Abstract
We examined the role of soluble poliovirus receptor on the transition of native poliovirus (160S or N particle) to an infectious intermediate (135S or A particle). The viral receptor behaves as a classic transition state theory catalyst, facilitating the N-to-A conversion by lowering the activation energy for the process by 50 kcal/mol. In contrast to earlier studies which demonstrated that capsid-binding drugs inhibit thermally mediated N-to-A conversion through entropic stabilization alone, capsid-binding drugs are shown to inhibit receptor-mediated N-to-A conversion through a combination of enthalpic and entropic effects.
Poliovirus is a nonenveloped virus of the family Picornaviridae. Picornaviruses share an icosahedral capsid architecture consisting of 60 copies of four proteins, VP1, VP2, VP3, and VP4. The surface of the virion is dominated by prominent star-shaped mesas at the fivefold axes and three-bladed propeller-like features at the threefold axes. These surface features are separated by deep canyons encircling the fivefold axes. These canyons are involved in many essential aspects of capsid function. Structural studies have shown that the receptor footprints for major group rhinoviruses (19) and poliovirus (2, 10, 27) map to the canyon. At the base of the canyon underneath the receptor footprint, there is an entry to a long, narrow hydrophobic pocket within the β-barrel core of VP1. For most entero- and rhinoviruses, crystallographic studies have revealed that this pocket is occupied by an unidentified fatty acid-like moiety, or pocket factor (6, 12, 17, 18, 23), which can be displaced by a family of capsid-binding antiviral drugs (9, 22). Interestingly, the pocket factor and the antiviral drugs can exert large-scale, global effects on the capsid's conformational dynamics, which play a critical role in the viral life cycle.
When poliovirus attaches to its receptor, the particle converts irreversibly from the N (native or 160S) to the A (infectious [4] intermediate, or 135S) conformation. In the course of this uncoating transition, normally internal components, including VP4 and the N-terminal extension of VP1, are externalized. Externalization of these components has been shown to facilitate the attachment of the A particle to liposomes in vitro (7), suggesting a mechanism for the entry of virus or viral RNA to the cell (1, 2). Transient and reversible exposure of portions of VP4 and the N terminus of VP1 also occurs naturally at physiological temperatures (14). This “breathing” process suggests that the particle is primed to undergo the N-to-A transition but cannot complete the transition in the absence of a trigger, i.e., the receptor. We have previously proposed that the receptor acts like an enzyme, accelerating the rate of the N-to-A transition at physiological temperature by lowering the activation energy (Ea) for the transition. Later in the cell entry process, the A particle undergoes further changes, which result in the release of the viral RNA and formation of an empty particle that sediments at 80S. The trigger RNA release is unknown. The N-to-A transition also can be induced by exposure of the virus to detergent-solubilized receptor (8, 11) or to the soluble ectodomain of the receptor at physiological temperature (see below), and both the N-to-A and A-to-empty transitions can also be induced in vitro by warming in hypotonic buffers containing millimolar levels of divalent cations (4, 25, 26). Regardless of the mechanism used to induce the transition, capsid-binding antiviral drugs inhibit the N-to-A transition (3, 8, 25).
Genetic data suggest that the pocket factor normally serves to regulate the stability of the virion (6), including regulating the N-to-A transition (16). Direct experimental studies demonstrate that the capsid-binding drugs inhibit both receptor- and heat-induced N-to-A transition. In the absence of receptor, the Ea for the N-to-A transition is very large (145 kcal/mol) and is unaffected by the presence of antiviral drugs (25). Thus, the drugs must inhibit the N-to-A transition via entropic stabilization of the native virion. This experimental observation is consistent with computational modeling studies that suggest that drug binding in the closely related rhinovirus 14 is accompanied by an increase in the compressibility of the capsid (20, 21, 24). Others have shown that binding of antivirals to the rhinovirus VP1 pocket decreases the ability of the virus to undergo breathing at room temperature (13).
In this work, we extend these studies by characterizing the effect of soluble poliovirus receptor (sPvr) on the kinetics of the N-to-A conversion. The results confirm the prediction that the receptor acts much like an enzyme, demonstrating that the receptor lowers the activation energy by approximately 50 kcal/mol. The results also demonstrate that in the presence of receptor, drugs inhibit the conversion by a combination of enthalpic and entropic effects. Curiously, the Ea for receptor-mediated conversion of a virus-drug complex with one of the drugs tested was higher than that for the virus-drug complex in the absence of receptor. Together, the results allow us to propose a kinetic model for the receptor-mediated N-to-A transition.
