passaged and, at different passages, treated with hygromycin B. DFJ8 cells transfected with RCASBP(Eco73)Hyg and passaged one to two times formed a monolayer in the presence of hygromycin B. These cells produced a viral stock with a titer of up to 5 × 106 CFU per ml (data not shown). In contrast, the DF-1 cells transfected with RCASBP-M2C(4070A)Hyg showed signs of cytotoxicity. The cells died rapidly during selection with hygromycin B, and we were not able to establish a permanently infected vector-producing cell line (data not shown). We believe that the difference in the behavior of RCASBP(Eco73)Hyg- and RCASBP-M2C(4070A)Hyg-infected cells is due to a difference in the cytopathogenicity of the retroviral vectors.
Processing of the transmembrane protein p15E in the adapted RCASBP(Eco73) virions.
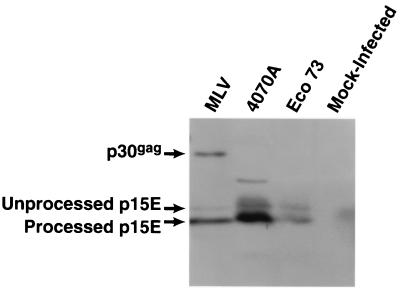
Earlier work showed that in the particles of amphotropic vector RCASBP-M2C(4070A), the transmembrane protein p15E is efficiently processed (6). In the ecotropic vector RCASBP(Eco73), the aberrant C-terminal amino acid sequence of p15E was changed to IEYGAGR during passage in DFJ8 cells. We examined whether this change affected the cleavage of p15E. Virus particles from DFJ8 cells infected with RCASBP(Eco73) were analyzed for the presence of processed p15E by immunoblotting with anti-p15E antibodies. As shown in Fig. 4, in the virions of RCASBP(Eco73), p15E is processed with approximately the same efficiency as in the virions of amphotropic vector RCASBP-M2C(4070) or amphotropic MLV. This suggests that the processing of the modified p15E in the chimeric ecotropic retrovirus particles is relatively efficient.
FIG. 4.
Proteolytic processing of the pre-p15E in RCASBP(Eco73) virions. Virus particles produced by infected DFJ8 cells were recovered by ultracentrifugation. Viral proteins were fractionated on SDS–16% polyacrylamide gels and transferred to Immobilon P membranes (see Materials and Methods). Virion-associated transmembrane protein was detected with antibodies against p15E. MLV, wild-type amphotropic MLV (positive control); 4070A, RCASBP-M2C(4070A) (positive control); Eco 73, ecotropic vector RCASBP(Eco73).
Passaging of RCASBP-M2C(4070A) in chicken embryos selects viruses with reduced cytopathogenicity.
The cytopathic effect of RCASBP-M2C(4070A) limits its usefulness. We first tried to select a less cytopathogenic virus in vitro by passaging of RCASBP-M2C(4070A) in DF-1 cells. However, even prolonged passaging of the virus in culture failed to produce a virus that had significantly reduced cytotoxicity (data not shown). We decided to try to select a less cytotoxic virus in embryonated eggs.
DF-1 cells were transfected with RCASBP-M2C(4070A), and the cells were passaged several times to generate a high-titer virus stock. Culture medium containing the RCASBP-M2C(4070A) virus was injected into EV-0 chicken embryos at day 6 of incubation. EV-0 embryos do not have endogenous retroviruses that are closely related to RCASBP. After 6 days of incubation in the embryos, the virus was recovered and used to infect DF-1 cells. Separately, control DF-1 cells were infected with the original cytopathic virus. At each passage, the morphology of infected cells was monitored. In parallel, the culture medium was collected and tested for the production of virus particles by p27 ELISA. To prove that the recovered virus was the amphotropic vector and not a recombinant retrovirus that could have been generated by recombination between the RCASBP-M2C(4070A) and a distantly related endogenous chicken retrovirus, we also tested the viral particles for the presence of both ASLV p27 capsid protein and amphotropic MLV gp70.
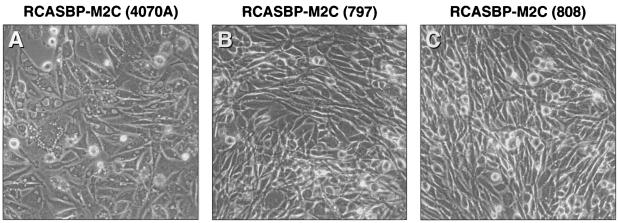
As shown in Fig. 5A, RCASBP-M2C(4070A) caused considerable vacuolization of cytoplasm in the infected DF-1 cells by passage 4. In contrast, viruses isolated from two separately infected chicken embryos caused only minor alterations of the morphology in the cells (compare the cells in Fig. 5B and C with the cells in Fig. 5A). These viruses were designated RCASBP-M2C(797) and RCASBP-M2C(808).
FIG. 5.
Cytotoxic effect of RCASBP-M2C(4070A) and chicken embryo-adapted viruses RCASBP-M2C(797) and RCASBP-M2C(808) in DF-1 cells. The cells were infected either with the cytotoxic amphotropic vector RCASBP-M2C (4070A) or with viruses recovered from two of the infected chicken embryos. The morphology of the infected cells at passage 4 is shown.
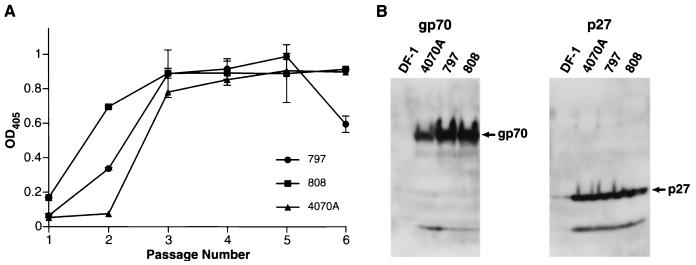
The data in Fig. 6A show that all three viruses grew rapidly in infected DF-1 cells. All virus-infected cell cultures reached the same level of virus production at passage 3. Particles of both RCASBP-M2C(797) and RCASBP-M2C(808) contained the amphotropic glycoprotein gp70, as shown by immunoblotting (Fig. 6B). This confirmed that the viruses recovered from chicken embryos were adapted versions of the amphotropic vector RCASBP-M2C(4070A) with substantially reduced cytotoxicity.
