Abstract
Worldwide, around 50 million people live with dementia, and this number is projected to triple by 2050. It has been estimated that 20% of all dementia cases have a predominant cerebrovascular pathology, while perhaps another 20% of vascular diseases contribute to a mixed dementia picture. Therefore, the vascular contribution to dementia affects 20 million people currently and will increase markedly in the next few decades, particularly in lower- and middle-income countries.
In this review, we discuss the mechanisms of vascular cognitive impairment (VCI) and review management. VCI refers to the spectrum of cerebrovascular pathologies that contribute to any degree of cognitive impairment, ranging from subjective cognitive decline, to mild cognitive impairment, to dementia. While acute cognitive decline occurring soon after a stroke is the most recognized form of VCI, chronic cerebrovascular disease, in particular cerebral small-vessel disease, can cause insidious cognitive decline in the absence of stroke. Moreover, cerebrovascular disease not only commonly co-occurs with Alzheimer’s disease (AD) and increases the probability that AD pathology will result in clinical dementia, but may also contribute etiologically to the development of AD pathologies.
Despite its enormous health and economic impact, VCI has been a neglected research area, with few adequately powered trials of therapies, resulting in few proven treatments. Current management of VCI emphasizes prevention and treatment of stroke and vascular risk factors, with most evidence for intensive hypertension control. Reperfusion therapies in acute stroke may attenuate the risk of VCI. Associated behavioral symptoms such as apathy and poststroke emotionalism are common. We also highlight novel treatment strategies that will hopefully lead to new disease course-modifying therapies. Finally, we highlight the importance of including cognitive endpoints in large cardiovascular prevention trials and the need for an increased research focus and funding for this important area.
Keywords: Vascular cognitive impairment, small-vessel disease, Alzheimer’s disease, stroke, dementia, vascular risk factors
In our rapidly aging society, the management and research of cerebrovascular disease (CVD) are of global importance not only because of its association with stroke but also because of its contribution to cognitive impairment. Vascular cognitive impairment (VCI) refers to the entire spectrum of vascular brain pathologies that contribute to any degree of cognitive impairment, ranging from subjective cognitive decline, to mild cognitive impairment (MCI), to dementia. 1 While acute cognitive decline occurring soon after a stroke is the most recognized form of VCI, CVD, in particular cerebral small-vessel disease (SVD), can also induce insidious cognitive decline in the absence of stroke. 2 Moreover, studies conducted over the recent decades have shown that CVD not only commonly co-occurs with Alzheimer’s disease (AD) and increases the probability that AD pathology will result in clinical dementia, but may also contribute etiologically to the development of AD pathologies. Since recent forecasts predict that the number of people living with dementia will triple from around 57.4 million currently to 152.8 million by 2050 and the greatest increase will be at low-income and middle-income countries (LMICs) where control of CVD is suboptimal, 3 tackling CVD is of paramount importance in combating this coming dementia tsunami. In this review, we provide an update on the mechanisms and management of VCI, as well as highlighting a number of articles published in this special edition of the International Journal of Stroke (IJS) on the vascular contribution to dementia.
Mechanisms of VCI
Poststroke cognitive impairment
Stroke commonly leads to acute onset of cognitive decline soon after the stroke event. Systematic reviews show that the prevalence of poststroke MCI and dementia are around 38% and 18.4%, respectively.4–6 Age and vascular risk factors/cardiovascular diseases (e.g. hypertension, hyperlipidemia, diabetes, atrial fibrillation (AF), physical inactivity, smoking, nutrition, obesity, and air pollutants) explain the majority of stroke risk. Whether the stroke event induces cognitive impairment depends on a complex interplay between the features of the stroke lesion itself and the brain resilience of the individual. Large lesions or lesions located at “strategic” sites (e.g. left frontotemporal lobe, left thalamus, or right parietal lobe) are more prone to result in cognitive impairment.4,7,8 Brain resilience depends on a host of factors, including pre-existing brain diseases (e.g. AD and SVD), cognitive reserve (e.g. educational level and cognitive activity), and other demographic or medical conditions (e.g. age, diabetes, and frailty). 9 Even a small lesion located in a non-strategic region, or a transient ischemic attack, may trigger poststroke cognitive impairment in someone with low brain resilience. The importance of pre-existing brain disease is emphasized by a meta-analysis by Ball and colleagues who in this month’s issue of IJS show that that pre-existing extent of magnetic resonance imaging (MRI) markers of SVD (white matter hyperintensities (WMHs), cerebral microbleeds (CMBs), and brain atrophy) present at the acute stage of stroke were associated with poststroke cognitive impairment. 10 It is notable that in contrast they did not find an association with features of the acute stroke lesion itself. In another study, also published in this issue of IJS, using the data from the Meta VCI Map consortium (n = 1568), Coenen et al. 11 found that apart from the total volume of pre-existing WMH volume, WMH located at “strategic” white matter tracts (left anterior thalamic radiation, forceps major) was also associated with poststroke cognitive impairment. This finding implies that the concept of “strategic” lesions extends beyond acute stroke lesions and involves pre-existing WMH as well. It emphasizes the importance of white matter damage in causing cognitive impairment, a view reinforced by results from diffusion tensor imaging (DTI) showing that diffuse white matter damage correlates with the degree of cognitive impairment. 12 Further studies using DTI data to construct brain networks have shown the central role of network disruption in mediating the effect of multiple different SVD pathologies, including WMH and lacunar infarcts, on causing VCI.13,14
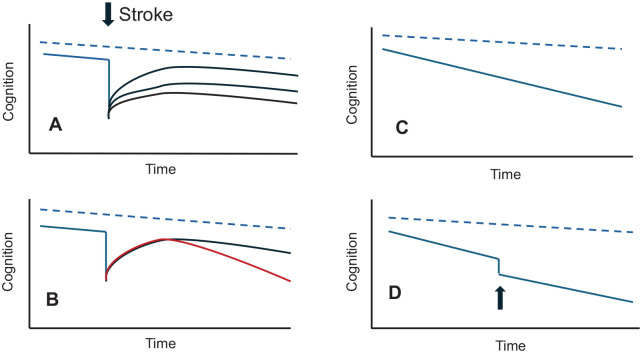
It is also noteworthy that even after the cognitive decline associated with an acute stroke has settled, the long-term rate of cognitive decline over subsequent years is faster when compared to that in stroke-free individuals and such a decline occurs even in the absence of recurrent stroke25,26 (Figure 1). One factor driving this delayed-onset insidious cognitive decline is that the stroke episode triggers the progression of pre-existing brain diseases (e.g. AD and SVD). Other possible mechanisms may include concurrent medical conditions (e.g. diabetes and frailty), or stroke triggering an immune response that leads to on-going inflammation.9,27,28
Figure 1.
Trajectories of different types of vascular dementia. In all figures, the dotted blue line represents cognitive decline with age in the absence of cerebrovascular disease: (A) Following a stroke cognition declines, and then recovers to a variable extent. The extent of the poststroke cognitive decline will depend both on the site and extent of the stroke, as well as brain resilience. Brain resilience depends on pre-existing brain diseases (e.g. AD and SVD), cognitive reserve (e.g. educational level and cognitive activity), and other demographic or medical conditions (e.g. age, diabetes, and frailty); (B) following stroke cognition can continue to decline in the longer term at a rate faster than that prestroke as illustrated by the red line; (C) cerebrovascular disease can result in gradual decline in cognition in the absence of stroke. This pattern is particularly seen with SVD; (D) other insults such as delirium and intercurrent infection (arrowed) can result in a decline in cognition which does not return to the pre-insult level.
Source: © Hugh Markus.
From a management perspective, it is important to determine the contribution of various brain diseases (e.g. vascular, AD, and mixed vascular/AD) in accounting for the cognitive impairment in an individual patient. Clinical guidelines from the American Heart Association/American Stroke Association (AHA/ASA) 1 and the Diagnostic and Statistical Manual of Mental Disorders–Fifth Edition (DSM-V) 29 have been proposed to determine whether the cognitive impairment has a strong vascular component. Both criteria suggest that if (1) the cognitive impairment has a clear temporal relationship with the cerebrovascular event, (2) neuroimaging shows significant relevant CVD lesions, and (3) there is no apparent progressive cognitive decline before or after the cerebrovascular event which may imply underlying neurodegenerative diseases (e.g. AD), then the cognitive impairment probably has a predominant vascular etiology. Yet these criteria have not yet been validated using neuroimaging. In this issue of IJS, Folloso and colleagues performed MRI and amyloid positron emission tomography (PET) imaging in 186 subjects recruited from a memory clinic and found that both criteria can identify cases with predominant vascular pathologies with little or no concomitant amyloid pathology. Rates of amyloid positivity of “probable” vascular MCI (3.8%) and “probable” vascular dementia (15%) were identical between the two criteria and were significantly lower than that of “possible” vascular MCI and “possible” vascular dementia, respectively. 30
Concurrent medical conditions may also affect poststroke outcome. Also in this issue, using the Japan Stroke Databank (n = 56,230), Miwa et al. 31 found that functional outcome following ischemic or hemorrhagic stroke was significantly associated with body mass index (BMI), in that a lower BMI (<18.5 kg/m2) was associated with greater disability and mortality, while a high BMI (>30 kg/m2) similarly associated with a worse outcome after large artery atherosclerosis disease (LAD)-related stroke. Although this study did not specifically look at cognitive outcome, it is possible that poststroke cognitive outcome could be worse among those who are underweight. A previous systematic review suggested that although mid-life obesity is associated with late-life dementia, being underweight was found to be associated with dementia when the BMI was assessed a few years before dementia diagnosis. 32 Low body weight likely reflects a poor underlying physical condition, which could be related to underlying brain diseases (e.g. AD and SVD) at the predementia phase, thereby increasing the risk of poorer functional outcome poststroke. To date, studies on the association between body weight and poststroke cognitive impairment showed conflicting results.33,34 Further study is needed to investigate the association between BMI and poststroke cognitive impairment.
Given that poststroke cognitive impairment depends on multiple factors, researchers have attempted to design a clinical model that can predict its risk.35,36 In this issue of IJS, Ashburner and colleagues present a risk score based on simple clinical and demographic information that are easily available on electronic health records that can identify patients at risk of poststroke cognitive impairment more than 5 years. 37 The C-statistic for predicting poststroke cognitive impairment was 0.731 in the internal validation cohort (n = 1925) and 0.724 in the external validation cohort (n = 2237). This risk score includes the following data: age, type of insurance, mobility problems, prior falls, delirium, peripheral vascular disease, Parkinson’s disease, depression, chronic kidney disease, abnormal weight loss and anorexia, and discharge to facility. Such a risk score could be used to risk stratify and identify individuals at increased risk for poststroke cognitive impairment for preventive efforts.