MATERIALS AND METHODS
Growth, propagation, and purification of virus.
The Mahoney strain of type 1 poliovirus (P1/M) was grown in HeLa cells grown in suspension and purified by differential centrifugation and CsCl density gradient fractionation as described previously (28). [3H]leucine-labeled P1/M was prepared as described previously (14). Purified virus was dialyzed into phosphate-buffered saline (PBS) and concentrated to 5 mg/ml or greater in a microconcentrator (Microcon).
sPvr.
Purified, mammalian cell-expressed sPvr (comprising residues 1 to 337 of the receptor's ectodomain and a C-terminal His6 tag) was produced and purified as described previously (15). Purified receptor was dialyzed into conversion buffer prior to use and stored at 4°C at a working concentration of 1.25 μM.
Differential scanning calorimetry of sPvr.
One-half milliliter of 1.25 μM sPvr in conversion buffer (10 mM HEPES, 2 mM CaCl2, 0.1% Triton X-100, 0.1% dimethyl sulfoxide [DMSO] [pH 7.5]) was injected into a MicroCal VP-DSC microcalorimeter. Temperature scans were done at 0.5°C/min from 25 to 80°C. Prior to loading of the sPvr sample, two conversion buffer blank scans from 25 to 80°C were executed to stabilize the instrument and provide a baseline.
Determination of rate constants for the N-to-A transition.
Concentrated virus stocks (11 μg) were incubated overnight at 4°C in 20-μl volumes of conversion buffer containing either no drug, 40 μM R77975, or 40 μM R78206 (8). The mixtures were equilibrated at room temperature for 15 min, and then the 20-μl incubations were transferred to 1.5-μl Eppendorf tubes containing 980 μl of conversion buffer + 0.25 μM sPvr (see below) containing either no drug, 40 μM R77975, or 40 μM R78206 respectively. The tubes and their contents had been preequilibrated to a desired temperature in a Fisher Scientific model 9100 Isotemp refrigerated circulator. The temperatures of the samples were monitored by inserting a thermocouple probe into another 1.5-ml Eppendorf tube containing conversion buffer only in the water bath. In general, the temperature did not fluctuate by more than 0.1°C during any experiment.
At specific time intervals, 80-μl aliquots of the reaction mixture were transferred to low-binding 500-μl tubes (Marsh Products) containing 50 μl of PBS+ buffer (PBS, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.5 mg of bovine serum albumin/ml) at 4°C. The extent of conversion to the A particle was assayed by immunoprecipitation using an A-particle-specific monoclonal antibody as described previously (25).
The first-order rate constant for the receptor-mediated N-to-A conversion of virus and virus-drug complexes at each temperature was estimated from the slope of the log of percent remaining native virus versus time. Data for each temperature point were collected in triplicate, and standard deviations were determined for the time points. The average value for each time point was plotted, and a straight line was fit to the points by linear regression. The slopes were calculated using Microsoft Excel.
Sedimentation analysis of products of the poliovirus transitions.
About 20,000 cpm of [3H]Leu-labeled virus or virus-drug complex in the presence of 0.25 μM sPvr was incubated at the highest temperature and longest time required to achieve the rate constants in Table 1: for virus in 0.1% DMSO, 37°C and 200 for virus and R78206, 45°C for 100 s; and for virus and R77975, 43°C for 110 s. The 135S marker was generated by incubating native virus to 50°C for 2 min (4); the 80S marker was generated by incubating native virus to 60°C for 10 min. All incubations were rapidly quenched by addition of an equal volume of ice-cold PBS+ buffer and immediate transfer to ice. The samples were then overlaid onto 12-ml 15 to 30% sucrose gradients. The gradients were developed at 39,000 rpm for 2.3 h at 4°C and then fractionated from the top.
TABLE 1.