FIG. 6.
Replication of chicken embryo-adapted amphotropic vectors in DF-1 cells. (A) p27 capture ELISA. The cells were infected with viruses obtained from infected chicken embryos or, separately, with the cytotoxic amphotropic virus RCASBP-M2C(4070A) and passaged every other day. At each passage, virus production was measured by quantitation of the p27 level in samples of the culture medium by ELISA. Values are averages of two independent determinations. (B) Virus was recovered from culture medium by ultracentrifugation, fractionated on SDS-gradient (5 to 20%) polyacrylamide gels, and detected by immunoblotting. Virion-associated Env and capsid proteins were analyzed by immunoblotting with antibodies against gp70 and p27. DF-1, supernatant from uninfected DF-1 cells; 4070A, supernatant from cells infected with RCASBP-M2C(4070A); 797 and 808, supernatant from cells infected with viruses recovered from chicken embryos. OD405, optical density at 405 nm.
Cytotoxicity and sequence analysis of the env gene of the chicken embryo-adapted viruses RCASBP-M2C(797) and RCASBP-M2C(808).
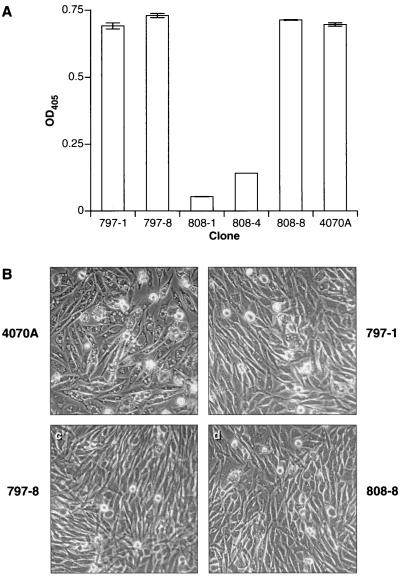
Based on our previous work with adaptation of RCASBP-M2C(4070A) in CEF (6), we expected that the adapted variants of the virus would contain mutations in their env genes. The original amphotropic RCASBP replicated poorly in chicken cells; RCASBP-M2C(4070A) has a single mutation in gp70, P242I, that substantially enhances the replication of the chimeric virus. Molecular clones of the viruses RCASBP-M2C(797) and RCASBP-M2C(808) were prepared from Hirt-fractioned DNA isolated from infected DF-1 cells as described previously (6). DF-1 cells were transfected with two clones of RCASBP-M2C(797), 797-1 and 797-8, and three clones of RCASBP-M2C(808), 808-1, 808-4, and 808-8, and then passaged. Separately, the DF-1 cells were transfected with the control plasmid, RCASBP-M2C(4070A). Cells transfected with clones 808-1 and 808-4 produced very limited amounts of viral particles at passage 4 as determined by p27 ELISA (Fig. 7A), suggesting that these clones were replication defective. In contrast, the cells transfected with clones 797-1, 797-8, and 808-8 produced about the same amount of p27 as those transfected with the parental virus, RCASBP-M2C(4070A) (Fig. 7A). As can be seen in Fig. 7B, the cells transfected with clones 797-1, 797-8, and 808-8 grew normally, and there was only limited vacuolization of the cytoplasm. In contrast, cells transfected with RCASBP-M2C(4070A) stopped growing and displayed substantial vacuolization. We easily established continuously growing cultures from the cells transfected with adapted viral clones. In contrast, the majority of cells transfected with RCASBP-M2C(4070) died at passage 5 after transfection. This shows that the clones 797-1, 797-8, and 808-8 are variants of the vector RCASBP-M2C(4070A) that have a reduced cytopathic effect.
FIG. 7.
Replication and cytotoxic effects of molecularly cloned chicken embryo-adapted amphotropic vectors in DF-1 cells. (A) p27 capture ELISA. DF-1 cells were transfected with plasmid DNA clones of chicken embryo-adapted vectors and passaged. Separately, the cells were transfected with cytotoxic amphotropic vector RCASBP-M2C(4070A). At passage 4, the amount of p27 capsid protein in the culture medium of the transfected cells was quantified by ELISA. Values are averages of two independent determinations. OD405, optical density at 405 nm. (B) Cytotoxic effect in DF-1 cells at passage 4. 4070A, cells transfected with RCASBP-M2C (4070A); 797-1, 797-8, 808-1, 808-4, and 808-8, cells transfected with molecular clones of chicken embryo-adapted vectors.
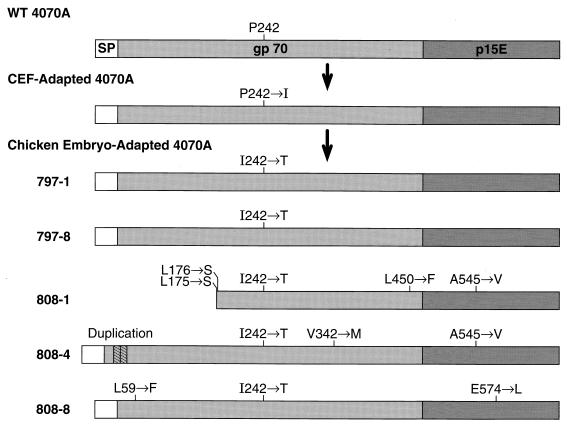
The env genes of the adapted viral clones were sequenced to determine the genetic changes responsible for the reduced cytopathogenicity of the RCASBP-M2C(4070A) variant. In all five clones, the isoleucine at position 242 of gp70 was replaced with threonine (Fig. 8). Clones 797-1 and 797-8 were identical and carried only the I242T mutation. Clone 808-8 carried two additional mutations, L59F in the N-terminal portion of gp70 and E574L in the transmembrane protein p15E. These mutations, which were present in only the one clone, apparently have no effect on the ability of the virus to replicate in DF-1 cells (Fig. 7A). One of the replication-defective clones, 808-1, carried a large deletion in the N-terminal segment of gp70-coding region and had several additional mutations (L175S, L176S, and L450F) in the gp70 and one mutation, A545V, in the p15E-coding region. The other replication-defective clone, 808-4, carried a small duplication in the N-terminal portion of gp70, as well as mutations in gp70 (V342M) and in p15E (A545V). Both of the replication-defective clones also carried the I242T mutation, which was present in all clones derived from viruses that had been passaged in chicken embryos. The mutation I242T was the only difference between the env gene of adapted viruses 797-1 and 797-8 and the env gene of the cytopathic RCASBP-M2C(4070A).
