Abstract
Background
Toxoplasmosis is a chronic protozoan parasitic infection that affects nearly one-third of the global population. During the COVID-19 pandemic, cases were observed in patients with COVID-19 and toxoplasmosis. Therefore, this systematic review and meta-analysis aimed to determine the frequency of Toxoplasma gondii exposure in patients with COVID-19.
Methods
A literature search was conducted in six databases or search tools (PubMed, Scopus, Embase, Web of Science, ScienceDirect, and Google Scholar) until March 3, 2024. Study selection, quality assessment, and data extraction were performed independently by three investigators. Statistical analysis was performed using R version 4.3, applying a random-effects model. The quality of the included observational studies was assessed using the “JBI-MAStARI”.
Results
A total of 5,936 studies were retrieved, 13 of which were included in the final meta-analysis. The sample included a total of 2,947 patients with COVID-19 from four countries, of whom approximately 43.3% were men and 49.4% were women. Among the patients, 1,323 showed evidence of exposure to T. gondii through IgG detection, while 1,302 COVID-19 patients were explicitly examined for T. gondii by IgM detection, and 36 positive cases were identified. The frequency of exposure to T. gondii, determined by the presence of IgG in patients with COVID-19, reached 49% (95% CI: 34–63%; 2,947 participants; 13 studies; I2 = 98%, p < 0.01). In addition, the frequency of exposure to T. gondii, evaluated by IgM presence in patients with COVID-19, was 2% (95% CI: 0–6%; 1,302 participants; 6 studies; I2 = 94%, p < 0.01).
Conclusion
It was shown that almost half of COVID-19 patients had previous exposure to T. gondii through the presence of IgG, and a small percentage, 2%, showed active infection through IgM detection. Although the results indicate a possible correlation between exposure to T. gondii and the presence of COVID-19, it is essential to note that this study is based on observational research, which precludes establishing a causal relationship. Consequently, further research is required to deepen understanding of the interaction between the two conditions.
Terms used
The Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument (JBI-MAStARI), Prospective International Registry of Systematic Reviews (PROSPERO), and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20334-x.
Keywords: COVID-19, Toxoplasmosis, T. gondii, Epidemiology, Toxoplasma gondii
Introduction
The COVID-19 pandemic has triggered a global public health crisis, highlighting the need to understand the complexity of the interactions between the immune system and various pathogens [1]. In this context, the presence of co-infections that could influence the immune response during SARS-CoV-2 infection (severe acute respiratory syndrome coronavirus 2), such as dengue, malaria, and toxoplasmosis, has become evident [2, 3].
Toxoplasmosis is caused by the intracellular parasite Toxoplasma gondii (T. gondii), and its transmission occurs through oocysts, with the most common route being zoonotic transmission, followed by congenital transmission [4]. The prevalence varies by region, with the highest rates in Africa at 61.4%, Oceania at 38.5%, and South America at 31.2% [5]. In pregnant women, the prevalence has also been estimated at 33.8%, being more frequent in South America at 56% and in low-income areas with low human development indices [6]. That makes T. gondii a globally distributed parasite, and although clinical manifestations are rare, they can present with fever, myalgia, cervical lymphadenopathy, fatigue, and ocular involvement in the congenital form [7].
It is noteworthy that SARS-CoV-2 and T. gondii can stimulate innate immunity using a similar pathway. Indeed, both pathogens activate toll-like receptors (TLRs), such as TLR 2, TLR4, and TLR7, through the canonical pathway [8, 9]. In addition, some cytokines produced in patients with toxoplasmosis may aggravate the severity of COVID-19 [8–10].
The link between COVID-19 infection and T. gondii parasite exposure has emerged as an essential focus of the current study. This study aimed to determine the frequency of exposure to T. gondii in patients with COVID-19, marking a significant milestone in understanding interactions between different infectious diseases.
We have collected and analyzed relevant information through a comprehensive systematic review and meta-analysis. This process has shed light on the frequency of exposure, thus providing a clearer picture of the immune response in individuals with both conditions.