Summary of rate constants for the N-to-A conversion in the presence of 0.1% DMSO, 40 μM R77975, or 40 μM R78206
| Temp (°C) |
k (10−5 s−1)a
|
||
|---|---|---|---|
| 0.1% DMSO | R77975 | R78206 | |
| 29 | 5.4 (0.2) | ||
| 30 | 17 (3) | ||
| 32 | 54 (14) | ||
| 33 | 94 (12) | ||
| 35 | 136 (1.5) | ||
| 36 | 351 (42) | ||
| 37 | 438 (53) | 22 (1.2) | |
| 38 | 94 (24) | ||
| 39 | 141 (22) | ||
| 40 | 230 (4) | ||
| 41 | 379 (17) | ||
| 42 | 681 (22) | 15 (2.5) | |
| 42.5 | 21 (2.9) | ||
| 42.8 | 37 (7.7) | ||
| 43 | 1,166 (122) | 80 (12) | |
| 44 | 319 (49) | ||
| 45 | 925 (129) | ||
Each value is the average of three separate experiments; in parentheses are standard deviations.
Kinetic analysis.
The Ea as for receptor-mediated N-to-A conversion of virus and virus-drug complexes were determined from the slopes of the Arrhenius plots, in which the natural log of the first-order rate constant was plotted versus −1/RT. Since the temperature data were collected in triplicate, the average values and standard deviations were plotted, and lines were fit to the data by linear regression. The slopes were calculated using Microsoft Excel.
RESULTS AND DISCUSSION
sPvr-mediated N-to-A transitions are first order.
To examine the effect of sPvr on the rate constant of the N-to-A transition, virus and virus-drug complexes were incubated at various temperatures in the presence of 0.25 μM sPvr. At the concentrations of sPvr and virus used in the conversions, the receptor-to-binding site ratio is ∼450:1. The kinetics and thermodynamics of poliovirus-sPvr interactions are complex. Surface plasmon resonance studies have identified two binding modes with KDs of 0.67 and 0.11 μM at 20°C (15). The relative abundance of high-affinity sites increases from 12% at 5°C to 46% at 20°C (15). Interference due to the N-to-A transition precludes determining the affinities and relative abundance of the two binding modes at physiological temperatures. Extrapolation of the available data suggests that the levels of receptor used in this study should be sufficient to guarantee high occupancy of the available sites but are probably insufficient to guarantee full occupancy (60 sites/virion). Indeed, preliminary titrations with sPvr suggested that further increases in the rate of conversion of virus could be achieved at higher concentrations (data not shown). However, limitations in the availability of sPvr preclude working at concentrations sufficient to guarantee full occupancy and maximal rates, and with minor caveats as noted, the implications of the results presented below are expected to be independent of changes in occupancy.
Since the working temperature range of the experiments went beyond physiological temperatures, we first determined the heat stability of sPvr by differential scanning calorimetry (Fig. 1). The rate of the scan (0.5 C/min) was chosen such that the time the sample spends at elevated temperature was similar to the total time course of the N-to-A conversion of virus-R78206 complex at high temperature. At this scan rate, the heat denaturation of sPvr begins at 43°C and peaks at 57°C. However, the rate of thermal denaturation is negligible at temperatures below 45°C. This observation together with the linearity of the kinetics of the N-to-A conversion suggests that thermal inactivation of the receptor is insignificant over the entire range of temperatures used in this study and can be ignored.
FIG. 1.
Differential scanning calorimetry profile of sPvR. For details, see Materials and Methods. The bold line corresponds to sPvR in the sample chamber; the lighter line corresponds to conversion buffer only in the sample chamber.
In the presence of receptor, the N-to-A transition obeys first-order kinetics (Fig. 2A). When the antiviral compounds R77975 and R78206 (8) were added to the conversion reactions at 40 μM in 0.1% DMSO, the reactions still obeyed first-order kinetics, but the rate constants were lower than for the sample with virus alone, as expected (Fig. 2B and C). R77975 and R78206 have MICs of 3.061 μM and 8 nM, respectively (8). The observed reduction in rate constants with respect to virus in the absence of drug is consistent with this ordering, because R78206 reduces the rate significantly more than R77975. In all cases, the rate at any given temperature is significantly higher than the rate in the absence of receptor, confirming that the receptor facilitates the conversion.
FIG. 2.
Rate constant determination for receptor-mediated transitions. The published experimental procedure (22) was modified to include sPvR at 0.25 μM. The plots show the natural logarithm of the concentration of unconverted 160S particle versus time. Each point is the average of three separate experiments; bars indicate the standard deviation of the average of the measurements. The data for the low-temperature reactions for all graphs were truncated for the sake of clarity of the higher-temperature data. Plots were generated using Microsoft Excel.