FIG. 8.
Structures of Env proteins encoded by wild-type amphotropic MLV (WT 4070A) and by molecular clones of the chicken embryo-adapted amphotropic vectors.
Amphotropic MLV passaged in the DF-1 cells contains P242 in the env gene.
The vector RCASBP-M2C(4070A) was derived by replacing avian subgroup A env gene in RCASBP(A) by the env gene from the amphotropic MLV 4070A. To better understand the nature of the mutation in the env gene of the adapted retroviral vector RCASBP-M2C(4070A), we transfected the DF-1 cells with the infectious molecular clone of the amphotropic MLV that contained the 4070A env gene and passaged the cells to allow the virus to spread. Unlike the unadapted RCASBP-M(4070A), the amphotropic MLV replicated rapidly in DF-1 cells (data not shown). After five passages, the genomic DNA was isolated from the infected cells, and the region of the amphotropic env gene encoding amino acid 242 was amplified by PCR and sequenced. After passage in DF-1 cells, the env gene of the passaged MLV contained a proline codon in the position 242. These data suggest that the amphotropic MLV grows well on DF-1 cells and that the change seen at position 242 of the RCASBP-M2C viruses is not related to binding to the avian version of the amphotropic receptor.
Gene transfer into mammalian cells by the chicken embryo-adapted RCASBP-M2C(797-8) vector.
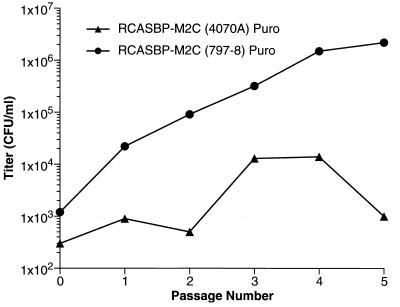
The vector RCASBP-M2C(797-8) is substantially less cytotoxic for DF-1 cells than RCASBP-M2C(4070A), making it easier to derive a high-titer stock. However, the mutation I242T in RCASBP-M2C(797-8) could potentially affect the ability of mutant envelope protein to recognize the mammalian amphotropic receptor, which could reduce the titer of the virus on mammalian cells. To address this issue, we constructed RCASBP-M2C(797-8)Puro, a version of the vector that expresses the puromycin resistance gene. DF-1 cells were transfected with the vector and passaged. At each passage, a sample of culture medium was harvested. Tenfold serial dilutions of all samples were prepared and used to infect D17 cells, which were then cultured in the presence of puromycin to select for puromycin-resistant colonies. In parallel, D17 cells were infected with serial dilutions of tissue culture medium obtained from DF-1 cells that were infected with the vector RCASBP-M2C(4070A)Puro (6). Figure 9 shows that the titer of RCASBP-M2C(4070A)Puro rose initially but at passage 5 dropped significantly. At passage 5, the infected culture contained large numbers of dead or dying cells. In contrast, DF-1 cells infected with RCASBP-M2C(797-8)Puro showed no significant cytotoxic changes at passage 5. The titer of the RCASBP-M2C(797-8)Puro virus was 1.1 × 106 CFU per ml. We passaged the infected DF-1 cells several more times and established permanent DF-1 cell culture that produced RCASBP-M2C(797-8)Puro stock with titer of 3 × 106 to 6 × 106 CFU/ml (data not shown). In addition, we passaged DF-1 cells infected with RCASBP-M2C(797-8)Puro in the presence of puromycin. The cells grew rapidly and produced virus with the titer of 5 × 106 CFU/ml (data not shown).
FIG. 9.
Passage of cells infected with RCASBP-M2C(4070A)Puro and RCASBP-M2C(797-8)Puro. Infection was initiated by transfection. Cells were passaged, and the virus was titered at each passage (see Materials and Methods). The lower titer of the RCASBP-M2C(4070A)Puro virus was a result of death of the cells infected with this virus.
DISCUSSION
Retroviruses readily incorporate envelope glycoproteins from other retroviruses and from viruses that belong to other taxonomic groups. Pseudotypes between the groups such as avian and murine leukemia/sarcoma viruses, murine and feline retroviruses, and murine retroviruses and lentiviruses, between different groups of lentiviruses, and between retroviruses and nonretroviruses have been described (1, 2, 4, 7, 9, 10, 12, 16, 19–21, 23, 26, 27, 29, 30, 35, 36, 39, 41, 44, 45, 52–54, 61, 65, 70, 73, 77, 78, 81–84). These pseudotyped retroviruses acquire the host range of the incorporated envelope glycoprotein.