SVD—the predominant pathology underlying VCI
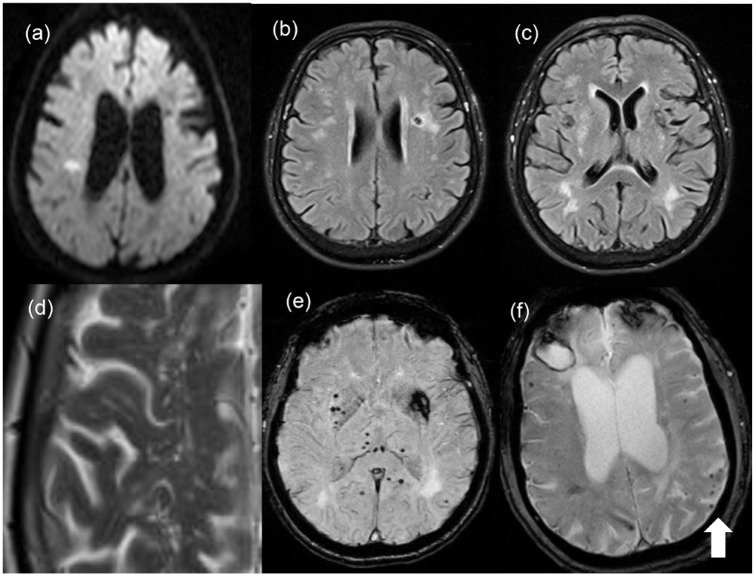
Sporadic non-amyloid cerebral SVD (arteriosclerosis) and sporadic cerebral amyloid angiopathy (CAA) are the two commonest subtypes of SVD and both can induce cognitive impairment even in the absence of causing overt stroke episodes. SVD is considered the most common substrate for VCI. SVD affects the perforating arterioles and venules, as well as capillaries that encompass the neurovascular unit and blood–brain barrier (BBB). Aging, vascular risk factors (e.g. hypertension), increase in pulsatile flow being transmitted to the cerebral small vessels secondary to stiffness of systemic arterial vasculature, 38 and/or genetic factors2,39–41 may lead to various abnormal structural and functional changes in the cerebral small vessels (e.g. luminal narrowing/occlusion, vessel rupture, endothelial dysfunction, BBB leakage, neuroinflammation, and impairment of glymphatic-meningeal lymphatic system), leading to varying severity levels of tissue damage, and secondary neurodegeneration, and eventually stroke and/or cognitive impairment. In general, cognitive impairment is associated with increasing extent or severity of tissue damage, lesions located at strategic regions or disrupting network connectivity, and secondary neurodegeneration. Traditionally on structural MRI, these SVD-related tissue damage may be visualized as recent small subcortical infarcts, WMH, lacunes, CMBs, enlarged perivascular space (PVS), and brain atrophy 42 (Figure 2). Other research-orientated MRI methods can detect more subtle brain white matter alterations on DTI, BBB leakage using contrast-enhanced imaging, and impaired cerebrovascular reactivity.43,44 Sporadic SVD commonly affects small vessels supplying deep subcortical, brainstem, or cerebellar regions. Apart from stroke (lacunar syndromes, deep intracerebral hemorrhage (ICH)) and cognitive impairment, other clinical manifestations of SVD include Parkinsonism and behavioral problems including apathy. 45
Figure 2.
MRI appearances of SVD: Note the right side of the brain is represented on the left-hand side of the images: (A) An right hemisphere acute lacunar infarct visible as high signal on diffusion-weighted imaging; (B) a chronic lacunar infarct on the left visible as an area of low signal on FLAIR MRI; (C) white matter hyperintensities particularly in the posterior periventricular white matter; (D) a close-up of a T2-weighted MRI showing enlarged PVSs; (E) gradient echo MRI from a patient with sporadic hypertensive SVD showing multiple CMB visible as small dots of low intensity. These as predominantly subcortical, in contrast to mainly cortical CMB seen in CAA. There is also a larger area of low density in the left hemisphere representing changes from an old subcortical intracerebral hemorrhage; (F) gradient echo MRI from a patient with CAA showing multiple cortical CMB (arrowed), as well as a right frontal lobar intracerebral hemorrhage. © Hugh Markus.
The severity of SVD-related lacunar stroke is commonly mild and is not always visible on computed tomography (CT) imaging particularly in the acute setting. It is much better seen on MRI but this is not always available making clinical tools for diagnosis useful particularity in less-resourced settings. In this issue, Arba and colleagues validated such a clinical score to identify lacunar stroke in the acute setting based on the presence of a lacunar syndrome and a low National Institute of Health Stroke Scale score (<7). Using data from the WAKE-UP trial (n = 503), the lacunar score had a very good specificity (0.82) and negative predictive value (0.84). However, the sensitivity (0.44) and positive predictive value (0.39) were low. Overall, the score could reliably exclude up to 9 of 10 patients with acute non-lacunar infarct. 46 This simple score may not only help to guide patient selection in clinical trials in hyperacute stroke setting, but it may also help to facilitate research on SVD in low-income regions where MRI is not easily accessible.
Recently, two more MRI SVD lesion types have been more widely recognized and were added to the recent updated neuroimaging standards for SVD research guidelines, 43 namely cortical cerebral microinfarcts (CCMs) 47 and incidental diffusion-weighted imaging (DWI)-positive lesions. 47 In a recent review in the IJS, Huang et al. discuss potential mechanisms by which these minute CCMs may induce cognitive impairment. The authors suggest mechanisms include (1) causing loss of function of neural tissues surrounding the microinfarct core with interruption of cortico-cortical and cortico-subcortical circuits, (2) disruption of brain structural network connectivity, and/or (3) secondary remote cortical neurodegeneration. 47
Sporadic CAA is associated with age and is relatively less associated with vascular risk factors when compared to sporadic SVD. Genetic factors, namely apolipoprotein E (APOE) ε4/ε2, were found to be associated with CAA and may contribute to the deposition of beta-amyloid (Aβ) in cerebral small vessels. CAA affects small vessels supplying cerebral cortex and leptomeninges. Since Aβ weakens the vessel wall, CAA commonly results in recurrent lobar ICH, convex subarachnoid hemorrhage, and cortical superficial siderosis. Other MRI lesions similar to that of sporadic SVD (e.g. WMH, CMB, and enlarged PVS) are also common in CAA, 48 and these can induce cognitive impairment even in the absence of stroke, with a recent study reporting that altered white matter diffusivity on DTI, cerebral atrophy, and altered cerebrovascular reactivity accounted for about half the effect of CAA on cognition. 49 It has been suggested that sex may also influence the clinical presentation of CAA, in that male sex was associated with an earlier age of first ICH and a more hemorrhagic disease course than women possibly because man has high greater global Aβ load than women. 50 In this issue, Koemans and colleagues investigated such a hypothesis using histopathological markers associated with Aβ burden and hemorrhage in two autopsy databases (n = 6139). However, they failed to find any sex difference with respect to vascular Aβ (CAA) and even observed significantly more CMB on ex vivo MRI in women. Yet, they found a higher cortical iron in men. They concluded that previously observed sex differences in hemorrhage onset and progression in CAA patients are likely not due to difference in global CAA severity between men and women. Other factors, such as vascular remodeling, may contribute as unique pattern of vascular remodeling is associated with cortical superficial siderosis. 51
The interaction of vascular risk and CVD with AD
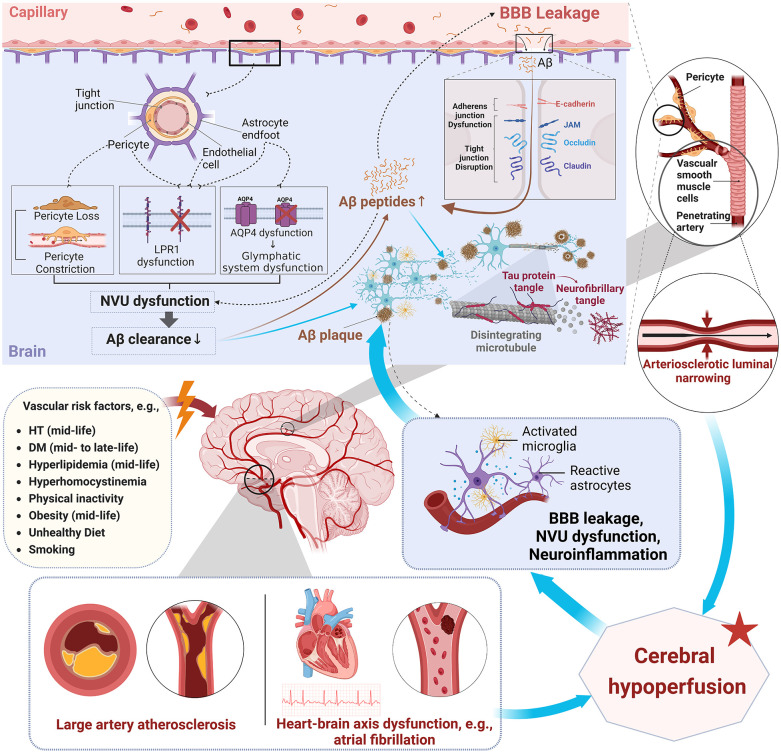
The latest Lancet standing Commission reported that vascular risk factors (e.g. mid-life hypertension, mid-life hyperlipidemia, and mid-life diabetes) are associated not only with all-cause dementia but also with AD. 52 Early pathology studies showed that various CVD lesions (e.g. arteriosclerosis, lacune, white matter changes, and LAD large infarcts) commonly co-occur in the majority of AD patients (~80%) and exert additive or interactive effects in inducing cognitive impairment.53,54 In addition, preclinical and clinical evidence showed that SVD is an early initiating event in the development of Aβ. Longitudinal studies with serial amyloid PET imaging showed that arterial stiffness 55 and WMH on MRI 56 were associated with increased Aβ deposition. A number of mid-life vascular risk factors (e.g. hypertension, diabetes, hyperlipidemia, obesity, and smoking) are associated with greater amyloid deposition in late life, 57 while greater physical activity and lower vascular risk independently attenuated the negative association of Aβ burden with cognitive decline and neurodegeneration in asymptomatic individuals. 58 Recent longitudinal studies showed that vascular risk factors interact with Aβ to promote cognitive decline by accelerating tau accumulation 59 and brain atrophy. 60 The presence of retinal microvasculopathy (e.g. increased retinal arteriolar tortuosity) was found to associate with incident dementia more than 10 years. 61 A recent pathology study showed that higher healthy lifestyle scores (physical activity, smoking status, diet quality, cognitive activity, alcohol consumption) were associated with lower Aβ load and better cognitive functioning proximate to death. 62 It is most likely that BBB integrity, neurovascular unit function, and the glymphatic system are compromised by aging and vascular risk factors, leading to reduced clearance or increased influx of Aβ, triggering the pathological cascades of AD (Figure 3).63–66
Figure 3.