This analysis lays the groundwork for forthcoming research, enriching the scientific understanding of clinical care for COVID-19 patients. Examining the potential coexistence of other infections, such as toxoplasmosis, sheds light on significant implications that may influence the diagnosis and treatment of these individuals.
Materials and methods
Protocol and registration
The present research followed a protocol registered with PROSPERO and assigned the identification number CRD42023424490. The research followed PRISMA guidelines (see Additional file, Table S1).
Eligibility criteria
Inclusion criteria
Observational studies that investigated the frequency of T. gondii exposure in patients with COVID-19 were incorporated without imposing restrictions related to language, time period, or geographic location. We evaluated those fully available studies that provided relevant details on the frequency of T. gondii exposure detected by immunoglobulin G (IgG), immunoglobulin M (IgM), and polymerase chain reaction (PCR).
Exclusion criteria
Studies that did not provide information on our variables of interest were excluded. Likewise, articles that did not comply with an observational design, such as editorials, letters to the editor, randomized clinical trials, conference abstracts, narrative reviews, systematic reviews, and meta-analyses, were excluded. Those with incomplete or inaccessible information were also discarded.
Information sources and search strategy
Two investigators conducted comprehensive searches using six databases or search tools (PubMed, Scopus, Web of Science, Embase, ScienceDirect, and Google Scholar) to identify relevant articles on the frequency of T. gondii exposure in patients with COVID-19. The strategy used combined Medical Subject Headings (MeSH) phrases based on the Boolean operators (“COVID-19” OR “SARS-CoV-2 Infection” OR “COVID-19 Pandemic”) AND (“toxoplasmosis” OR “Toxoplasma gondii” OR “Toxoplasma”). The specific search strategies used for each database are detailed in the Additional file, Table S2. The initial search was performed on June 5, 2023, and updated on March 3, 2024. To ensure the reliability of the results, two other independent investigators performed a comprehensive review.
Study selection
Endnote software was used to construct a database based on the findings of the electronic search. Duplicate articles were eliminated at an initial stage. Then, three investigators independently reviewed article titles and abstracts to identify those that met the inclusion criteria. Subsequently, two other investigators comprehensively reviewed the full reports to determine their alignment with the inclusion criteria. Any discrepancies were resolved with the intervention of a sixth investigator.
Main result
To evaluate the frequency of T. gondii exposure detected by immunoglobulin IgG, IgM, and PCR in patients with COVID-19.
Quality assessment
The JBI-MAStARI was used to evaluate the quality of the articles included in the meta-analysis. The evaluation considered several aspects: the study context, outcome and explanatory variables, specific inclusion criteria, measurement standards, topic description, and exact statistical analysis. The quality of the studies was classified as high (≥ 7 points), moderate (4 to 6 points), or low (< 4 points) based on their score, and any discrepancies were resolved through dialogue among the researchers (see Additional file, Table S3).
Data collection process and data items
Two independent investigators were responsible for collecting the relevant data from the included articles and recording it in an Excel spreadsheet. The information collected included various details such as author, year of publication, study design, country of study and continent, number of participants affected by COVID-19, frequency of T. gondii in patients with COVID-19 identified by IgG, IgM, and PCR, gender, and age of participants, as well as comorbidities or signs and symptoms of the patients involved in the study. The methods used to diagnose COVID-19 and the number of deaths were also considered. It should be noted that data extraction was subjected to exhaustive review and verification by a third investigator to avoid the inclusion of duplicate data or incorrect information.
Data analysis
The data obtained from the selected articles was entered into a Microsoft Excel spreadsheet for subsequent analysis using R, version 4.2.3. Tables and narrative graphs were used to present the results of the investigation. The pooled frequency of T. gondii exposure in patients with COVID-19 was estimated using the inverse variance-weighted random effects model. Between-study heterogeneity was assessed using Cochrane’s Q statistic, and the I2 index determined its measure. Values of 25%, 50%, and 75% were considered low, moderate, and high heterogeneity indicators, respectively. Funnel plots and Egger’s test were used to analyze publication bias. The presence of a possible publication bias was interpreted when the p-value was less than 0.05.