It is possible to permanently change the host range of a retrovirus by replacing its env gene with the env gene from another retrovirus. Replacing the Env-coding sequence of RCASBP(A) with the envelope gene from an amphotropic MLV and adapting the virus by passage in avian cells allowed us to generate the stable chimeric retroviral vector RCASBP-M2C(4070A) (6). Relative to the parental amphotropic envelope, the envelope gene in this adapted vector contains a point mutation, P242I, in the surface glycoprotein gp70. The adapted virus replicates efficiently in CEF and DF-1 cells and infects, but does not replicate in, mammalian cells. However, this version of the amphotropic RCASBP is cytopathic in avian cells. Two RCASBP derivatives that can infect mammalian cells, but are much less cytopathic, have been developed. One virus uses the ecotropic envelope glycoprotein. The other virus uses a version of the amphotropic envelope that has undergone a second round of selection. This amphotropic virus has a second amino acid change at position 242; the isoleucine found in the cytotoxic version of the amphotropic virus was converted to threonine. The chimeric ecotropic retrovirus maintained the original proline residue at position 242; there were no genetic changes in the ecotropic envelope gene. Passage of an ecotropic virus with an aberrant C-terminal amino acid sequence of p15E selected for a virus in which the C terminus of p15E was modified.
How do the mutations in the amphotropic envelope help the RCASBP viruses to replicate? The chicken amphotropic receptor appears to be different from its mammalian counterpart (47, 74). In the case of the amphotropic envelope, one possibility was that the envelope was selected so that it could better use the avian amphotropic receptor. In contrast, the ecotropic vectors RCASBP(Eco) and RCASBP(Eco1) use the normal mammalian ecotropic receptor that is expressed in DFJ8 cells. However, amphotropic MLV replicated rapidly in the DF-1 cells with no delay. In addition, there was no change at position 242 upon passage of MLV in DF-1 cells. This suggests that the wild-type 4070A Env glycoprotein interacts efficiently with the chicken amphotropic receptor, permitting infection by both amphotropic MLV and the amphotropic chimeric retroviral vector RCASBP-M2C(4070A). Thus, it appears unlikely that the mutation P242I is involved in adapting the chimeric retrovirus to the avian amphotropic receptor.
The fact that the ecotropic envelope does not need to adapt suggests that there is nothing fundamentally wrong with the interaction of a murine envelope glycoprotein and an ASLV virion. The data also shows that both the naturally occurring (YEYEP) and mutant (YEYGAGR) variants of the C-terminal sequence of p15E are suitable for the formation of infectious ASLV particles. Moreover, we previously showed that the unadapted amphotropic Env glycoprotein is efficiently incorporated into the RCASBP particles (6). Therefore, it appears to be unlikely that the P242I mutation significantly increases the level of incorporation of the amphotropic Env glycoprotein into ASLV particles. What is the effect of the P242I mutation on the amphotropic envelope? The P242I mutation is in the surface glycoprotein but is at the C-terminal amino acid position in the available crystal structure (17). It is unclear from the structure how the mutation would enhance the ability of the amphotropic envelope to function in an ASLV particle. The fact that a similar mutation is not required for the ecotropic envelope to function in an ASLV particle only complicates the problem.
Env proteins are among the determinants responsible for the cytopathic effect of retroviruses in infected cells (11, 13, 14, 32–34, 37, 38, 42, 43, 48, 50, 56, 59, 60, 62, 69, 80). Among the ASLV, the subgroup B viruses are substantially more cytopathic than subgroup A viruses. Replacing the subgroup A env gene of the noncytopathic retrovirus RCASBP(A) with the env gene of an amphotropic MLV produced, after one round of adaptation in cell culture, a highly cytopathic retrovirus, RCASBP-M2C(4070A). The virus was passaged in chicken embryos to select a variant that was less cytopathic. In doing this experiment, we hoped not only to obtain a more useful vector but also that the mutations in the embryo-adapted virus might help us understand the role of the P242I mutation. The genetic variant of the vector RCASBP-M2C(4070A), isolated after passage in chicken embryos, had the isoleucine residue in position 242 of the gp70 converted to threonine, which markedly reduced the cytotoxicity of the virus for avian cells. These data have two implications. First, they demonstrate a strong selective pressure in vivo for a formation of less cytopathic retroviruses, suggesting that this strategy could be used to improve other retroviral vectors. Second, they underscore the potential structural and functional importance of position 242 in gp70, since the single amino acid change in this position appears not only to adapt the amphotropic murine retrovirus Env glycoprotein to allow it to function in the chimeric ASLV particles but also to affect the cytotoxicity of chimeric retroviruses in avian cells. Unfortunately, it does not make it easier to understand why this residue is so important.
Numerous retroviral vector systems that exploit the ability of retroviruses to form pseudotypes to alter the host range of the vector have been described (15, 18, 29, 31, 40, 45, 46, 49, 66, 75, 76). The majority of retroviral vectors of this type are replication defective. Replication-defective vectors are able to infect target cells; however, our data suggest that at least in some cases, an unmodified Env may not be optimal. The amphotropic RCASBP vector initially replicated very slowly in avian cells. Passaging the chimeric retrovirus selected variants that grew rapidly in avian cells. In contrast, replication-defective retroviruses cannot be passaged to select variants with enhanced infectivity. Since our current understanding of the structure-function relationship of retroviral Env proteins is limited, it is not possible to rationally construct an optimized Env protein. This suggests a general strategy that can be employed to optimize env genes for use with particular retroviruses, whether the ultimate goal is the development of a replication-competent or a replication-defective vector. The strategy takes advantage of the ability of replication-competent chimeric retroviruses to spread, even if the initial replication efficiency is low. Mutations arise, and those that increase the replication of the chimeric virus are selected. Our results suggest that at least in some cases, selection may be more efficient in a developing embryo or in an intact animal. Once the appropriate adapted virus is selected, its genome can be molecularly cloned and used in the construction of more efficient vectors. In some cases, identification of sequence changes in the adapted chimeric retroviruses could potentially provide information that could be used to engineer optimized Env proteins. We have identified one type of sequence change that can adapt the ASLV-MLV chimeric amphotropic retroviruses and alter their cytopathogenesis. We hope that further studies of chimeric retroviruses would allow us to develop other chimeric retroviral vectors with various viral surface glycoproteins providing expanded host range. Such retroviral vectors could potentially be used for targeted gene delivery.