Interplay of vascular dysfunction and neurodegenerative pathways in Aβ and tau pathology. This figure highlights the complex relationship between vascular dysfunction and pathways leading to the accumulation of Aβ and tau protein formation. Modifiable vascular risk factors probably cause dysfunction of the neurovascular unit (NVU), leakage of the BBB, and neuroinflammation. The disruption of tight junctions facilitates the increased entry of Aβ from the bloodstream into the brain. Features of NVU dysfunction, such as pericyte constriction and loss, along with AQP4 mis-localization and decreased levels of LRP1, lead to inefficient Aβ clearance and promote its accumulation in the brain. In addition, activated microglia and reactive astrocytes intensify neuroinflammation, which further exacerbates BBB leakage and NVU dysfunction. All these changes contribute to the formation of Aβ plaques and the hyperphosphorylation of tau proteins, resulting in neurofibrillary tangles. The figure also connects vascular risk factors with changes in vascular smooth muscle cells that lead to arteriosclerotic luminal narrowing. Furthermore, conditions like LAD and heart–brain axis dysfunction, exemplified by atrial fibrillation, also aggravate cerebral hypoperfusion. The cerebral hypoperfusion may exacerbate NVU dysfunction, BBB leakage, and neuroinflammation, further promoting Aβ deposition and tau pathology. Aβ deposition in turn reverberates with neuroimmune and vascular dysfunction, further exacerbating the progression of this process. Aβ: amyloid-beta; BBB: blood–brain barrier; NVU: neurovascular unit; AQP4: aquaporin 4; LRP1: low-density lipoprotein receptor-related protein 1; HT: hypertension; DM: diabetes mellitus.
An early pathology study observed an association between LAD and AD pathologies 67 while clinical studies showed an association between AF and AD. 68 A recent clinical study further showed an association between atrial volume/atrial cardiopathy with increased cerebral Aβ load. 69 A possible explanation for such findings is that LAD and heart–brain dysfunctions may associate with cerebral hypoperfusion, hence affecting BBB integrity and neurovascular unit function, resulting in Aβ/tau accumulation (Figure 2). 70
Management of VCI
Considering its global health importance, there is a paucity of well-designed adequately power clinical trials to guide therapy in poststroke cognitive impairment and vascular dementia. In this section, we review the available data and consider how treatments for acute stroke could reduce poststroke cognitive impairment, long-term preventive therapies for VCI and vascular dementia, and symptomatic therapies for those already suffering from VCI and vascular dementia, and finally management of associated behavioral symptoms such as apathy and poststroke emotionalism.
Acute stroke treatments
Acute ischemic stroke and reperfusion therapy
Given stroke commonly induces acute onset cognitive impairment, any measures (e.g. intravenous thrombolytic (IVT) and endovascular thrombectomy (EVT)) that can mitigate brain damage during the acute stage should reduce poststroke cognitive impairment. The Virtual International Stroke Trial (n = 6268) suggested that cognitive outcome was possibly better in thrombolysed compared with non-thrombolysed patients with ischemic stroke. 71 Secondary analysis of the REVASCAT (n = 206) showed that Solitaire thrombectomy was associated with better cognitive performance than the best medical treatment at 3 months and 1 year after stroke. 72 The most recent secondary analysis of the ESCAPE trial (n = 315) showed that the odds of favorable cognitive outcome was higher with EVT than with control. 73
In this issue of IJS, Gallucci et al. 74 investigated the prevalence of early poststroke cognitive impairment among survivors with first-ever anterior circulation ischemic stroke without prestroke cognitive decline, the majority of whom had received acute reperfusion therapy. Although they found that the rate of cognitive impairment remained high (69.3%), they noted that when compared to previous studies, the prevalence of aphasia and neglect in this study (18%) was much lower than that of previous studies (15–63%). They found that risk factors for reduced poststroke cognitive impairment were lesser stroke severity, right hemispheric lesions, higher education, and the absence of hyperlipidemia. Moreover, poststroke cognitive impairment was associated with poor functional outcome at 3 months poststroke. In another observational study, using the Ontario Stroke Registry, the authors found that among patients with first-ever ischemic stroke who had not been previously diagnosed with dementia, thrombolysis was associated with a 24% reduced rate of dementia. 75
A common clinical question is whether to perform reperfusion therapy in patients with pre-existing cognitive impairment and dementia. It has been suggested that they may benefit less, but also that the pathology causing dementia might increase the risk of bleeding after thrombolysis, particularly in patients with CAA. These patients were often excluded from clinical trials meaning there are less data to guide us. In this issue of IJS, Fouzi and colleagues examined this question by performing a meta-analysis of five observational studies of IVT use in patients with (n = 1078) and without prestroke dementia (n = 2805). 76 They found no significant differences in favorable outcome, mortality, ICH, and symptomatic ICH for patients undergoing IVT with prestroke dementia versus those without. One EVT study (n = 615 with dementia vs n = 9600 without) also found no significant differences in outcomes apart from an increased odds of ICH for those with pre-existing dementia. Overall, they concluded that there are no substantial safety issues in the use of IVT or EVT for patients with pre-existing dementia or cognitive impairment. 76
Acute ICH treatment
Recent randomized-controlled trials (RCTs) showed positive effects in certain acute surgical and medical management for ICH (e.g. intensive care bundle with blood pressure reduction, early minimal invasive hematoma evacuation, and the use of andexanet alfa in factor Xa inhibitors associated ICH), resulting in better functional outcome, reduced mortality, and reduced hematoma volume. 77 Since poststroke cognitive impairment correlates with size of hematoma, it is likely that these treatments will result in less cognitive impairment post-ICH. To date, cognition was not assessed in those RCTs, and cognitive endpoints should be added to further trials of acute ICH treatments.
Long-term preventive treatments
Given the strong association between vascular risk factors and cognitive impairment, 52 measures that can improve control of vascular risk factors should prevent cognitive impairment that is associated with stroke, SVD, and/or AD. Here, we provide an update on the effects of several established pharmacological treatments (anti-hypertensive agents, lipid-lowering agents, anti-diabetic agents, anti-thrombotic agents) in the primary/secondary prevention of cardiovascular disease/stroke upon incident dementia or cognitive decline with reference to recent RCTs or meta-analysis of RCTs among at-risk subjects without dementia (if such data are available). Moreover, since vascular-related (e.g. physical activity and diet) and other non-vascular-related lifestyle factors (e.g. cognitive/social activities) may affect manifestation of VCI, recent RCTs have investigated effects of multidomain lifestyle intervention upon cognitive impairment. These recent RCTs will be discussed here. The effects of individual domain lifestyle intervention upon cognition are beyond the scope of this review.
Anti-hypertensive agents
Meta-analysis of recent RCTs (e.g. SPRINT-MIND) showed that blood pressure lowering with anti-hypertensives was associated with approximately 10% reduction in incident dementia/cognitive decline and the effect was greater with trials achieving a greater systolic blood pressure difference between randomized arms (e.g. ⩾ 10 mmHg).78–82 The SPRINT-MIND in patients with hypertension but without stroke reported that reducing blood pressure to 120 mmHg systolic was associated with a reduction in both incident MCI and a combined MCI plus dementia endpoint; it was also associated with reduced WMH progression on MRI suggesting the benefit is at least partially mediated by a reduction in SVD progression. 81 The PROGRESS study showed that blood pressure lowering was associated with a reduced risk of incident dementia and cognitive decline. 83 Pooled analysis including PROGRESS, ProFESS, SCOPE, ACCORD MIND, PreDIVA, and SPRINT-MIND confirmed that anti-hypertensive agents were associated with reduced WMH progression. 84
With respect to the class of anti-hypertensive agents, a recent meta-analysis involving 649,790 participants found that the use of angiotensin II receptor blockers (ARBs) or calcium channel blockers (CCBs) was associated with reduced risk of dementia. 85 In another study among those with MCI and hypertension, 1-year treatment with candesartan (ARB) had superior cognitive outcomes compared with lisinopril (angiotensin-converting enzyme inhibitor) and such effects were achieved independent of the blood pressure-lowering effect of candesartan. 86 In a recent study in the IJS, Henley and colleagues investigated the impact of modulating the renin–angiotensin system upon cerebrovascular reactivity in individuals with MCI based on findings from two RCTs (n = 102) that compared candesartan with lisinopril or placebo. 87 They found that candesartan improved cerebrovascular reactivity and these findings were independent of its blood pressure effect. In contrast, another RCT showed that treatment with losartan (ARB), amlodipine (CCB), or atenolol did not differ in their effects on cerebrovascular reactivity in sporadic SVD. 88 A meta-analysis of RCTs (n = 12,849) comparing BBB-crossing renin–angiotensin drugs with non-crossing drugs found that older adults taking BBB-crossing drugs (e.g. candesartan and lisinopril) exhibited better memory recall over up to 3 years of follow-up, relative to those taking BBB non-penetrant medications (e.g. losartan and enalapril). 89 Taken together, these data suggest CCB and ARB are suitable agents to use in stroke patients to help reduce dementia risk, but further studies are required to determine which anti-hypertensive regimens are most effective.