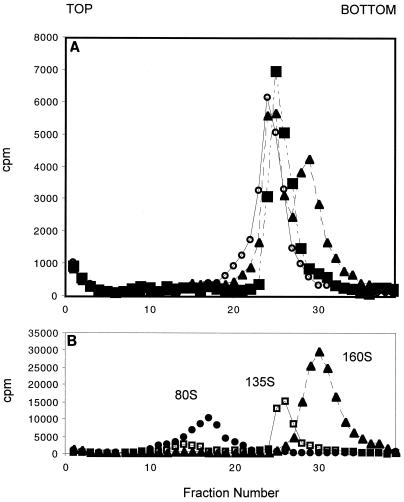
To ensure that the precipitated counts were due to A particles and not 80S particles, aliquots from the highest temperature and longest incubation time for each data set were analyzed on 15 to 30% sucrose gradients. The dominant products were A particles, and 80S particles were not observed even under the most extreme conditions (Fig. 3).
FIG. 3.
(A) Sucrose gradient analysis of virus products from conversions. 3H-labeled P1/M (1.8 μg; ∼20,000 cpm) and 0.25 μM sPvr were incubated under the most extreme conditions for each compound tested: 0.1% DMSO, 200 s and 37°C (squares); R77975, 110 s and 43°C (open circles); and R78206, 110 s and 45°C (triangles). (B) Positions of 80S, 135S, and 160S markers on equivalent gradients. The gradients were fractionated from the top.
sPvr reduces the activation energy for the N-to-A transition.
The Arrhenius equation states that for a first-order reaction obeying simple transition state kinetics, the rate constant for a reaction is exponentially dependent on the temperature: k = A exp (−Ea/RT), where k is the rate constant, Ea is the activation energy, R is the gas constant (1.98 cal/mol deg), and T is the temperature in kelvins. The preexponential factor A is described by the relation A = (kbT/h) exp (ΔS†/R), where kb is Boltzmann's constant, h is Planck's constant, and ΔS† is the entropy difference between the ground state and the activated complex. Thus, a plot of the natural logarithm of the first-order rate constant versus −1/RT should yield a line with a slope that is equivalent to Ea and a y intercept that is proportional to ΔS†.
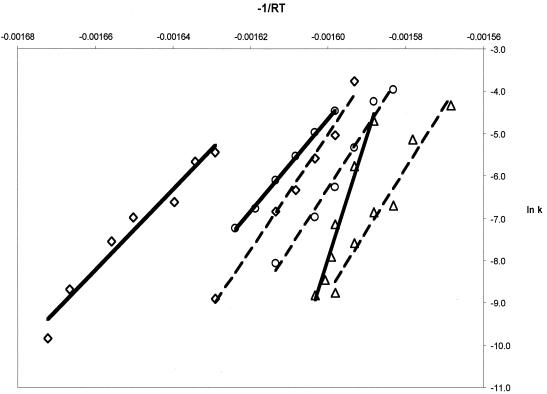
In the presence of receptor, the virus and virus-drug complexes produce linear Arrhenius plots, indicating that the first-order rate constants are dependent on a single exponential function as required by simple transition state theory (Fig. 4). The linearity of the Arrhenius plots is maintained over a significant range of temperatures, suggesting that facilitation of the conversion occurs via a single mechanism over a wide range of temperatures. Similar behavior has been previously reported for the N-to-A transition of virus and virus-drug complexes in the absence of receptor (25). The addition of sPvr reduces the Ea of the N-to-A transition by 50 kcal/mol (95 kcal/mol for virus plus sPvr, versus 145 kcal/mol for virus alone) (Fig. 4 and Table 2). Increased occupancy of the receptor might be expected to induce a further decrease in Ea. Thus, as predicted, the receptor is behaving like a classic transition state theory catalyst, accelerating the rate of the transition by lowering the activation barrier.
FIG. 4.
Arrhenius plots for conversion in the presence and absence of sPvR. The averaged values of the natural logarithm of k from Table 1 were plotted as a function of −1/RT, so that the slope of each line is equivalent to Ea. Solid lines correspond to data collected in the presence of sPvR; dashed lines represent data collected in the absence of sPvR. No drug (0.1% DMSO), R77975, and R78206 are denoted by ◊, ○, and ▵, respectively. Temperature increases from left to right on the chart.
TABLE 2.