Subgroup analyses were also performed, considering each study’s country of origin and continent. The frequency of T. gondii exposure in patients with COVID-19 was represented using a forest plot format, incorporating 95% confidence intervals.
Results
Study selection
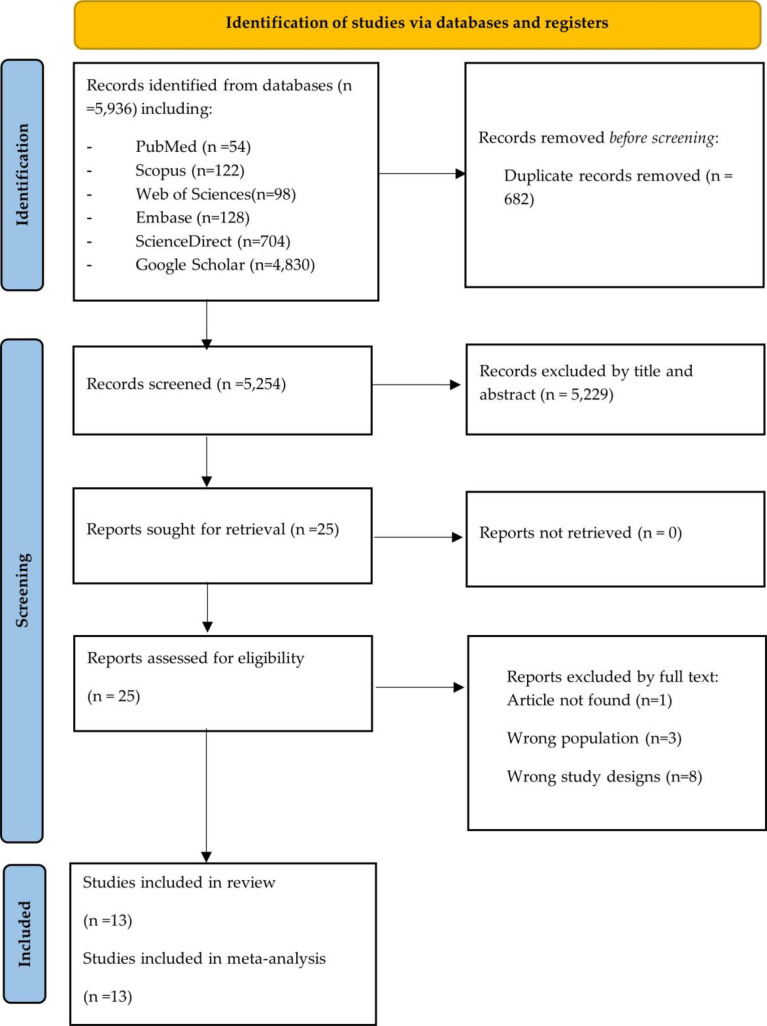
After applying the search method, 5,936 articles were identified, as described in the PRISMA flow diagram in Fig. 1. After eliminating duplicates (n = 682), the remaining 5254 articles were analyzed. Subsequently, a review of titles and abstracts was carried out, resulting in the selection of 25 articles for detailed full-text evaluation. Finally, after this rigorous process, 13 articles that met the established inclusion criteria were selected for further evaluation in this systematic review and meta-analysis [9, 11–22] (Fig. 1and see Additional file Table S4).
Fig. 1.