Anti-diabetic agents
Although earlier studies showed no effects of intensive glucose-lowering upon risk of dementia/cognitive decline in diabetic patients and hypoglycemic episodes during treatment were associated with increased risk of dementia,90,91 meta-analysis of recent RCTs suggested that anti-diabetic agents were associated with less cognitive decline in diabetic patients. 92 Observational studies suggested that biguanide (metformin), thiazolidinedione (e.g. rosiglitazone and pioglitazone), glucagon-like peptide-1 receptor agonists (GLP-1RA) (e.g. liragludie, exenatide, and dulaglutide), and dipeptidyl peptidase-4 (DPP-4) (e.g. sitagliptin and linagliptin) were associated with less dementia.93–96 The CARMELINA study, however, showed that linagliptin (DPP-4) did not modulate cognitive decline more than 2.5 years, 97 while the REWIND study showed that the use of dulaglutide (GLP-1RA) was associated with a 14% reduction in the risk of cognitive decline over a median follow-up of 5.4 years. 98 Smaller clinical trials using liraglutide (GLP-1RA) also yielded positive effects on cognitive outcomes among diabetic patients.99,100 In this issue of IJS, Adamou and colleagues conducted a systematic review and meta-analysis of cardiovascular outcome RCTs comparing GLP-1RA versus placebo in patients with or without diabetes. Among a total of 82,140 subjects of 11 RCTs, they found that GLP1-RAs demonstrated a 16% relative reduction in stroke compared with placebo. 101 Such finding supports that GLP-1RA prevents CVD even in those without diabetes, which may explain at least partially the potential cognitive benefits of GLP-1RA. In another post hoc analysis of two RCTs among diabetic patients, semaglutide (GLP-1RA) associated with reduced incidence of any first stroke, primarily driven by prevention of small-vessel occlusion. 102 Findings of a phase II RCT presented at the Alzheimer’s Association International Conference 2024 (July 28 to August 1) showed that the use of liraglutide (GLP-1RA) for 1 year was able to slow both brain atrophy and cognitive decline when compared to placebo among patients with early AD. Overall, both preclinical and clinical studies suggested that GLP1-1RA may exhibit beneficial impact upon the brain and cognition beyond its glucose-lowering effects.103,104 To date, there are limited data based on RCTs evaluating the effects of anti-diabetic agents upon SVD progression. The ACCORD MIND showed that a combination of intensive treatment of diabetes, blood pressure, and lipids among diabetic patients was associated increased WMH volume at 40 months and reduced brain atrophy. However, such differences were lost at 80 months.105,106 There was no difference in cognitive performance between both treatment arms at any time point. Future study is needed to confirm the effects of GLP-1RA upon the brain and cognition in at-risk individuals (e.g. early SVD/AD, mixed SVD/AD, and poststroke) among those with or without diabetes, although results to date suggest this is a very promising area.
Lipid-lowering agents
Despite early concerns regarding the use of statins and risk of cognitive impairment, 107 meta-analysis of observational studies has shown that the long-term use of statins is associated with a reduced risk of dementia. 108 A recent observational study among patients with heart failure showed that the use of statins was associated with a lower risk of all-cause dementia and its subtypes (AD, vascular dementia, and unspecified dementia). 109 However, the majority of RCTs evaluating cognitive outcomes after statin therapy failed to show a cognitive benefit with exception of one study. 110 Similar concerns were also raised with respect to the association between the use of proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors and cognitive impairment. 111 Subsequent large-scale RCTs showed that evolocumab (PCSK9) when used with statins was not associated with cognitive decline.112,113 A recent meta-analysis of RCTs (n = 128,691) involving contemporary lipid-lowering agents (statins, PCSK9 inhibitors, and ezetimibe) found that these agents were not associated with cognitive impairment, and that a low low-density lipoprotein cholesterol level did not influence the incidence of cognitive disorder or global cognitive performance. 114 In another meta-analysis involving both observational studies and an RCT among stroke patients, poststroke statin use was associated with decreased risk of cognitive impairment, with a larger potential effect of higher dose and longer duration of statin use on prevention of dementia or cognitive impairment poststroke. 115 Therefore, overall, compelling evidence has shown that lipid-lowering agents do not harm cognition. 116 Meta-analysis including telmisartan/rosuvastatin study, ROCAS, and PROSPER showed that statin use was associated with less WMH progression and incident lacunes. 117 Whether lipid-lowering agents may prevent incident dementia or cognitive decline in certain at-risk population (e.g. poststroke, early SVD or AD, LAD, and heart failure) requires further investigation.
Anti-thrombotics
Aspirin is an established treatment for secondary prevention of cardiovascular disease or non-cardioembolic ischemic stroke. It has an anti-inflammatory effect that may potentially benefit SVD or AD given both of these diseases are associated with neuroinflammation. Its efficacy and safety as a primary preventive agent for those without cardiovascular disease or stroke were investigated by the ASPREE trial, which showed that the use of low-dose aspirin (100 mg daily) over a median period of 4.7 years was not associated with reduced incident cardiovascular disease nor dementia among elderly (>70 years) who did not have cardiovascular disease/stroke, dementia, or disability at baseline. Moreover, it was associated with higher risk of major hemorrhage. 118 In a more detailed subgroup analysis of the ASPREE, aspirin was not associated with risk of overall dementia nor with probable AD, MCI, or cognitive decline. 119 The JPAD trial also showed that low-dose aspirin was not associated with reduced dementia among diabetic patients over a period of 7 years. 120 Similarly, the ASCEND trial showed that the use of low-dose aspirin over a period of 7.4 years was not associated with reduced dementia among diabetic patients without history of cardiovascular disease. 121 A recent meta-analysis on five RCTs (n = 46,804) showed that aspirin was not associated with reduced dementia, MCI, or cognitive decline. 122 Therefore despite its established benefit in secondary prevention in cardiovascular disease and non-cardioembolic ischemic stroke, data based on RCTs have failed to show any cognitive benefit of aspirin in those without cardiovascular diseases or stroke.
Cilostazol is a phosphodiesterase (PDE)-3 inhibitor with anti-platelet properties that may also have endothelial stabilizing properties. Among subjects with subclinical SVD (confluent WMH on MRI), cilostazol was not associated with reduced progression of WMH or of cognitive decline when compared to placebo. 123 In another RCT among subjects with mildly symptomatic SVD (confluent SVD and at least one lacune on MRI) with or without history of stroke, although cilostazol was associated with reduced incident stroke, it was not associated with reduced WMH progression when compared to aspirin. 124 In the recent LACI-2 trial with a 2 × 2 factorial design, cilostazol alone did not reduce cognitive decline. 125 However, the isosorbide mononitrate (ISMN)-cilostazol combination was associated with reduced composite of adverse vascular, dependence, and cognitive outcomes in patients with clinical lacunar ischemic stroke. 125 ISMN on its own was associated with less cognitive decline. ISMN is a nitric oxide (NO) donor which augments the NO-cyclic guanosine monophosphate (cGMP) PDE5-inhibitor pathway and may improve endothelial function. The phase 3 LACI-3 trial to evaluate the effectiveness of this drug combination is on-going.
RCTs completed more than a decade ago showed that long-term dual anti-platelet therapies when compared to single anti-platelet therapy (SPS3: aspirin and clopidogrel vs aspirin; PRoFESS: aspirin plus extended-release dipyridamole vs clopidogrel) were not associated with better cognitive outcomes.126,127 Moreover, hemorrhagic complications were higher with long-term dual anti-platelet agents. In contrast, among AF patients, the use of oral anti-coagulants was found not only to be associated with lower risk of stroke but also with dementia,128,129 possibly through the prevention of subclinical infarcts or microinfarcts. A recent meta-analysis of observational studies showed that direct oral anti-coagulants (DOAC) were associated with lower risk of dementia (hazard ratio = 0.88) compared with warfarin, particularly in Asian patients and among patients younger than 75 years. 130 RCTs are needed to confirm the superior effects of DOAC over warfarin upon reducing incident dementia/cognitive decline and to compare the efficacy of various DOACs.
Polypill (anti-hypertensive agents and statins)
In TIPS-3 trial in which older subjects (>65 years) with vascular risk factors were recruited, a polypill (anti-hypertensive agents and statins) with or without aspirin was not associated with reduced cognitive outcomes as measured by neuropsychological tests, but was associated with reduced functional decline over a mean follow-up period of 5 years. 131 The reduced functional decline might possibly be partially accounted for by less cognitive decline in the polypill group, which was not be captured by the neuropsychological tests used in the study. Note further that the blood pressure was lowered by only a mean of 5.7 mmHg during the study period, which might not be sufficient to induce a benefit upon cognition.
Multidomain lifestyle intervention
The FINGER study showed that a multidomain lifestyle (vascular risk monitoring, Mediterranean-like diet, physical exercise, cognitive training) intervention (Table 1) could improve or maintain cognitive functioning in at-risk elderly people without dementia. 132 Meta-analysis suggested that multidomain (e.g. physical exercise plus cognitive training) was associated with more improvement in cognitive function when compared to single-domain intervention.133,134 The SMARRT study recruited elderly subjects with two or more of the eight AD-modifiable risk factors that were not well controlled and randomized them into a personalized multidomain risk-reduction intervention or to general education. The study showed that after 2 years, the personalized multidomain risk-reduction intervention led to modest improvements in cognition, dementia risk factors, and quality of life. 24 A few other similar studies also showed positive effects of multidomain intervention upon cognition.16,19 However, the AgeWell.de study and J-MINT study failed to show that a multidomain intervention was effective in improving cognitive performance among at-risk elderly individuals more than 2 years and 18 months, respectively.20,23 Subgroup analyses from the J-MINT study suggested that interventions appeared to be particularly effective for individuals with high attendance during exercise sessions, and those with APOE ε4 polymorphism and elevated plasma glial fibrillary acidic protein (GFAP) levels. The J-MIND-Diabetes study also failed to show that a multidomain intervention could prevent cognitive decline among diabetic patients with MCI. 135 A meta-analysis including RCTs of longer duration showed that multidomain intervention could induce only slight improvement in cognitive function but was not able to reduce incident dementia. 136 Another meta-analysis including only two RCTs among stroke patients showed that multidomain intervention improved processing speed and attention. 137 To date, available evidence based on subgroup analysis suggested that subjects with following features may benefit more from multidomain interventions: 138 low education, 139 poor control in modifiable risk factors,132,140 subtle or subjective cognitive decline,132,141 minimal brain atrophy, 142 ApoE ε4 carriers,23,143,144 positive AD biomarkers (e.g. Aβ, high GFAP),23,141 and good adherence to the lifestyle intervention. 23 A more intensive regime may also be more effective. 20 Future study is needed to investigate the profile of at-risk elderly individuals who may benefit most from multidomain intervention. Moreover, a reason explaining the negative studies is that the evolution from subclinical to clinical stages of SVD/AD commonly spans over years to decade(s). Therefore, it is likely that a large study of long duration is required to show a significant effect of intervention upon incident dementia. Epidemiological studies that used similar methods over sequential periods showed that dementia incidence has indeed decreased at the population level in several Western countries, 145 which could be at least partially explained by better control of vascular risk factors, reduced burden of both cerebral SVD Aβ, and better education when compared to that of previous cohorts. 145
Table 1.