ACKNOWLEDGMENTS
We thank Alan Rein for his kind gifts of plasmid pRR88 and anti-gp70 and anti-p15E antibodies, J. Cunningham for supplying plasmid pJET, and Donald Blair for his gift of the retroviral vector LRNL. We are grateful to James Resau and Erik Hudson for help with confocal microscopy and to Marilyn Powers and Mary Jane McWilliams for invaluable help with automatic DNA sequencing. We also thank Hilda Marusiodis for help with preparation of the manuscript.
REFERENCES
- 1.Ablashi D V, Armstrong G R, Turner W. Production and characterization of human cell-adapted murine Rauscher virus pseudotype of murine sarcoma virus. J Natl Cancer Inst. 1973;50:381–385. doi: 10.1093/jnci/50.2.381. [DOI] [PubMed] [Google Scholar]
- 2.Al-Adhami R, Chapman A L. Aberrant viruses in cells infected with murine sarcoma virus-feline leukemia virus. J Natl Cancer Inst. 1975;54:763–766. [PubMed] [Google Scholar]
- 3.Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 4.Arai T, Matsumoto K, Saitoh K, Ui M, Ito T, Murakami M, Kanegae Y, Saito I, Cosset F L, Takeuchi Y, Iba H. A new system for stringent, high-titer vesicular stomatitis virus G protein-pseudotyped retrovirus vector induction by introduction of Cre recombinase into stable prepackaging cell lines. J Virol. 1998;72:1115–1121. doi: 10.1128/jvi.72.2.1115-1121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker B W, Boettiger D, Spooncer E, Norton J D. Efficient retroviral-mediated gene transfer into human B lymphoblastoid cells expressing mouse ecotropic viral receptor. Nucleic Acids Res. 1992;20:5234. doi: 10.1093/nar/20.19.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barsov E V, Hughes S H. Gene transfer into mammalian cells by a Rous sarcoma virus-based retroviral vector with the host range of the amphotropic murine leukemia virus. J Virol. 1996;70:3922–3929. doi: 10.1128/jvi.70.6.3922-3929.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basombrio M A, Mayer A M, Pasqualini C D. Murine sarcoma virus pseudotypes used as immunogens against viral and chemical oncogenesis. Cancer Res. 1977;37:1768–1776. [PubMed] [Google Scholar]
- 8.Bertran J, Miller J L, Yang Y, Fenimore-Justman A, Rueda F, Vanin E F, Nienhuis A W. Recombinant adeno-associated virus-mediated high-efficiency, transient expression of the murine cationic amino acid transporter (ecotropic retroviral receptor) permits stable transduction of human HeLa cells by ecotropic retroviral vectors. J Virol. 1996;70:6759–6766. doi: 10.1128/jvi.70.10.6759-6766.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besmer P, Baltimore D. Mechanism of restriction of ecotropic and xenotropic murine leukemia viruses and formation of pseudotypes between the two viruses. J Virol. 1977;21:965–973. doi: 10.1128/jvi.21.3.965-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitman M, Prevec L. The use of vesicular stomatitis virus pseudotype production in the study of a temperature-sensitive murine leukemia virus. Virology. 1977;76:643–652. doi: 10.1016/0042-6822(77)90246-x. [DOI] [PubMed] [Google Scholar]
- 11.Cao J, Park I W, Cooper A, Sodroski J. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J Virol. 1996;70:1340–1354. doi: 10.1128/jvi.70.3.1340-1354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chieco-Bianchi L, Collavo D, Colombatti A, Biasi G. In vivo interactions between murine leukemia and sarcoma viruses. Bibl Haematol. 1975;40:613–620. doi: 10.1159/000397582. [DOI] [PubMed] [Google Scholar]
- 13.Dedera D, Ratner L. Demonstration of two distinct cytopathic effects with syncytium formation-defective human immunodeficiency virus type 1 mutants. J Virol. 1991;65:6129–6136. doi: 10.1128/jvi.65.11.6129-6136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiFronzo N L, Pise-Masison C A, Fernandez-Larsson R, Holland C A. Viral determinants of HIV-1 sufficient to extend tropism to macrophages are distinct from the determinants that control the cytopathic phenotype in HL-60 cells. AIDS. 1997;11:1681–1688. doi: 10.1097/00002030-199714000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Eglitis M A, Kohn D B, Moen R C, Blaese R M, Anderson W F. Infection of human hematopoietic progenitor cells using a retroviral vector with a xenotropic pseudotype. Biochem Biophys Res Commun. 1988;151:201–206. doi: 10.1016/0006-291x(88)90579-7. [DOI] [PubMed] [Google Scholar]
- 16.Emi N, Friedmann T, Yee J K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65:1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fass D, Davey R A, Hamson C A, Kim P S, Cunningham J M, Berger J M. Structure of a murine leukemia virus receptor-binding glycoprotein at 2.0 angstrom resolution. Science. 1997;277:1662–1666. doi: 10.1126/science.277.5332.1662. [DOI] [PubMed] [Google Scholar]
- 18.Federico M, Nappi F, Ferrari G, Chelucci C, Mavilio F, Verani P. A nonproducer, interfering human immunodeficiency virus (HIV) type 1 provirus can be transduced through a murine leukemia virus-based retroviral vector: recovery of an anti-HIV mouse/human pseudotype retrovirus. J Virol. 1995;69:6618–6626. doi: 10.1128/jvi.69.11.6618-6626.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischinger P J, Haapala D K. Quantitative interactions of feline leukaemia virus and its pseudotype of murine sarcoma virus in cat cells: requirement for DNA synthesis. J Gen Virol. 1971;13:203–214. doi: 10.1099/0022-1317-13-2-203. [DOI] [PubMed] [Google Scholar]