Selection process of studies according to the PRISMA flow diagram
Characteristics of the included studies
The analysis comprised thirteen observational studies published between 2021 and 2024, which investigated the frequency of T. gondii exposure in patients affected by COVID-19. A total of 2,947 patients with COVID-19 from four countries (Egypt, Iran, Saudi Arabia, and Mexico) were evaluated. Of this group, 1,323 patients had evidence of T. gondii exposure by IgG detection. Likewise, among the 1,302 COVID-19 patients tested, 36 cases were identified with the presence of T. gondii by IgM detection. Finally, of the 533 patients with COVID-19 screened by PCR, 21 cases were found to have T. gondii (Table 1) [9, 11–22]. Of the total number of patients, approximately 43.3% (1275) were male, 49.4% (1456) were female, and 7.3% (216) had no data recorded. The mean age of the participants was 58.48 years. The two most common comorbidities were arterial hypertension and diabetes mellitus. In addition, a total of 180 deaths were recorded. The technique used for the diagnosis of COVID-19 was reverse transcription-polymerase chain reaction (RT-PCR), while immunoglobulins (IgG and IgM) were used for the detection of T. gondii (Table 1) [9, 11–22].
Table 1.
Characteristics of the included studies on the frequency of exposure to Toxoplasma Gondii in patients with COVID-19
| Authors | Year | Study design | Country and continent | Participants with COVID-19 (n) | T. gondii in patients with COVID-19 | Sex | Age (Years) |
Comorbidities / Signs and symptoms | COVID19 diagnosis | Deaths | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | PCR | M | F | |||||||||||
| Abdel-Hamed EF, et al. (11) | 2021 | Cross sectional | Egypt/Africa | 375 | 58 | NS | NR | 246 | 129 | Mean: 44.72 | HTN (12), DM (12), DM and HTN (8), Chronic heart disease (21), and Chronic lung disease (74) | RT-PCR | NR | ||
| Sharaf-El-Deen SA, et al. (12) | 2021 | Case-control | Egypt/Africa | 100 | 54 | NS | NR | 54 | 46 | Over 50 years old | 58 patients with comorbidities | RT-PCR | NR | ||
| Ghaffari S, et al. (13) | 2021 | Cross sectional | Iran/Asia | 269 | 226 | NR | NR | 105 | 164 | Mean: 60.9 | Bleeding (58), Trombose (13), Cough (217), Dyspnea (150), Fever (221), Gastrointestinal signs (45), Muscular Pain (150), and Headache (156) | RT-PCR | NR | ||
| Halawi M, et al. (14) | 2021 | Cross sectional | Saudi Arabia/Asia | 417 | 268 | 2 | NR | 150 | 267 | < 30 (153), 30–50 (234), > 50 (30) | NR | RT-PCR | NR | ||
| Geraili A, et al. (15) | 2022 | Cross sectional | Iran/Asia | 161 | 42 | 8 | NR | 80 | 81 | Mean: 50.0 |
Fever > 38 °C (39), Cough (41), Shortness of breath (60) HTN (20), Chronic heart disease (25), and Chronic pulmonary disease (13) |
RT-PCR | 113 | ||