Recent multidomain intervention RTCs on cognitive decline.
| Trial name/ID number | Region | Population/sample size | Intervention | Intervention given outcomes |
|---|---|---|---|---|
| TIGER, NCT03528005 15 | Taiwan, China | Community-dwelling outpatients aged 65 years or older with at least three chronic medical conditions/398 total (199 per group) | Integrated multidomain intervention (16 2-h sessions per year of exercise, cognitive training, nutrition, and disease education, plus individualized treatment) vs usual care for 12 months | Intervention group had significantly higher mean SF-36 physical component scores overall (difference = 0.8; 95% CI = 0.2–1.5; p = 0.010). SF-36 mental component scores were significantly higher in intervention group at 12 months (57.2 vs 55.3; p = 0.019) |
| SURPERBRAIN, NCT03980392 16 | Korea | Adults aged 60–79 years without dementia and with one or more modifiable dementia risk factors/152 total (51 FMI, 51 HMI, and 50 control) | Facility-based multidomain intervention (FMI) vs home-based multidomain intervention (HMI) vs general health advice control for 6 months | Retention rates were 88.2% (FMI) and 96.1% (HMI). Adherence was 94.5% (FMI) and 96.8% (HMI). RBANS total scale index score improved significantly in FMI (5.46 ± 7.50, p = 0.004) and HMI (5.50 ± 8.14, p = 0.004) compared with control (−0.74 ± 11.51) |
| Look AHEAD-MIND, NCT00017953 17 | The United States | Individuals with type 2 diabetes and overweight or obesity/3938 total (1984 intervention) | Intensive lifestyle intervention vs diabetes support and education for 10 years | No overall differences in cognitive decline rates between groups. Subgroup with baseline cardiovascular disease in the intervention arm performed worse on Stroop test |
| MEDEX, NCT02665481 18 | The United States | Older adults aged 65–84 with subjective cognitive concerns/585 total | Four groups: Mindfulness-based stress reduction (MBSR n = 150), exercise (n = 138), combined MBSR and exercise (n = 144), or health education control (n = 153) for 18 months | No significant effect of MBSR or exercise on episodic memory or executive function at 6 or 18 months. No significant effects at 18 months. No significant improvements in secondary outcomes (hippocampal volume, dorsolateral prefrontal cortex measures, functional cognitive capacity, self-reported cognitive concerns) |
| COMBAT, ChiCTR190002548. 19 | China | 209 community-dwelling older adults aged 60 years or older, with ⩾2 risk factors of cognitive decline/209 total (99 intervention), 192 completed | Multidomain intervention (meditation, cognitive training, exercise, nutrition counseling) vs usual care for 9 months, with 1-year follow-up | Intervention group showed significant enhancement in cognitive performance immediately after intervention (between-group difference in Z-score = 0.20, 95% CI = 0.053–0.35; Hedges’ g = 0.40, 95% CI = 0.29–0.50). Benefit not significant at 1-year follow-up |
| AgeWell.de, DRKS00013555 20 | Germany | 60 to 77 years and CAIDE risk score 14 of ⩾9 points/1030 participants at baseline (487 intervention), 819 completed | Multidomain intervention (nutrition, medication optimization, physical, social, and cognitive activity) vs general health advice for 2 years | No significant differences (average marginal effect = 0.010, 15.7% difference, p = 0.874) in global cognitive performance between groups. Intervention improved health-related quality of life and reduced depressive symptoms in women. |
| THISCE, NCT03056768 21 | Taiwan, China | Community-dwelling older adults/1054 community-dwelling older adults (529 intervention) | Multidomain intervention (exercise, cognitive training, nutritional counseling, chronic condition management) vs control for 1 year | Intervention significantly prevented cognitive declines and physical frailty, especially in those with ⩾3 impaired intrinsic capacity subdomains (MoCA: coefficient = 1.909, 95% CI = 0.736–3.083; CHS frailty scores: coefficient = −0.405, 95% CI = −0.715 to −0.095). Significant improvements in MoCA scores for those with poorer baseline cognition and vitality |
| GOIZ-ZAINDU, NCT06163716 22 | Spain | CU older adults, CAIDE risk score ⩾6/125 participants (61 intervention) | Multidomain intervention (MD-Int, including cardiovascular risk control, nutrition, physical activity, cognitive training) vs regular health advice (RHA) for 1 year | Higher proportion of participants declined in NTB executive function (64% RHA vs 40% MD-Int) and processing speed scores (61% RHA vs 39% MD-Int) |
| J-MINT, UMIN000038671 23 | Japan | Adults aged 65–85 years subjects with MCI/531 participants (265 in intervention), 406 completed the trial | Multidomain intervention (vascular risk management, exercise, nutrition, cognitive training) vs control for 18 months | No significant difference in preventing cognitive decline (between-group difference in composite score changes = 0.047, 95% CI = −0.029 to 0.124). Positive impacts on secondary health outcomes. More effective for those with high attendance in exercise sessions and those with APOE ε4 allele and elevated plasma GFAP levels |
| SMARRT, NCT03683394 24 | The United States | Adults aged 70–89 years with ⩾2 of 8 targeted dementia risk factors/172 total (68/82 in intervention and 81/90 in control completed) | Personalized risk-reduction goals with health coaching and nurse visits vs health education control for 2 years | Intervention group showed larger improvements in composite cognitive score (ATE of SD = 0.14; 95% CI = 0.03–0.25; p = 0.02), better composite risk factor score (ATE of SD = 0.11; 95% CI = 0.01–0.20; p = 0.03), and improved quality of life (ATE = 0.81 points; 95% CI = −0.21 to 1.84; p = 0.12) |
SF-36: 36-Item Short-Form Health Survey; CI: confidence interval; FMI: facility-based multidomain intervention; HMI: home-based multidomain intervention; RBANS: Repeatable Battery for the Assessment of Neuropsychological Status; SPPB: Short Physical Performance Battery; HR: hazard ratio; MBSR: mindfulness-based stress reduction; CAIDE: cardiovascular risk factors, aging and dementia; MoCA: Montreal Cognitive Assessment; CHS: Cardiovascular Health Study; NTB: neuropsychological test battery; MD-Int: multidomain intervention; RHA: regular health advice; MCI: mild cognitive impairment; ATE: average treatment effect; SD: standard deviation; CU: cognitively unimpaired.
Others
Minocycline is a tetracycline antibiotic with anti-neuroinflammatory and BBB-stabilizing properties based on preclinical studies.146–148 However, a phase II RCT showed that minocycline was not effective in reducing neuroinflammation or BBB permeability in patients with SVD as measured by 11 C-PK11195 PET and dynamic contrast-enhanced MRI, respectively. 149 DL-3-n-butylphthalide (NBP) is a synthetic compound with multiple pathological pathways related to ischemic stroke (e.g. oxidative stress, neuroplasticity, neuronal apoptosis, and autophagy). Its use at the acute stage of ischemic stroke in conjunction with reperfusion therapy was associated with improved outcomes. 150 It was approved by China Food and Drug Administration for use in stroke. An RCT more than 24 months showed that the use of NBP was associated with improved cognitive and daily function in patients with MCI associated with SVD. 151 A meta-analysis of 26 RCTs showed that it improved cognitive outcomes in poststroke cognitive impairment. 152 Future studies should investigate its effects upon slowing down progression of SVD, AD, and cognitive decline in the long term.
Limitations of current data
Much of the data discussed above is from studies examining the effect of acute stroke interventions and longer-term prevention measures to prevent the onset of cognitive decline and dementia. There are much less trial data available in patients with established poststroke dementia. 153 Even when looking at prevention trials, much of the data is from small underpowered studies with risk of bias, and larger, well-powered RCTs looking at prevention of dementia in patients with stroke are needed. 153 Studies such as SPRINT-MIND have demonstrated the benefit of adding cognitive outcomes to large RCTs with other cardiovascular endpoints, and this approach needs to be adopted widely in stroke trials.
Symptomatic treatment in poststroke dementia and VCI
The treatments discussed above may reduce cognitive impairment by preventing brain damage, but are their effect treatment to improve cognition in those with established VCI and vascular dementia? Both cholinesterase inhibitors and memantine are used as symptomatic treatments in AD, and it has been suggested they may have benefit in VCI perhaps due to denervation resulting in loss of cholinergic innervation. Their benefit was rigorously examined in the recent European Stroke Organisation Guidelines which concluded that “there is no robust data that pharmacological interventions including cholinesterase inhibitors and memantine improved symptoms or delayed progression to dementia.” 153 Almost all data were from trials of dementia rather than poststroke cognitive impairment and interpretation of these trials is complex, in particular because many cases labeled as vascular dementia may in fact have mixed dementia which is the predominant dementia type in the elderly. Indeed, there has been debate as to whether any benefit reported with cholinesterase inhibitors in vascular dementia trials is due to a true effect on vascular dementia, or an effect on concurrent Alzheimer’s pathology. To address this question, the efficacy of donepezil was examined in a RCT in a model of pure vascular dementia, CADASIL. Although there was a significant effect on the secondary endpoint of executive dysfunction, there was no improvement in the primary cognitive endpoint or in activities of daily living. 154 Therefore, currently, there is no clinical indication for cholinesterase inhibitors and memantine in poststroke cognitive impairment or vascular dementia, although they can be used in cases of suspected mixed dementia with co-existent AD.
Some studies have examined non-pharmacological interventions including acupuncture, transcranial magnetic stimulation, and cognitive interventions but these have all been small and have not proven benefit of any specific interventions. 155
Management of cognitive symptoms associated with dementia
VCI is often associated with other symptoms including depression, apathy, and emotionalism. It is important to look for depression which may respond well to treatment with anti-depressants and cognitive interventions. While apathy may co-exist with depression, the two are quite distinct, and apathy may occur without depression. Apathy is a reduction in goal-directed activity in the cognitive, behavioral, emotional, or social domains of a patient’s life and occurs in one of three patients after stroke. 45 It can be differentiated from depression by its manifestation as a reduction in initiative, while depression manifests as negative emotionality. It is particularly common in SVD, and recent data suggest it results from damage to brain networks from white matter tract disruption, by a similar mechanism to that which can cause VCI. 156 No drug treatments have been proven to improve apathy after stroke, and a recent secondary analysis of EFFECTS trial data showed no benefit of fluoxetine in preventing development of apathy after stroke. 157 Current management is limited to psychological support to help the patient, and particularly the carer, adapt to this disabling symptom.