- 20.Fischinger P J, Nomura S, Blevins C S, Bolognesi D P. Two levels of restriction by mouse or cat cells of murine sarcoma virus coated by endogenous xenotropic oncornavirus. J Gen Virol. 1975;29:51–62. doi: 10.1099/0022-1317-29-1-51. [DOI] [PubMed] [Google Scholar]
- 21.Gilden D H, Devlin M, Wroblewska Z. The use of vesicular stomatitis (visna virus) pseudotypes to demonstrate visna virus receptors in cells from different species. Arch Virol. 1981;67:181–185. doi: 10.1007/BF01318603. [DOI] [PubMed] [Google Scholar]
- 22.Graham F L, van der Eb A J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 23.Gregory S, Collman R, James W, Gordon S, Gonzalez-Scarano F, Nathanson N. HIV-1 pseudotype virus containing a Cocal virus genome and an HIV envelope: construction, assay and use. J Virol Methods. 1993;44:287–304. doi: 10.1016/0166-0934(93)90064-x. [DOI] [PubMed] [Google Scholar]
- 24.Gritz L, Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983;25:179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- 25.Himly M, Foster D N, Bottoli I, Iacovoni J S, Vogt P K. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology. 1998;248:295–304. doi: 10.1006/viro.1998.9290. [DOI] [PubMed] [Google Scholar]
- 26.Hoshino H, Weiss R A, Clapham P, Miwa M, Miyoshi I, Yoshida M, Sugimura T. Pseudotype viruses bearing envelope antigens of Japanese isolates of human T-cell leukemia viruses type I. Princess Takamatsu Symp. 1984;15:159–164. [PubMed] [Google Scholar]
- 27.Huebner R J, Hartley J W, Rowe W P, Lane W T, Capps W I. Rescue of the defective genome of Moloney sarcoma virus from a noninfectious hamster tumor and the production of pseudotype sarcoma viruses with various murine leukemia viruses. Proc Natl Acad Sci USA. 1966;56:1164–1169. doi: 10.1073/pnas.56.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igarashi T, Suzuki S, Takahashi M, Tamaoki T, Shimada T. A novel strategy of cell targeting based on tissue-specific expression of the ecotropic retrovirus receptor gene. Hum Gene Ther. 1998;9:2691–2698. doi: 10.1089/hum.1998.9.18-2691. [DOI] [PubMed] [Google Scholar]
- 29.Indraccolo S, Minuzzo S, Feroli F, Mammano F, Calderazzo F, Chieco-Bianchi L, Amadori A. Pseudotyping of Moloney leukemia virus-based retroviral vectors with simian immunodeficiency virus envelope leads to targeted infection of human CD4+ lymphoid cells. Gene Ther. 1998;5:209–217. doi: 10.1038/sj.gt.3300603. [DOI] [PubMed] [Google Scholar]
- 30.Kang C Y, Lambright P. Pseudotypes of vesicular stomatitis virus with the mixed coat of reticuloendotheliosis virus and vesicular stomatitis virus. J Virol. 1977;21:1252–1255. doi: 10.1128/jvi.21.3.1252-1255.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiem H P, Heyward S, Winkler A, Potter J, Allen J M, Miller A D, Andrews R G. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudotyped retroviral vectors in a competitive repopulation assay in baboons. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 32.Koga Y, Nakamura K, Sasaki M, Kimura G, Nomoto K. The difference in gp160 and gp120 of HIV type 1 in the induction of CD4 downregulation preceding single-cell killing. Virology. 1994;201:137–141. doi: 10.1006/viro.1994.1274. [DOI] [PubMed] [Google Scholar]
- 33.Koga Y, Sasaki M, Nakamura K, Kimura G, Nomoto K. Intracellular distribution of the envelope glycoprotein of human immunodeficiency virus and its role in the production of cytopathic effect in CD4+ and CD4− human cell lines. J Virol. 1990;64:4661–4671. doi: 10.1128/jvi.64.10.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koga Y, Sasaki M, Yoshida H, Oh-Tsu M, Kimura G, Nomoto K. Disturbance of nuclear transport of proteins in CD4+ cells expressing gp160 of human immunodeficiency virus. J Virol. 1991;65:5609–5612. doi: 10.1128/jvi.65.10.5609-5612.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristal B S, Reinhart T A, Hoover E A, Mullins J I. Interference with superinfection and with cell killing and determination of host range and growth kinetics mediated by feline leukemia virus surface glycoproteins. J Virol. 1993;67:4142–4153. doi: 10.1128/jvi.67.7.4142-4153.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Landau N R, Page K A, Littman D R. Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurent-Crawford A G, Coccia E, Krust B, Hovanessian A G. Membrane-expressed HIV envelope glycoprotein heterodimer is a powerful inducer of cell death in uninfected CD4+ target cells. Res Virol. 1995;146:5–17. doi: 10.1016/0923-2516(96)80585-1. [DOI] [PubMed] [Google Scholar]
- 38.Laurent-Crawford A G, Krust B, Riviere Y, Desgranges C, Muller S, Kieny M P, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 39.Le Guern M, Levy J A. Human immunodeficiency virus (HIV) type 1 can superinfect HIV-2-infected cells: pseudotype virions produced with expanded cellular host range. Proc Natl Acad Sci USA. 1992;89:363–367. doi: 10.1073/pnas.89.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindemann D, Bock M, Schweizer M, Rethwilm A. Efficient pseudotyping of murine leukemia virus particles with chimeric human foamy virus envelope proteins. J Virol. 1997;71:4815–4820. doi: 10.1128/jvi.71.6.4815-4820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu M L, Winther B L, Kay M A. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)-Moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Y Y, Koga Y, Tanaka K, Sasaki M, Kimura G, Nomoto K. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J Virol. 1994;68:390–399. doi: 10.1128/jvi.68.1.390-399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynn W S, Tweedale A, Cloyd M W. Human immunodeficiency virus (HIV-1) cytotoxicity: perturbation of the cell membrane and depression of phospholipid synthesis. Virology. 1988;163:43–51. doi: 10.1016/0042-6822(88)90232-2. [DOI] [PubMed] [Google Scholar]