| Montazeri M, et al. (16) | 2022 | Cross sectional | Iran/Asia | 133 | 109 | 0 | 0 | 63 | 70 | Mean: 58.95 | DM/HTN/CHD (76) | RT-PCR | 14 | ||
| Galván-Ramírez MdlL, et al. (9) | 2023 | Cross sectional | Mexico/America | 384 | 105 | 26/191 | NR | 142 | 242 | Mean: 47.31 | HTN (2), DM (24), respiratory diseases (2), and Cardiac Diseases (1) | IgG | NR | ||
| Habib S, et al. (17) | 2023 | Cross sectional | Egypt/Africa | 44 | 33 | NR | NR | 31 | 13 | Range: 50-75.5 | HTN (3), DM (4), Renal (2), Hepatic (4), Thyroid (2), deep vein thrombosis (1), Hernia (1), Gout (1), Cardiac (1), and Oncology (1) | RT-PCR | NR | ||
| Mahmoudi MR, et al. (18) | 2023 | Cross sectional | Iran/Asia | 210 | 155 | NR | NR | 111 | 99 | Mean: 60.9 | NR | RT-PCR | 48 | ||
| Gouda MA, et al. (19) | 2023 | Cross sectional | Egypt/Africa | 330 | 114 | 0 | 11 | 42/114 | 72/114 |
Mean: 77,14 (M) Mean: 94.90 (F) |
HTN (18), DM (11), Asthma (2) and Cardiac (5) | RT-PCR | 3 | ||
| Gouda MA, et al. (20) | 2023 | Case-control | Egypt/Africa | 375 | 108 | NS | NR | 154 | 221 | Range: 14–80 | HTN (40), DM (20), Asthma (8), and Cardiac (8) | RT-PCR | 2 | ||
| Hasanzadeh M, et al. (21) | 2024 | Case-control | Iran/Asia | 70 | 25 | 0 | 10 | 56 | 14 | Mean: 36 | NR | RT-PCR | NR | ||
| Abdeltawab MSA, et al. (22) | 2024 | Case-control | Egypt/Africa | 79 | 26 | NR | NR | 41 | 38 | Mean: 56,4 | NR | RT-PCR | NR | ||
M/F: Male/Female¸ NS: Not specified; RT-PCR: real-time polymerase chain reaction, IgG: Immunoglobulin G, IgM: Immunoglobulin M, NR: Not reported; HTN: Hypertension; DM: Diabetes
Quality of the included studies and publication bias
The studies’ quality was evaluated using the JBI Critical Appraisal Tools for cross-sectional research. All the studies were of moderate quality (see Additional file, Table S3) [9, 11–22]. Egger’s test for evaluating publication bias obtained a value of p = 0.4301 (t = 0.82, df = 11). This result suggests that the null hypothesis of symmetry is accepted, indicating no evidence of publication bias in the studies examined (see Additional file Figure S1).
Frequency of T. Gondii exposure in patients with COVID-19
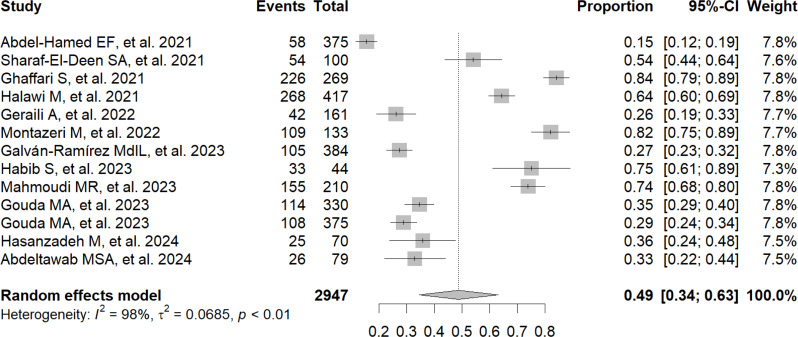
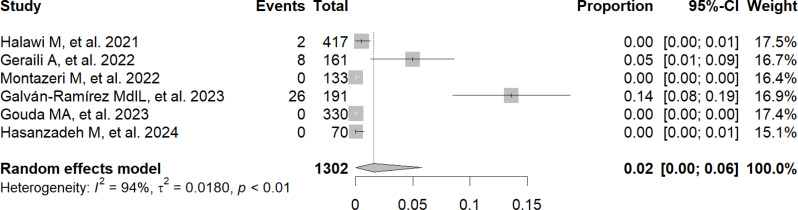
The frequency of exposure to T. gondii, detected through the presence of IgG in patients with COVID-19, reached 49% (95% CI: 34–63%; 2,947 participants; 13 studies; I2 = 98%, p < 0.01) (Fig. 2) [9, 11–22]. In addition, the frequency of exposure to T. gondii, detected through the presence of IgM in patients with COVID-19, reached 2% (95% CI: 0–6%; 1,302 participants; 6 studies; I2 = 94%, p < 0.01) (Fig. 3) [9, 14–16, 20, 21].
Fig. 2.
Forest plot showing the frequency of exposure to Toxoplasma gondii by IgG antibody detection in patients with COVID-19
Fig. 3.