A related symptom is poststroke emotionalism which is also common, occurring in one in five stroke patients and in those with VCI, particularly due to SVD. This topic is covered by an excellent review by Broomfield and colleagues in this issue of IJS. They describe how TEARS-Q (Testing for Emotionalism after Recent Stroke–Questionnaire) has recently been developed to allow standardized assessment. Treatment options are limited, and there have been few adequately powered treatment trials. Anti-depressants may reduce severity, but more trial data are required. There have been no RCTs of non-pharmacological interventions. 158
Conclusion
Worldwide, around 50 million people live with dementia, and this number is projected to triple by 2050, rising particularly in LMIC where around two-thirds of people with dementia live. 3 Vascular factors contribute to many of these cases. It has been estimated 20% of all dementia cases have a predominant vascular pathology—so 10 million worldwide—while perhaps another 20% of vascular diseases may contribute to a mixed dementia picture. Therefore, the vascular contribution to dementia affects 20 million people currently and will increase markedly in the next few decades particularly in LMICs. Despite this enormous health and economic impact, it has been a neglected research area, with limited adequately powered trials of therapies, resulting in few proven treatments. Current management of VCI emphasizes prevention and treatment of stroke and vascular risk factors, with intensive hypertension control being the most robust treatment in preventing cognitive and radiological decline due to SVD and possibly preventing development of AD. Early IVT/EVT in acute stroke may also attenuate the risk of VCI. Novel treatment strategies that target cellular and molecular pathophysiology of cSVD are actively being evaluated and will hopefully lead to new disease course-modifying therapies. Meanwhile, we need an increased research focus and funding for this important area.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: H.S.M.’s research receives infrastructural support from the Cambridge British Heart Foundation Centre of Research Excellence (RE/24/130011) and the Cambridge University Hospitals NIHR Biomedical Research Centre (NIHR203312). The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or UK Department of Health and Social Care.
References
- 1. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Markus HS, de Leeuw FE. Cerebral small vessel disease: recent advances and future directions. Int J Stroke 2023; 18: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patterson C. World Alzheimer report 2018, 2018, https://www.alzint.org/resource/world-alzheimer-report-2018/
- 4. Rost NS, Brodtmann A, Pase MP, et al. Post-stroke cognitive impairment and dementia. Circ Res 2022; 130: 1252–1271. [DOI] [PubMed] [Google Scholar]
- 5. Sexton E, McLoughlin A, Williams DJ, et al. Systematic review and meta-analysis of the prevalence of cognitive impairment no dementia in the first year post-stroke. Eur Stroke J 2019; 4: 160–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Craig L, Hoo ZL, Yan TZ, et al. Prevalence of dementia in ischaemic or mixed stroke populations: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2022; 93: 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weaver NA, Kuijf HJ, Aben HP, et al. Strategic infarct locations for post-stroke cognitive impairment: a pooled analysis of individual patient data from 12 acute ischaemic stroke cohorts. Lancet Neurol 2021; 20: 448–459. [DOI] [PubMed] [Google Scholar]
- 8. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 9. Mok VC, Lam BY, Wong A, Ko H, Markus HS, Wong LK. Early—onset and delayed—onset poststroke dementia—revisiting the mechanisms. Nat Rev Neurol 2017; 13: 148–159. [DOI] [PubMed] [Google Scholar]
- 10. Ball EL, Shah M, Ross E, et al. Predictors of post-stroke cognitive impairment using acute structural MRI neuroimaging: a systematic review and meta-analysis. Int J Stroke 2023; 18: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coenen M, de Kort FA, Weaver NA, et al. Strategic white matter hyperintensity locations associated with post-stroke cognitive impairment: a multicenter study in 1568 stroke patients. Int J Stroke 2024; 19: 916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawrence AJ, Patel B, Morris RG, et al. Mechanisms of cognitive impairment in cerebral small vessel disease: multimodal MRI results from the St George’s cognition and neuroimaging in stroke (SCANS) study. PLoS ONE 2013; 8: e61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lawrence AJ, Chung AW, Morris RG, et al. Structural network efficiency is associated with cognitive impairment in small-vessel disease. Neurology 2014; 83: 304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tuladhar AM, Tay J, van Leijsen E, et al. Structural network changes in cerebral small vessel disease. J Neurol Neurosurg Psychiatry 2020; 91: 196–203. [DOI] [PubMed] [Google Scholar]
- 15. Lee WJ, Peng LN, Lin CH, et al. Effects of incorporating multidomain interventions into integrated primary care on quality of life: a randomised controlled trial. Lancet Healthy Longev 2021; 2: e712–e723. [DOI] [PubMed] [Google Scholar]
- 16. Moon SY, Hong CH, Jeong JH, et al. Facility-based and home-based multidomain interventions including cognitive training, exercise, diet, vascular risk management, and motivation for older adults: a randomized controlled feasibility trial. Aging (Albany NY) 2021; 13: 15898–15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayden KM, Neiberg RH, Evans JK, et al. Legacy of a 10-year multidomain lifestyle intervention on the cognitive trajectories of individuals with overweight/obesity and type 2 diabetes mellitus. Dement Geriatr Cogn Disord 2021; 50: 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lenze EJ, Voegtle M, Miller JP, et al. Effects of mindfulness training and exercise on cognitive function in older adults: a randomized clinical trial. JAMA 2022; 328: 2218–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu X, Ma Z, Zhu X, et al. Cognitive benefit of a multidomain intervention for older adults at risk of cognitive decline: a cluster-randomized controlled trial. Am J Geriatr Psychiatry 2023; 31: 197–209. [DOI] [PubMed] [Google Scholar]
- 20. Zülke AE, Pabst A, Luppa M, et al. A multidomain intervention against cognitive decline in an at-risk-population in Germany: results from the cluster-randomized AgeWell.de trial. Alzheimers Dement 2024; 20: 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang CK, Lee WJ, Chou MY, et al. Roles of baseline intrinsic capacity and its subdomains on the overall efficacy of multidomain intervention in promoting healthy aging among community-dwelling older adults: analysis from a nationwide cluster-randomized controlled trial. J Prev Alzheimers Dis 2024; 11: 356–365. [DOI] [PubMed] [Google Scholar]
- 22. Tainta M, Ecay-Torres M, de Arriba M, et al. GOIZ ZAINDU study: a FINGER-like multidomain lifestyle intervention feasibility randomized trial to prevent dementia in Southern Europe. Alzheimers Res Ther 2024; 16: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sakurai T, Sugimoto T, Akatsu H, et al. Japan-Multimodal Intervention Trial for the Prevention of Dementia: a randomized controlled trial. Alzheimers Dement 2024; 20: 3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yaffe K, Vittinghoff E, Dublin S, et al. Effect of personalized risk-reduction strategies on cognition and dementia risk profile among older adults: the SMARRT randomized clinical trial. JAMA Intern Med 2024; 184: 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015; 314: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mok VCT, Lam BYK, Wang Z, et al. Delayed-onset dementia after stroke or transient ischemic attack. Alzheimers Dement 2016; 12: 1167–1176. [DOI] [PubMed] [Google Scholar]
- 27. Sandvig HV, Aam S, Alme KN, et al. Plasma inflammatory biomarkers are associated with poststroke cognitive impairment: the Nor-COAST study. Stroke 2023; 54: 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tack RWP, Amboni C, van Nuijs D, et al. Inflammation, anti-inflammatory interventions, and post-stroke cognitive impairment: a systematic review and meta-analysis of human and animal studies. Transl Stroke Res. Epub ahead of print 28 November 2023. DOI: 10.1007/s12975-023-01218-5 [DOI] [PubMed] [Google Scholar]
- 29. American Psychiatric Association and American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 30. Folloso MC, Villaraza SG, Yi-Wen L, et al. The AHA/ASA and DSM-V diagnostic criteria for vascular cognitive impairment identify cases with predominant vascular pathology. Int J Stroke 2024; 19: 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miwa K, Nakai M, Yoshimura S, et al. Clinical impact of body mass index on outcomes of ischemic and hemorrhagic strokes. Int J Stroke 2024; 19: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kivimäki M, Luukkonen R, Batty GD, et al. Body mass index and risk of dementia: analysis of individual-level data from 1.3 million individuals. Alzheimers Dement 2018; 14: 601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Q, Liao X, Pan Y, Xiang X, Zhang Y. The obesity paradox: effect of body mass index and waist circumference on post-stroke cognitive impairment. Diabetes Metab Syndr Obes 2023; 16: 2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee M, Oh MS, Jung S, et al. Differential effects of body mass index on domain-specific cognitive outcomes after stroke. Sci Rep 2021; 11: 14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dong Y, Ding M, Cui M, et al. Development and validation of a clinical model (DREAM-LDL) for post-stroke cognitive impairment at 6 months. Aging (Albany NY) 2021; 13: 21628–21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chander RJ, Lam BYK, Lin X, et al. Development and validation of a risk score (CHANGE) for cognitive impairment after ischemic stroke. Sci Rep 2017; 7: 12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ashburner JM, Chang Y, Porneala B, et al. Predicting post-stroke cognitive impairment using electronic health record data. Int J Stroke 2024; 19: 898–906. [DOI] [PubMed] [Google Scholar]
- 38. Kim EJ, Park CG, Park JS, et al. Relationship between blood pressure parameters and pulse wave velocity in normotensive and hypertensive subjects: invasive study. J Hum Hypertens 2007; 21: 141–148. [DOI] [PubMed] [Google Scholar]
- 39. Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019; 18: 684–696. [DOI] [PubMed] [Google Scholar]
- 40. Wardlaw JM, Doubal FN, Valdes-Hernandez M, et al. Blood-brain barrier permeability and long-term clinical and imaging outcomes in cerebral small vessel disease. Stroke 2013; 44: 525–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bordes C, Sargurupremraj M, Mishra A, Debette S. Genetics of common cerebral small vessel disease. Nat Rev Neurol 2022; 18: 84–101. [DOI] [PubMed] [Google Scholar]
- 42. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duering M, Biessels GJ, Brodtmann A, et al. Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol 2023; 22: 602–618. [DOI] [PubMed] [Google Scholar]