- 44.Mandeville R, Rohan P, Wainberg M A. Neutralization of pseudotypes of vesicular stomatitis virus by sera from avian retrovirus-infected hosts. Int J Cancer. 1979;23:415–423. doi: 10.1002/ijc.2910230322. [DOI] [PubMed] [Google Scholar]
- 45.Manning W C, Murphy J E, Jolly D J, Mento S J, Ralston R O. Use of a recombinant murine cytomegalovirus expressing vesicular stomatitis virus G protein to pseudotype retroviral vectors. J Virol Methods. 1998;73:31–39. doi: 10.1016/s0166-0934(98)00034-2. [DOI] [PubMed] [Google Scholar]
- 46.Miletic H, Bruns M, Tsiakas K, Vogt B, Rezai R, Baum C, Kuhlke K, Cosset F L, Ostertag W, Lother H, von Laer D. Retroviral vectors pseudotyped with lymphocytic choriomeningitis virus. J Virol. 1999;73:6114–6116. doi: 10.1128/jvi.73.7.6114-6116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller D G, Edwards R H, Miller A D. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukemia virus. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mor-Vaknin N, Turgeman H, Torgeman A, Wolfson M, Huleihel M, Aboud M. Rapid syncytium formation between human T-cell leukaemia virus type-I (HTLV-I)-infected T-cells and human nervous system cells: a possible implication for tropical spastic paraparesis/HTLV-I associated myelopathy. Cell Biol Int. 1998;22:95–103. doi: 10.1006/cbir.1998.0241. [DOI] [PubMed] [Google Scholar]
- 49.Movassagh M, Desmyter C, Baillou C, Chapel-Fernandes S, Guigon M, Klatzmann D, Lemoine F M. High-level gene transfer to cord blood progenitors using gibbon ape leukemia virus pseudotype retroviral vectors and an improved clinically applicable protocol. Hum Gene Ther. 1998;9:225–234. doi: 10.1089/hum.1998.9.2-225. [DOI] [PubMed] [Google Scholar]
- 50.Mulligan M J, Kumar P, Hui H X, Owens R J, Ritter G D, Jr, Hahn B H, Compans R W. The env protein of an infectious noncytopathic HIV-2 is deficient in syncytium formation. AIDS Res Hum Retroviruses. 1990;6:707–720. doi: 10.1089/aid.1990.6.707. [DOI] [PubMed] [Google Scholar]
- 51.Nathwani A C, Persons D A, Stevenson S C, Frare P, McClelland A, Nienhuis A W, Vanin E F. Adenovirus-mediated expresssion of the murine ecotropic receptor facilitates transduction of human hematopoietic cells with an ecotropic retroviral vector. Gene Ther. 1999;6:1456–1468. doi: 10.1038/sj.gt.3300974. [DOI] [PubMed] [Google Scholar]
- 52.Okabe H, Gilden R V, Hatanaka M. Murine sarcoma virus related nucleic acid sequences in a non-transforming virus derived from an interspecies pseudotype sarcoma virus. Int J Cancer. 1975;5:849–859. doi: 10.1002/ijc.2910150517. [DOI] [PubMed] [Google Scholar]
- 53.Okazaki T. Restriction of host range of xenotropic pseudotype murine sarcoma virus by helper leukemia virus. Acta Med Okayama. 1983;37:273–282. doi: 10.18926/AMO/32394. [DOI] [PubMed] [Google Scholar]
- 54.Otten J A, Myer F E, Tennant R W, Brown A. Effect of the Fv-1 locus in vivo: host range pseudotypes of murine sarcoma virus. J Natl Cancer Inst. 1978;60:875–880. doi: 10.1093/jnci/60.4.875. [DOI] [PubMed] [Google Scholar]
- 55.Petropoulos C J, Hughes S H. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991;65:3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poss M L, Quackenbush S L, Mullins J I, Hoover E A. Characterization and significance of delayed processing of the feline leukemia virus FeLV-FAIDS envelope glycoprotein. J Virol. 1990;64:4338–4343. doi: 10.1128/jvi.64.9.4338-4345.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci USA. 1987;84:156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qing K, Bachelot T, Mukherjee P, Wang X S, Peng L, Yoder M C, Leboulch P, Srivastava A. Adeno-associated virus type 2-mediated transfer of ecotropic retrovirus receptor cDNA allows ecotropic retroviral transduction of established and primary human cells. J Virol. 1997;71:5663–5667. doi: 10.1128/jvi.71.7.5663-5667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Resnick-Roguel N, Burstein H, Hamburger J, Panet A, Eldor A, Vlodavsky I, Kotler M. Cytocidal effect caused by the envelope glycoprotein of a newly isolated avian hemangioma-inducing retrovirus. J Virol. 1989;63:4325–4330. doi: 10.1128/jvi.63.10.4325-4330.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rey-Cuille M A, Galabru J, Laurent-Crawford A, Krust B, Montagnier L, Hovanessian A G. HIV-2 EHO isolate has a divergent envelope gene and induces single cell killing by apoptosis. Virology. 1994;202:471–476. doi: 10.1006/viro.1994.1364. [DOI] [PubMed] [Google Scholar]
- 61.Rhim J S. Characterization of sarcoma-positive, leukemia-negative (S+L−) human cells induced by the feline leukemia virus pseudotype of Moloney sarcoma virus. Proc Soc Exp Biol Med. 1981;167:597–606. doi: 10.3181/00379727-167-41221. [DOI] [PubMed] [Google Scholar]