Forest plot showing the frequency of exposure to Toxoplasma gondii by IgM antibody detection in patients with COVID-19
Subgroup analysis by country and continent according to IgG antibody detection
A subgroup analysis by country was performed, which revealed that the frequency of T. gondii exposure in patients with COVID-19 in Iran reached 62% (95% CI: 37–83%; 843 participants; 5 studies; I2 = 98%, p < 0.01) [13, 15, 16, 18, 21], while in Egypt it was 38% (95% CI: 26–52%; 1303 participants; 6 studies; I2 = 96%, p < 0.01) [11, 12, 17, 19, 20, 22] (see Additional file, Figure S2). Subgroup analysis by continent showed that the frequency of T. gondii exposure in patients with COVID-19 in Africa was 38% (95% CI: 26–52%; 1303 participants; 6 studies; I² = 96%, p < 0.01) [11, 12, 17, 19, 20, 22], while in Asia it reached 62% (95% CI: 44–79%; 1260 participants; 6 studies; I² = 98%, p < 0.01) [13–16, 18, 21] (see Additional file, Figure S3).
Discussion
Over the past four years, COVID-19 has triggered a global crisis, impacting multiple aspects. One of them is the uncertainty of co-infections with other viral diseases, such as chikungunya [23], Zika [23], dengue [2], and Mpox [24, 25], as well as parasitic diseases such as malaria [26], Chagas disease [27, 28], and even toxoplasmosis [3]. This complex and multifaceted scenario has accentuated the challenges in public health management, especially in low- and middle-income countries, underscoring the need for integrated strategies and coordinated approaches to address the various dimensions of this pandemic and its interactions with other prevalent diseases [29, 30].
According to IgG detection, 49% of COVID-19 patients were exposed to T. gondii in this systematic review and meta-analysis. When analyzing the data in detail by country, it was found that the frequency of exposure in patients with COVID-19 in Iran reached 62%, compared to 38% in Egypt. The detection of IgG in these patients with COVID-19 is interpreted as evidence of previous exposure to T. gondii. In previous investigations, a 54% prevalence of toxoplasmosis was observed among COVID-19 patients registered in Egypt [12]. Another study conducted by Habib S. et al. in the same country noted that 75% of patients with COVID-19 had toxoplasmosis [17]. In Iran, Ghaffari et al. found that 84% of patients with COVID-19 had anti-Toxoplasma gondii antibodies (IgG) [13]. In contrast, Geraili et al. reported a prevalence rate of 26.1% in northern Iran [15]. In Saudi Arabia, Halawi M. et al. reported that 64.3% of patients with COVID-19 had toxoplasmosis [14]. On the other hand, a similar study was proposed by Galván-Ramírez MdlL. et al. in Mexico and revealed that 21.9% of patients with COVID-19 showed the presence of T. gondii [9].
Another important result was that 2% of patients diagnosed with COVID-19 showed evidence of active T. gondii infection, which was assessed by IgM detection. In their study, Galván-Ramírez MdlL et al. reported that, when analyzing 191 patients with COVID-19 by IgM detection, 13.6% were found to have an active T. gondii infection [9]. However, studies by Gouda MA et al. [19] and Hasanzadeh M et al. [21] indicated the absence of active T. gondii infection in patients with COVID-19 by IgM detection.
The variations in the prevalence of toxoplasmosis in patients with COVID-19 observed in different studies can be explained by a combination of environmental factors, such as temperature, humidity, and geographical characteristics of the regions studied, as well as sociodemographic factors, including the age distribution of the populations analyzed [19, 31].
The results of this investigation document a remarkable prevalence of previous or active toxoplasmosis in patients with COVID-19 but do not allow us to establish how T. gondii and SARS-CoV-2 interact. These findings underscore the need for further research exploring potential interactions between the two pathogens and their impact on public health. One hypothesis to explore is that SARS-CoV-2 infection could reduce the number of T cells, natural killer (NK) cells, monocytes, and dendritic cells, as well as the production of interferon-gamma (IFN-γ), essential for the immune response against T. gondii, which would increase the risk of acute toxoplasmosis or its reactivation [9].