- 44. van den Brink H, Doubal FN, Duering M. Advanced MRI in cerebral small vessel disease. Int J Stroke 2023; 18: 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tay J, Morris RG, Markus HS. Apathy after stroke: diagnosis, mechanisms, consequences, and treatment. Int J Stroke 2021; 16: 510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arba F, Rinaldi C, Jensen M, et al. Validation of a simple clinical tool for screening of acute lacunar stroke-a substudy of the WAKE-UP trial. Int J Stroke 2024; 19: 935–941. [DOI] [PubMed] [Google Scholar]
- 47. Huang J, Biessels GJ, de Leeuw FE, et al. Cerebral microinfarcts revisited: detection, causes, and clinical relevance. Int J Stroke 2024; 19: 7–15. [DOI] [PubMed] [Google Scholar]
- 48. Schwarz G, Banerjee G, Hostettler IC, et al. MRI and CT imaging biomarkers of cerebral amyloid angiopathy in lobar intracerebral hemorrhage. Int J Stroke 2023; 18: 85–94. [DOI] [PubMed] [Google Scholar]
- 49. Durrani R, Wang M, Cox E, et al. Mediators of cognitive impairment in cerebral amyloid angiopathy. Int J Stroke 2023; 18: 78–84. [DOI] [PubMed] [Google Scholar]
- 50. Koemans EA, Castello JP, Rasing I, et al. Sex differences in onset and progression of cerebral amyloid angiopathy. Stroke 2023; 54: 306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koemans EA, Perosa V, Freeze WM, et al. Sex differences in histopathological markers of cerebral amyloid angiopathy and related hemorrhage. Int J Stroke 2024; 19: 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Livingston G, Huntley J, Liu KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024; 404: 572–628. [DOI] [PubMed] [Google Scholar]
- 53. Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013; 136: 2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer’s disease—lessons from pathology. BMC Med 2014; 12: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hughes TM, Kuller LH, Barinas-Mitchell EJ, et al. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol 2014; 71: 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moscoso A, Rey-Bretal D, Silva-Rodríguez J, et al. White matter hyperintensities are associated with subthreshold amyloid accumulation. Neuroimage 2020; 218: 116944. [DOI] [PubMed] [Google Scholar]
- 57. Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017; 317: 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rabin JS, Klein H, Kirn DR, et al. Associations of physical activity and beta-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol 2019; 76: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yau WW, Shirzadi Z, Yang HS, et al. Tau mediates synergistic influence of vascular risk and abeta on cognitive decline. Ann Neurol 2022; 92: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rabin JS, Pruzin J, Scott M, et al. Association of beta-amyloid and vascular risk on longitudinal patterns of brain atrophy. Neurology 2022; 99: e270–e280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rebouças SCL, Cougnard-Gregoire A, Arnould L, et al. Retinal microvasculature and incident dementia over 10 years: the Three-City-Alienor cohort. Alzheimers Dement (Amst) 2023; 15: e12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dhana K, Agarwal P, James BD, et al. Healthy lifestyle and cognition in older adults with common neuropathologies of dementia. JAMA Neurol 2024; 81: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ko H, Lam BYK, Mok VCT. Pathophysiology of vascular cognitive impairment (II): amyloid contribution in vascular cognitive impairment. In: Lee S-H, Lim J-S. (eds) Stroke revisited: vascular cognitive impairment. Singapore: Springer, 2020, pp. 87–97. [Google Scholar]
- 64. Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement 2019; 15: 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kapasi A, Yu L, Petyuk V, et al. Association of small vessel disease with tau pathology. Acta Neuropathol 2022; 143: 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tian Y, Zhao M, Chen Y, et al. Correction: Tian et al. The underlying role of the glymphatic system and meningeal lymphatic vessels in cerebral small vessel disease. Biomolecules 2022, 12, 748. Biomolecules 2023; 13: 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Beach TG, Wilson JR, Sue LI, et al. Circle of Willis atherosclerosis: association with Alzheimer’s disease, neuritic plaques and neurofibrillary tangles. Acta Neuropathol 2007; 113: 13–21. [DOI] [PubMed] [Google Scholar]
- 68. Proietti R, AlTurki A, Vio R, et al. The association between atrial fibrillation and Alzheimer’s disease: fact or fallacy? A systematic review and meta-analysis. J Cardiovasc Med (Hagerstown) 2020; 21: 106–112. [DOI] [PubMed] [Google Scholar]
- 69. Johansen MC, Mosley TH, Knopman DS, et al. Associations between atrial cardiopathy and cerebral amyloid: the ARIC-PET study. J Am Heart Assoc 2020; 9: e018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gupta A, Iadecola C. Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci 2015; 7: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hajjar K, Fulton RL, Diener HC, et al. Does the cognitive measure Cog-4 show improvement among patients treated with thrombolysis after acute stroke. Int J Stroke 2013; 8: 652–656. [DOI] [PubMed] [Google Scholar]
- 72. López-Cancio E, Jovin TG, Cobo E, et al. Endovascular treatment improves cognition after stroke: a secondary analysis of REVASCAT trial. Neurology 2017; 88: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Joundi RA, Smith EE, Mandzia J, et al. Effect of endovascular thrombectomy for acute ischemic stroke on cognitive outcomes: a secondary analysis of the ESCAPE trial. Neurology 2024; 102: e209270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gallucci L, Sperber C, Guggisberg AG, et al. Post-stroke cognitive impairment remains highly prevalent and disabling despite state-of-the-art stroke treatment. Int J Stroke 2024; 19: 888–897. [DOI] [PubMed] [Google Scholar]
- 75. Cerasuolo JO, Mandzia J, Cipriano LE, et al. Intravenous thrombolysis after first-ever ischemic stroke and reduced incident dementia rate. Stroke 2022; 53: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 76. Bala F, Betzner W, Beland B, et al. Reperfusion therapies for ischemic stroke in dementia and cognitive impairment: a systematic review and meta-analysis. Int J Stroke 2024; 19: 867–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Seiffge DJ, Anderson CS. Treatment for intracerebral hemorrhage: dawn of a new era. Int J Stroke 2024; 19: 482–489. [DOI] [PubMed] [Google Scholar]
- 78. Peters R, Breitner J, James S, et al. Dementia risk reduction: why haven’t the pharmacological risk reduction trials worked? An in-depth exploration of seven established risk factors. Alzheimers Dement (N Y) 2021; 7: e12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA 2020; 323: 1934–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Peters R, Warwick J, Anstey KJ, et al. Blood pressure and dementia: what the SPRINT-MIND trial adds and what we still need to know. Neurology 2019; 92: 1017–1018. [DOI] [PubMed] [Google Scholar]
- 81. Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019; 321: 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Peters R, Xu Y, Fitzgerald O, et al. Blood pressure lowering and prevention of dementia: an individual patient data meta-analysis. Eur Heart J 2022; 43: 4980–4990. [DOI] [PubMed] [Google Scholar]
- 83. Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005; 112: 1644–1650. [DOI] [PubMed] [Google Scholar]
- 84. Su C, Wu H, Yang X, et al. The relation between antihypertensive treatment and progression of cerebral small vessel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 2021; 100: e26749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. den Brok MGHE, van Dalen JW, Abdulrahman H, et al. Antihypertensive medication classes and the risk of dementia: a systematic review and network meta-analysis. J Am Med Dir Assoc 2021; 22: 1386–1395.e1315. [DOI] [PubMed] [Google Scholar]
- 86. Hajjar I, Okafor M, McDaniel D, et al. Effects of candesartan vs lisinopril on neurocognitive function in older adults with executive mild cognitive impairment: a randomized clinical trial. JAMA Netw Open 2020; 3: e2012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Henley B, Okafor M, Kulshreshtha A, Trammell A, Hajjar I. Effects of candesartan on cerebral microvascular function in mild cognitive impairment: results of two clinical trials. Int J Stroke 2023; 18: 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kopczak A, Stringer MS, van den Brink H, et al. Effect of blood pressure-lowering agents on microvascular function in people with small vessel diseases (TREAT-SVDs): a multicentre, open-label, randomised, crossover trial. Lancet Neurol 2023; 22: 991–1004. [DOI] [PubMed] [Google Scholar]
- 89. Ho JK, Moriarty F, Manly JJ, et al. Blood-brain barrier crossing renin-angiotensin drugs and cognition in the elderly: a meta-analysis. Hypertension 2021; 78: 629–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Areosa Sastre A, Vernooij RW, González-Colaço Harmand M, et al. Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev 2017; 6: CD003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Whitmer RA, Karter AJ, Yaffe K, et al. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009; 301: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lin Y, Gong Z, Ma C, Wang Z, Wang K. Relationship between glycemic control and cognitive impairment: a systematic review and meta-analysis. Front Aging Neurosci 2023; 15: 1126183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Akimoto H, Negishi A, Oshima S, et al. Antidiabetic drugs for the risk of Alzheimer disease in patients with type 2 DM using FAERS. Am J Alzheimers Dis Other Demen 2020; 35: 1533317519899546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Campbell JM, Stephenson MD, de Courten B, Chapman I, Bellman SM, Aromataris E. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J Alzheimers Dis 2018; 65: 1225–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ha J, Choi DW, Kim KJ, et al. Pioglitazone use and reduced risk of dementia in patients with diabetes mellitus with a history of ischemic stroke. Neurology 2023; 100: e1799–e1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou JB, Tang X, Han M, Yang J, Simó R. Impact of antidiabetic agents on dementia risk: a Bayesian network meta-analysis. Metabolism 2020; 109: 154265. [DOI] [PubMed] [Google Scholar]
- 97. Biessels GJ, Verhagen C, Janssen J, et al. Effect of linagliptin on cognitive performance in patients with type 2 diabetes and cardiorenal comorbidities: the CARMELINA randomized trial. Diabetes Care 2019; 42: 1930–1938. [DOI] [PubMed] [Google Scholar]
- 98. Cukierman-Yaffe T, Gerstein HC, Colhoun HM, et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol 2020; 19: 582–590. [DOI] [PubMed] [Google Scholar]
- 99. Li Q, Jia M, Yan Z, et al. Activation of glucagon-like peptide-1 receptor ameliorates cognitive decline in type 2 diabetes mellitus through a metabolism-independent pathway. J Am Heart Assoc 2021; 10: e020734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cheng H, Zhang Z, Zhang B, et al. Enhancement of impaired olfactory neural activation and cognitive capacity by liraglutide, but not dapagliflozin or acarbose, in patients with type 2 diabetes: a 16-week randomized parallel comparative study. Diabetes Care 2022; 45: 1201–1210. [DOI] [PubMed] [Google Scholar]
- 101. Adamou A, Barkas F, Milionis H, et al. Glucagon-like peptide-1 receptor agonists and stroke: a systematic review and meta-analysis of cardiovascular outcome trials. Int J Stroke 2024; 19: 876–887. [DOI] [PubMed] [Google Scholar]