Summary of kinetic parameters
In contrast to previous studies that showed that the capsid-binding drugs do not alter the Ea for the thermally mediated N-to-A transition, both R77975 and R78206 significantly elevate the Ea for the receptor-mediated N-to-A transition (Fig. 4 and Table 2). The relatively small increase in Ea for the R77975 complex could be attributed to a reduction in the affinity of the receptor for the virus-drug complex. However, the Ea for the virus-R78206 complex in the presence of receptor (290 kcal/mol) is significantly higher than the Ea for the virus-R78206 complex in the absence of receptor, and the increase must therefore reflect an enthalpic contribution of drug binding to the reduction in the rate. Significantly, the rate of the N-to-A conversion of the virus-R78206 complex in the presence of receptor is still much higher than that observed for the complex in absence of receptor. The energy that is driving this process forward in the presence of receptor must therefore also have a significant entropic component, corresponding either to a decrease in the entropy of the virus-R78206-receptor complex or an increase in the entropy of the transition state.
Kinetic model for the receptor-mediated N-to-A conversion.
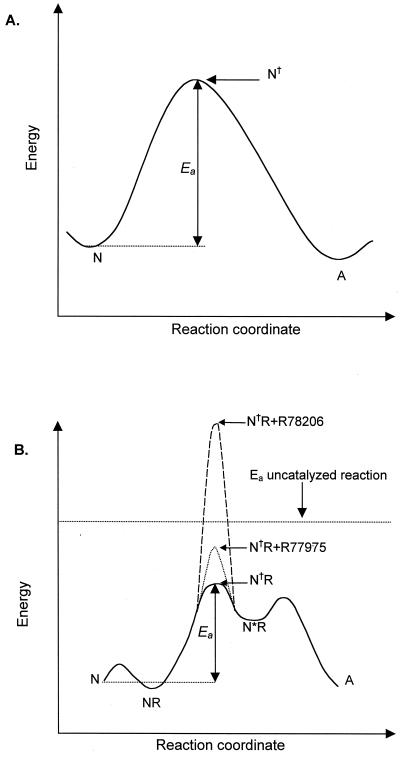
The results presented above raise two apparent paradoxes. (i) Drug binding has a significant effect on the Ea of the receptor-mediated, but not the thermally mediated, N-to-A conversion. (ii) The Ea for the N-to-A transition of the virus-R78206 complex is actually much higher in the presence of receptor. In the uncatalyzed (thermally mediated) conversion, there is a single transition state, N†, whose activation energy is independent of bound drug (25) (Fig. 5A). To rationalize the receptor-catalyzed data, we propose a more complex kinetic model (Fig. 5B), wherein receptor binding introduces additional intermediates and alters the rate-determining step of the reaction. The model contains three assumptions: (i) There is an activated intermediate (N∗R) in the receptor-mediated reaction pathway that includes virus, receptor, and perhaps ligand; (ii) the transition between the initial virus-receptor complex (NR) and the activated virus-receptor complex (N∗R; denoted by the transition state N†R in Fig. 5B) is rate limiting (at least for the drug complexes); and (iii) the drugs significantly increase the enthalpy of activation (and thus Ea) for this step in the reaction pathway. The simplest model for how drugs might increase Ea for this step is one in which the formation of activated virus-receptor complex requires a conformational adjustment that can only occur if the drug (or pocket factor) is expelled from the pocket. The absence of the drug (or pocket factor) would be expected to substantially reduce the stability of the virus (16). Expulsion of the drug would require energy, and the energy requirement would be lowest for pocket factor, intermediate for R77975 (which is a relatively poor drug), and highest for R78206 (which is a nanomolar inhibitor of poliovirus replication). A recent study demonstrates that drug binding interferes with receptor binding at low temperature (4°C) but not at room temperature or physiological temperature, suggesting that formation of a tight-binding complex between virus and receptor requires enthalpically regulated conformational adjustments of the virus or receptor (5). These conformational adjustments required for tight binding may be related to the proposed activation of the receptor.
FIG. 5.
Model of the reaction pathway for the N-to-A transition. (A) Reaction pathway for the uncatalyzed (thermally mediated) conversion. The pathway proceeds through a single transition state, N†, whose Ea is independent of drug binding (25). (B) Reaction pathway for the receptor-mediated conversion. Binding of the receptor to N produces an initial virus receptor complex, NR. The receptor-mediated reaction goes through an intermediate, the activated virus receptor complex N∗R. R77975 and R78206 raise the Ea of the transition state for this step, N†R, such that it becomes rate limiting. The horizontal dashed line represents the energy barrier for the uncatalyzed reaction, which is 145 kcal/mol.