- 62.Rohn J L, Moser M S, Gwynn S R, Baldwin D N, Overbaugh J. In vivo evolution of a novel, syncytium-inducing and cytopathic feline leukemia virus variant. J Virol. 1998;72:2686–2696. doi: 10.1128/jvi.72.4.2686-2696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 64.Schaefer-Klein J, Givol I, Barsov E V, Whitcomb J M, VanBrocklin M, Foster D N, Federspiel M J, Hughes S H. The EV-O-derived cell line DF-1 supports the efficient replication of avian leukosis-sarcoma viruses and vectors. Virology. 1998;248:305–311. doi: 10.1006/viro.1998.9291. [DOI] [PubMed] [Google Scholar]
- 65.Scher C D. Effect of pseudotype on Abelson virus and Kirsten sarcoma virus-induced leukemia. J Exp Med. 1978;147:1044–1053. doi: 10.1084/jem.147.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schnierle B S, Stitz J, Bosch V, Nocken F, Merget-Millitzer H, Engelstadter M, Kurth R, Groner B, Cichutek K. Pseudotyping of murine leukemia virus with the envelope glycoproteins of HIV generates a retroviral vector with specificity of infection for CD4-expressing cells. Proc Natl Acad Sci USA. 1997;94:8640–8645. doi: 10.1073/pnas.94.16.8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scholz A, Beato M. Transient transfection of ecotropic retrovirus receptor permits stable gene transfer into non-rodent cells with murine retroviral vectors. Nucleic Acids Res. 1996;24:979–980. doi: 10.1093/nar/24.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scott-Taylor T H, Gansbacher B, Sadelain M. Efficient retroviral infection of human cells utilising an adenoviral vector expressing the ecotropic receptor. Adv Exp Med Biol. 1998;451:423–430. doi: 10.1007/978-1-4615-5357-1_65. [DOI] [PubMed] [Google Scholar]
- 69.Sela-Donenfeld D, Korner M, Pick M, Eldor A, Panet A. Programmed endothelial cell death induced by an avian hemangioma retrovirus is density dependent. Virology. 1996;223:233–237. doi: 10.1006/viro.1996.0472. [DOI] [PubMed] [Google Scholar]
- 70.Spector D H, Wade E, Wright D A, Koval V, Clark C, Jaquish D, Spector S A. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol. 1990;64:2298–2308. doi: 10.1128/jvi.64.5.2298-2308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stuhlmann H, Jaenisch R, Mulligan R C. Construction and properties of replication-competent murine retroviral vectors encoding methotrexate resistance. Mol Cell Biol. 1989;9:100–108. doi: 10.1128/mcb.9.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stuhlmann H, Jaenisch R, Mulligan R C. Transfer of a mutant dihydrofolate reductase gene into pre- and postimplantation mouse embryos by a replication-competent retrovirus vector. J Virol. 1989;63:4857–4865. doi: 10.1128/jvi.63.11.4857-4865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takeuchi Y, Simpson G, Vile R G, Weiss R A, Collins M K. Retroviral pseudotypes produced by rescue of a Moloney murine leukemia virus vector by C-type, but not D-type, retroviruses. Virology. 1992;186:792–794. doi: 10.1016/0042-6822(92)90049-u. [DOI] [PubMed] [Google Scholar]
- 74.van Zeijl M, Johann S V, Closs E, Cunningham J, Eddy R, Shows T B, O'Hara B. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukemia virus receptor family. Proc Natl Acad Sci USA. 1994;91:1168–1172. doi: 10.1073/pnas.91.3.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang R F, Wang X, Johnston S L, Zeng G, Robbins P F, Rosenberg S A. Development of a retrovirus-based complementary DNA expression system for the cloning of tumor antigens. Cancer Res. 1998;58:3519–3525. [PubMed] [Google Scholar]
- 76.Wang S, Beattie G M, Hayek A, Levine F. Development of a VSV-G protein pseudotyped retroviral vector system expressing dominant oncogenes from a lacO-modified inducible LTR promoter. Gene. 1996;182:145–150. doi: 10.1016/s0378-1119(96)00536-7. [DOI] [PubMed] [Google Scholar]
- 77.Weiss R A, Boettiger D, Murphy H M. Pseudotypes of avian sarcoma viruses with the envelope properties of vesicular stomatitis virus. Virology. 1977;76:808–825. doi: 10.1016/0042-6822(77)90261-6. [DOI] [PubMed] [Google Scholar]
- 78.Weiss R A, Wong A L. Phenotypic mixing between avian and mammalian RNA tumor viruses. I. Envelope pseudotypes of Rous sarcoma virus. Virology. 1977;76:826–834. doi: 10.1016/0042-6822(77)90262-8. [DOI] [PubMed] [Google Scholar]
- 79.Xu L, Yee J K, Wolff J A, Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989;171:331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- 80.Yahi N, Fantini J, Hirsch I, Chermann J C. Structural variability of env and gag gene products from a highly cytopathic strain of HIV-1. Arch Virol. 1992;125:287–298. doi: 10.1007/BF01309645. [DOI] [PubMed] [Google Scholar]
- 81.Yoshikura H. Adaptation of N-tropic Friend leukaemia virus and its murine sarcoma virus pseudotype of non-permissive B-type C57BL/6 mouse cell line. J Gen Virol. 1975;29:1–9. doi: 10.1099/0022-1317-29-1-1. [DOI] [PubMed] [Google Scholar]
- 82.Zavada J, Dickson C, Weiss R. Pseudotypes of vesicular stomatitis virus with envelope antigens provided by murine mammary tumor virus. Virology. 1977;82:221–231. doi: 10.1016/0042-6822(77)90045-9. [DOI] [PubMed] [Google Scholar]
- 83.Zavada J, Cerny L, Altstein A D, Zavadova Z. Pseudotype particles of vesicular stomatitis virus with surface antigens of bovine leukaemia virus-VSV (BLV) as a sensitive probe for detecting antibodies in the sera of spontaneously infected cattle. Acta Virol. 1978;22:91–96. [PubMed] [Google Scholar]
- 84.Zavadova Z, Zavada J. Pseudotypes of vesicular stomatitis virus with coat antigen of bovine leukaemia virus-VSV (BLV): antigenic surface mosaic and the roles of precipitating antibodies and polycations. Acta Virol. 1980;24:166–174. [PubMed] [Google Scholar]