- 102. Strain WD, Frenkel O, James MA, et al. Effects of semaglutide on stroke subtypes in type 2 diabetes: post hoc analysis of the randomized SUSTAIN 6 and PIONEER 6. Stroke 2022; 53: 2749–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li Z, Chen X, Vong JSL, et al. Systemic GLP-1R agonist treatment reverses mouse glial and neurovascular cell transcriptomic aging signatures in a genome-wide manner. Commun Biol 2021; 4: 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zhao L, Li Z, Vong JSL, et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat Commun 2020; 11: 4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Launer LJ, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol 2011; 10: 969–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Murray AM, Hsu FC, Williamson JD, et al. ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia 2017; 60: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Muldoon MF, Ryan CM, Sereika SM, et al. Randomized trial of the effects of simvastatin on cognitive functioning in hypercholesterolemic adults. Am J Med 2004; 117: 823–829. [DOI] [PubMed] [Google Scholar]
- 108. Olmastroni E, Molari G, De Beni N, et al. Statin use and risk of dementia or Alzheimer’s disease: a systematic review and meta-analysis of observational studies. Eur J Prev Cardiol 2022; 29: 804–814. [DOI] [PubMed] [Google Scholar]
- 109. Ren QW, Katherine Teng TH, Tse YK, et al. Statins and risks of dementia among patients with heart failure: a population-based retrospective cohort study in Hong Kong. Lancet Reg Health West Pac 2024; 44: 101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ip BYM, Ko H, Lam BYK, et al. Current and future treatments of vascular cognitive impairment. Stroke 2024; 55: 822–839. [DOI] [PubMed] [Google Scholar]
- 111. Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2016; 37: 536–545. [DOI] [PubMed] [Google Scholar]
- 112. Gencer B, Mach F, Guo J, et al. Cognition after lowering LDL-cholesterol with evolocumab. J Am Coll Cardiol 2020; 75: 2283–2293. [DOI] [PubMed] [Google Scholar]
- 113. Giugliano RP, Mach F, Zavitz K, et al. Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017; 377: 633–643. [DOI] [PubMed] [Google Scholar]
- 114. Ying H, Wang J, Shen Z, Wang M, Zhou B. Impact of lowering low-density lipoprotein cholesterol with contemporary lipid-lowering medicines on cognitive function: a systematic review and meta-analysis. Cardiovasc Drugs Ther 2021; 35: 153–166. [DOI] [PubMed] [Google Scholar]
- 115. Yang Z, Wang H, Edwards D, et al. Association of blood lipids, atherosclerosis and statin use with dementia and cognitive impairment after stroke: a systematic review and meta-analysis. Ageing Res Rev 2020; 57: 100962. [DOI] [PubMed] [Google Scholar]
- 116. Goldstein LB, Toth PP, Dearborn-Tomazos JL, et al. Aggressive LDL-C lowering and the brain: impact on risk for dementia and hemorrhagic stroke: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2023; 43: e404–e442. [DOI] [PubMed] [Google Scholar]
- 117. Katsanos AH, Lioutas VA, Charidimou A, et al. Statin treatment and accrual of covert cerebral ischaemia on neuroimaging: a systematic review and meta-analysis of randomized trials. Eur J Neurol 2020; 27: 1023–1027. [DOI] [PubMed] [Google Scholar]
- 118. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018; 379: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ryan J, Storey E, Murray AM, et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology 2020; 95: e320–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Matsumoto C, Ogawa H, Saito Y, et al. Sex difference in effects of low-dose aspirin on prevention of dementia in patients with type 2 diabetes: a long-term follow-up study of a randomized clinical trial. Diabetes Care 2020; 43: 314–320. [DOI] [PubMed] [Google Scholar]
- 121. Parish S, Mafham M, Offer A, et al. Effects of aspirin on dementia and cognitive function in diabetic patients: the ASCEND trial. Eur Heart J 2022; 43: 2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Xie X, Kou L, Chen X, et al. Association of aspirin with dementia or mild cognitive impairment: a systematic review and meta-analysis of randomized trials. Neuroepidemiology 2023; 57: 197–205. [DOI] [PubMed] [Google Scholar]
- 123. Ip BYM, Lam BYK, Hui VMH, et al. Efficacy and safety of cilostazol in decreasing progression of cerebral white matter hyperintensities-a randomized controlled trial. Alzheimers Dement (N Y) 2022; 8: e12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kim BC, Youn YC, Jeong JH, et al. Cilostazol versus aspirin on white matter changes in cerebral small vessel disease: a randomized controlled trial. Stroke 2022; 53: 698–709. [DOI] [PubMed] [Google Scholar]
- 125. Wardlaw JM, Woodhouse LJ, Mhlanga II, et al. Isosorbide mononitrate and cilostazol treatment in patients with symptomatic cerebral small vessel disease: the Lacunar Intervention Trial-2 (LACI-2) randomized clinical trial. JAMA Neurol 2023; 80: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012; 367: 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008; 359: 1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Bezabhe WM, Bereznicki LR, Radford J, et al. Oral anticoagulant treatment and the risk of dementia in patients with atrial fibrillation: a population-based cohort study. J Am Heart Assoc 2022; 11: e023098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rahman AA, Michaud J, Dell’Aniello S, et al. Oral anticoagulants and the risk of dementia in patients with nonvalvular atrial fibrillation: a population-based cohort study. Neurology 2023; 100: e1309–e1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fong KY, Chan YH, Wang Y, et al. Dementia risk of direct oral anticoagulants versus warfarin for atrial fibrillation: systematic review and meta-analysis. JACC Asia 2023; 3: 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bosch JJ, O’Donnell MJ, Gao P, et al. Effects of a polypill, aspirin, and the combination of both on cognitive and functional outcomes: a randomized clinical trial. JAMA Neurol 2023; 80: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–2263. [DOI] [PubMed] [Google Scholar]
- 133. Lauenroth A, Ioannidis AE, Teichmann B. Influence of combined physical and cognitive training on cognition: a systematic review. BMC Geriatr 2016; 16: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Salzman T, Sarquis-Adamson Y, Son S, et al. Associations of multidomain interventions with improvements in cognition in mild cognitive impairment: a systematic review and meta-analysis. JAMA Netw Open 2022; 5: e226744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sugimoto T, Araki A, Fujita H, et al. Multidomain intervention trial for preventing cognitive decline among older adults with type 2 diabetes: J-MIND-diabetes. J Prev Alzheimers Dis. Epub ahead of print January 2024. DOI: 10.14283/jpad.2024.117 [DOI] [Google Scholar]
- 136. Hafdi M, Hoevenaar-Blom MP, Richard E. Multi-domain interventions for the prevention of dementia and cognitive decline. Cochrane Database Syst Rev 2021; 11: CD013572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Teuschl Y, Ihle-Hansen H, Matz K, et al. Multidomain intervention for the prevention of cognitive decline after stroke–a pooled patient-level data analysis. Eur J Neurol 2018; 25: 1182–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 2018; 14: 653–666. [DOI] [PubMed] [Google Scholar]
- 139. Wong A, Yiu S, Lam BYK, et al. Physical activities attenuate the negative cognitive impact from white matter hyperintensities in stroke and TIA patients with low education. Int J Geriatr Psychiatry 2019; 34: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 140. Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016; 388: 797–805. [DOI] [PubMed] [Google Scholar]
- 141. Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 2017; 16: 377–389. [DOI] [PubMed] [Google Scholar]
- 142. Stephen R, Liu Y, Ngandu T, et al. Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Res Ther 2019; 11: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jensen CS, Simonsen AH, Siersma V, et al. Patients with Alzheimer’s disease who carry the APOE ε4 allele benefit more from physical exercise. Alzheimers Dement (N Y) 2019; 5: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol 2018; 75: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol 2017; 13: 327–339. [DOI] [PubMed] [Google Scholar]
- 146. Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaBalpha degradation in a stimulus-specific manner in microglia. J Neurochem 2006; 96: 314–323. [DOI] [PubMed] [Google Scholar]
- 147. Yang F, Zhou L, Wang D, et al. Minocycline ameliorates hypoxia-induced blood-brain barrier damage by inhibition of HIF-1α through SIRT-3/PHD-2 degradation pathway. Neuroscience 2015; 304: 250–259. [DOI] [PubMed] [Google Scholar]
- 148. Jalal FY, Yang Y, Thompson JF, Roitbak T, Rosenberg GA. Hypoxia-induced neuroinflammatory white-matter injury reduced by minocycline in SHR/SP. J Cereb Blood Flow Metab 2015; 35: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Brown RB, Tozer DJ, Loubière L, et al. MINocyclinE to Reduce inflammation and blood-brain barrier leakage in small Vessel diseAse (MINERVA): a phase II, randomized, double-blind, placebo-controlled experimental medicine trial. Alzheimers Dement 2024; 20: 3852–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Wang A, Jia B, Zhang X, et al. Efficacy and safety of butylphthalide in patients with acute ischemic stroke: a randomized clinical trial. JAMA Neurol 2023; 80: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Jia J, Wei C, Liang J, et al. The effects of DL-3-n-butylphthalide in patients with vascular cognitive impairment without dementia caused by subcortical ischemic small vessel disease: a multicentre, randomized, double-blind, placebo-controlled trial. Alzheimers Dement 2016; 12: 89–99. [DOI] [PubMed] [Google Scholar]
- 152. Fan X, Shen W, Wang L, Zhang Y. Efficacy and safety of DL-3-n-butylphthalide in the treatment of poststroke cognitive impairment: a systematic review and meta-analysis. Front Pharmacol 2021; 12: 810297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Quinn TJ, Richard E, Teuschl Y, et al. European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. Eur Stroke J 2021; 6: I–xxxviii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Dichgans M, Markus HS, Salloway S, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol 2008; 7: 310–318. [DOI] [PubMed] [Google Scholar]
- 155. Smith EE, Cieslak A, Barber P, et al. Therapeutic strategies and drug development for vascular cognitive impairment. J Am Heart Assoc 2017; 6: e005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tay J, Lisiecka-Ford DM, Hollocks MJ, et al. Network neuroscience of apathy in cerebrovascular disease. Prog Neurobiol 2020; 188: 101785. [DOI] [PubMed] [Google Scholar]
- 157. Tay J, EFFECTS Trial Collaboration, Mårtensson B, Markus HS, Lundström E. Does fluoxetine reduce apathetic and depressive symptoms after stroke? An analysis of the Efficacy oF Fluoxetine-a randomized Controlled Trial in Stroke trial data set. Int J Stroke 2023; 18: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Broomfield NM, Blake J, Gracey F, et al. Post-stroke emotionalism: diagnosis, pathophysiology, and treatment. Int J Stroke 2024; 19: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]