Low resolution cryoelectron microscopy structures of the virus-receptor complexes have been reported recently. These structures revealed no significant structural alterations at the resolution of the reported structures (∼22 Å), although one of the studies raised the possibility that pocket factor may have been expelled in the complex. Because the complexes used in the structural studies were formed at high virus and receptor concentrations in the cold, it is not yet clear whether the structures represent the initial complex or the tight-binding complex. Further studies characterizing the structure of the virus-receptor complexes at higher resolution as a function of temperature, or the structure of virus-drug-receptor complexes at low temperature, may resolve these questions.
ACKNOWLEDGMENTS
This work was supported by NIH grants to J.M.H. (AI20566) and to V.R.R. (AI20017).
We acknowledge Steve Miller, Dave Filman, and other members of the Hogle lab for valuable discussion.
REFERENCES
- 1.Belnap D M, Filman D J, Trus B L, Cheng N, Booy F P, Conway J F, Currey S, Hiremath C N, Tsang S K, Steven A C, Hogle J M. Molecular tectonic model of virus structural transitions: the putative cell-entry states of poliovirus. J Virol. 2000;74:1342–1354. doi: 10.1128/jvi.74.3.1342-1354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belnap D M, McDermott B M, Filman D J, Cheng N, Trus B L, Zuccola H J, Racaniello V R, Hogle J M, Steven A C. Three dimensional structure of poliovirus receptor bound to poliovirus. Proc Natl Acad Sci USA. 2000;97:73–78. doi: 10.1073/pnas.97.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caliguiri L A, McSharry J J, Lawrence G W. Effect of arildone on modification of poliovirus in vitro. Virology. 1980;105:86–93. doi: 10.1016/0042-6822(80)90158-0. [DOI] [PubMed] [Google Scholar]
- 4.Curry S, Chow M, Hogle J M. The poliovirus 135S particle is infectious. J Virol. 1996;70:7125–7131. doi: 10.1128/jvi.70.10.7125-7131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dove A W, Racaniello V R. An antiviral compound that blocks structural transitions of poliovirus prevents receptor binding at low temperatures. J Virol. 2000;74:3929–3931. doi: 10.1128/jvi.74.8.3929-3931.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filman D J, Syed R, Chow M, Macadam A J, Minor P D, Hogle J M. Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. EMBO J. 1989;8:1567–1579. doi: 10.1002/j.1460-2075.1989.tb03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fricks C E, Hogle J M. Cell-induced conformational change of poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990;64:1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez-Yafal A, Kaplan G, Racaniello V R, Hogle J M. Characterization of poliovirus conformational alteration mediated by soluble cell receptors. Virology. 1993;197:501–505. doi: 10.1006/viro.1993.1621. [DOI] [PubMed] [Google Scholar]
- 9.Grant R A, Hiremath C, Filman D J, Syed R, Andries K, Hogle J M. Structures of poliovirus complexes with antiviral drugs: implications for viral stability and drug design. Curr Biol. 1994;4:784–797. doi: 10.1016/s0960-9822(00)00176-7. [DOI] [PubMed] [Google Scholar]
- 10.He Y, Bowman V D, Mueller S, Bator C M, Bella J, Peng X, Baker T S, Wimmer E, Kuhn R J, Rossmann M G. Interaction of the poliovirus receptor with poliovirus. Proc Natl Acad Sci USA. 2000;97:79–84. doi: 10.1073/pnas.97.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan G, Freistadt M S, Racaniello V R. Neutralization of poliovirus by cell receptors expressed in insect cells. J Virol. 1990;64:4697–4702. doi: 10.1128/jvi.64.10.4697-4702.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S S, Smith T J, Chapman M S, Rossmann M C, Pevear D C, Dutko F J, Felock P J, Diana G D, McKinlay M A. Crystal structure of human rhinovirus serotype 1A (HRV1A) J Mol Biol. 1989;210:91–111. doi: 10.1016/0022-2836(89)90293-3. [DOI] [PubMed] [Google Scholar]