Another possible hypothesis would be that T. gondii infection may increase the risk of SARS-CoV-2 infection and aggravate the severity of COVID-19 due to its ability to modify the host immune response. This immunomodulation, through alteration of key cytokines such as interleukin (IL)-10, IL-5, IL-6, and transforming growth factor beta (TGF-β), could weaken the immune system’s ability to fight viral infection, thereby facilitating the replication and spread of SARS-CoV-2 [31].
It is essential to implement comprehensive epidemiological surveillance, adapted to the particularities of each geographical region, to obtain a more detailed knowledge of the associated risks. This will allow the development of more effective management strategies and the adoption of preventive measures appropriate to the specific circumstances of each region [32].
Several authors propose strengthening public health programs through a comprehensive approach that addresses not only the management of the COVID-19 pandemic but also the surveillance of concurrent infections such as toxoplasmosis [33–35]. Educational campaigns aimed at the population to promote specific preventive measures to reduce the transmission of both diseases are also highlighted. International collaboration is key in this context, as it facilitates the dissemination of data and good practices, the coordination of efforts in research, and the development of public health strategies [36, 37].
This study has several important limitations. First, few studies were identified that specifically address the frequency of exposure to T. gondii in patients with COVID-19. Second, the included studies presented variable sample sizes, ranging from 44 to 417 patients, which could generate heterogeneity, affecting the consistency of the results, making direct comparison between studies difficult, and limiting the robustness of the conclusions. Thirdly, the high heterogeneity between studies (I² > 75%) suggests variability in the methodologies and populations analyzed. Fourth, it is essential to consider the presence of possible biases and confounding factors in the included studies. Fifth, the analysis was limited to subgroups of countries and continents due to the lack of specific data on T. gondii exposure in COVID-19 patients, which precluded assessment by sex and age. Sixth, the studies do not provide detailed information on clinical manifestations in patients with COVID-19 and toxoplasmosis.
Concerning its positive aspects, this study is positioned as the first systematic review that comprehensively analyzes the frequency of exposure of T. gondii in patients with COVID-19, using a rigorous methodological approach following the guidelines proposed by the PRISMA guides. In addition, all the procedures used to select studies were carried out independently by two or more authors.
Conclusions
It was shown that almost half of COVID-19 patients had previous exposure to T. gondii through the presence of IgG, and a small percentage, 2%, showed active infection through IgM detection. Although the results indicate a possible correlation between exposure to T. gondii and the presence of COVID-19, it is essential to note that this study is based on observational research, which precludes establishing a causal relationship. Consequently, further research is required to deepen understanding of the interaction between the two conditions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Table S1: PRISMA Checklist (PRISMA 2020 Main Checklist and PRIMSA Abstract Checklist)
Supplementary Material 2: Table S2: The adjusted search terms as per searched electronic databases
Supplementary Material 3: Table S3: Quality of the included studies
Supplementary Material 4: Table S4: Articles excluded after a thorough review of the complete text
Supplementary Material 5: Figure S1: The funnel plot illustrates the publication bias of the included studies on the frequency of T. gondii exposure by detection of IgG antibodies in patients with COVID-19
Supplementary Material 6: Figure S2. Country-based subgroup analysis of the frequency of T. gondii exposure by IgG antibody detection in patients with COVID-19
Supplementary Material 7: Figure S3. Subgroup analysis by continent of the frequency of exposure to T. gondii by IgG antibody detection in patients with COVID-19
Acknowledgements
Not applicable.