- 13.Lewis J K, Bothner B, Smith T J, Siuzdak G. Antiviral agent blocks breathing of the common cold virus. Proc Natl Acad Sci USA. 1998;95:6774–6778. doi: 10.1073/pnas.95.12.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Yafal A G, Lee Y M-H, Hogle J, Chow M. Poliovirus neutralization by antibodies to internal epitopes of VP4 and VP1 results from reversible exposure of these sequences at physiological temperature. J Virol. 1994;68:3965–3970. doi: 10.1128/jvi.68.6.3965-3970.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott B M, Rux A H, Eisenberg R J, Cohen G H, Racaniello V R. Two distinct binding affinities of poliovirus for its cellular receptor. J Biol Chem. 2000;275:23089–23096. doi: 10.1074/jbc.M002146200. [DOI] [PubMed] [Google Scholar]
- 16.Mosser A G, Rueckert R R. WIN 51711-dependent mutants of poliovirus type 3: evidence that virions decay after release from cells unless drug is present. J Virol. 1993;67:1246–1254. doi: 10.1128/jvi.67.3.1246-1254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muckelbauer J K, Kremer M, Minor I, Diana G, Dutko F J, Groarke J, Pevear D C, Rossmann M G. The structure of coxsackievirus B3 at 3.5A resolution. Structure. 1995;3:653–667. doi: 10.1016/s0969-2126(01)00201-5. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira M A, Zhao R, Lee W-M, Kremer M J, Minor I, Rueckert R R, Diana G D, Pevear D C, Dutko F J, McKinlay M A, Rossmann M G. The structure of human rhinovirus 16. Structure. 1993;1:51–68. doi: 10.1016/0969-2126(93)90008-5. [DOI] [PubMed] [Google Scholar]
- 19.Olson N H, Kolatkar P R, Oliveira M A, Cheng R H, Greve J M, McClelland A, Baker T S, Rossmann M G. Structure of a human rhinovirus complexed with its receptor molecule. Proc Natl Acad Sci USA. 1993;90:507–511. doi: 10.1073/pnas.90.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phelps D K, Post C B. A novel basis for capsid stabilization by antiviral compounds. J Mol Biol. 1995;254:544–551. doi: 10.1006/jmbi.1995.0637. [DOI] [PubMed] [Google Scholar]
- 21.Phelps D K, Rossky P J, Post C B. Influence of an antiviral compound on the temperature dependence of viral protein flexibility and packing: a molecular dynamics study. J Mol Biol. 1998;276:331–337. doi: 10.1006/jmbi.1997.1542. [DOI] [PubMed] [Google Scholar]
- 22.Smith T J, Kremer M J, Luo M, Vriend G, Arnold E, Kamer G, Rossmann M G, McKinlay M A, Diana G D, Otto M J. The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986;233:1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- 23.Smyth M, Tate J, Hoey E, Lyons C, Martin S, Stuart D. Implications for viral uncoating from the structure of bovine enterovirus. Struct Biol. 1995;2:224–231. doi: 10.1038/nsb0395-224. [DOI] [PubMed] [Google Scholar]
- 24.Speelman B, Brooks B R, Post C B. Molecular dynamics simulations of human rhinovirus and an antiviral compound. Biophysi J. 2001;80:121–129. doi: 10.1016/S0006-3495(01)75999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang S K, Danthi P, Chow M, Hogle J M. Stabilization of poliovirus by capsid-binding antiviral drugs is due to entropic effects. J Mol Biol. 2000;296:335–340. doi: 10.1006/jmbi.1999.3483. [DOI] [PubMed] [Google Scholar]
- 26.Wetz K, Kucinski T. Influence of different ionic and pH environments on structural alterations of poliovirus and their possible relation to virus uncoating. J Gen Virol. 1991;72:2541–2544. doi: 10.1099/0022-1317-72-10-2541. [DOI] [PubMed] [Google Scholar]
- 27.Xing L, Tjarnlund K, Lindqvist B, Kaplan G G, Feigelstock D, Cheng R H, Casasnovas J M. Distinct cellular receptor interactions in poliovirus and rhinoviruses. EMBO J. 2000;19:1207–1216. doi: 10.1093/emboj/19.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeates T O, Jacobson D H, Martin A, Wychowski C, Girard M, Filman D J, Hogle J M. Three-dimensional structure of a mouse-adapted type 2 / type 1 poliovirus chimera. EMBO J. 1991;10:2331–2341. doi: 10.1002/j.1460-2075.1991.tb07772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]