Author contributions
Conceptualization, DALF; methodology, JJB and RS; software, DALF; validation, AS, RS and JGMR; formal analysis, DALF; investigation, MJVG; resources, RS; data curation, DALF; writing—original draft preparation, DALF, MJVG, SA, ARM, DKBA, JJB and RS; writing—review and editing, DALF, JJB, AS, EAM, CQV, MJVG, SA, ARM, JGMR, DKBA, AJRM and RS; visualization, RS; supervision, JJB and EAM. All au-thors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mallah SI, Ghorab OK, Al-Salmi S, Abdellatif OS, Tharmaratnam T, Iskandar MA, et al. COVID-19: breaking down a global health crisis. Ann Clin Microbiol Antimicrob. 2021;20(1):35. 10.1186/s12941-021-00438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.León-Figueroa DA, Abanto-Urbano S, Olarte-Durand M, Nuñez-Lupaca JN, Barboza JJ, Bonilla-Aldana DK, et al. COVID-19 and dengue coinfection in Latin America: a systematic review. New Microbes New Infect. 2022;49:101041. 10.1016/j.nmni.2022.101041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–75. 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prevention, CC for DC and. CDC - Toxoplasmosis - Epidemiology & Risk Factors [Internet]. 2019. Available online: https://www.cdc.gov/parasites/toxoplasmosis/epi.html (accessed on 21 May 2023).
- 5.Molan A, Nosaka K, Hunter M, Wang W. Global status of Toxoplasma gondii infection: systematic review and prevalence snapshots. Trop Biomed. 2019;36(4):898–925. [PubMed] [Google Scholar]
- 6.Rostami A, Riahi SM, Gamble HR, Fakhri Y, Nourollahpour Shiadeh M, Danesh M, et al. Global prevalence of latent toxoplasmosis in pregnant women: a systematic review and meta-analysis. Clin Microbiol Infect. 2020;26(6):673–83. 10.1016/j.cmi.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Dubey JP. Outbreaks of clinical toxoplasmosis in humans: five decades of personal experience, perspectives and lessons learned. Parasit Vectors. 2021;14(1):263. 10.1186/s13071-021-04769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jankowiak Ł, Rozsa L, Tryjanowski P, Møller AP. A negative covariation between toxoplasmosis and CoVID-19 with alternative interpretations. Sci Rep. 2020;10(1):12512. 10.1038/s41598-020-69351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galván-Ramírez M, de la Salas-Lais L, Muñoz-Medina AG, Fernandes-Matano JE, Pérez L. Franco De León K. Association of Toxoplasmosis and COVID-19 in a Mexican Population. Microorganisms. 2023;11(6):1441. 10.3390/microorganisms11061441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarovinsky F. Innate immunity to Toxoplasma Gondii infection. Nat Rev Immunol. 2014;14(2):109–21. 10.1038/nri3598. [DOI] [PubMed] [Google Scholar]
- 11.Abdel-Hamed EF, Ibrahim MN, Mostafa NE, Moawad HSF, Elgammal NE, Darwiesh EM, et al. Role of interferon gamma in SARS-CoV-2-positive patients with parasitic infections. Gut Pathog. 2021;13(1):29. 10.1186/s13099-021-00427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharaf-El-Deen SA. Toxoplasma Gondii as a possible risk factor for COVID-19 severity: a case-control study. Egypt J Med Microbiol. 2021;30(2):125–32. [Google Scholar]
- 13.Ghaffari S, Kalantari N, Gorgani-Firouzjaee T, Bayani M, Jalali F, Daroonkola MA. Is COVID-19 associated with latent toxoplasmosis? Environ Sci Pollut Res Int diciembre de. 2021;28(47):67886–90. 10.1007/s11356-021-17126-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halawi M, Al-Hazmi A, Aljuaid A, Allahyani M, Abdulaziz O, Almalki A. Seroprevalence of Toxoplasma Gondii, Rubella, Group A Streptococcus, CMV and HSV-1 in COVID-19 patients with Vitamin D Deficiency. Pak J Biol Sci PJBS. 2021;24(11):1169–74. 10.3923/pjbs.2021.1169.1174. [DOI] [PubMed] [Google Scholar]
- 15.Geraili A, Badirzadeh A, Sadeghi M, Mousavi SM, Mousavi P, Shahmoradi Z, et al. Toxoplasmosis and symptoms severity in patients with COVID-19 in referral centers in Northern Iran. J Parasit Dis off Organ Indian Soc Parasitol. 2022;47(1):1–7. 10.1007/s12639-022-01556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montazeri M, Nakhaei M, Fakhar M, Pazoki H, Pagheh AS, Nazar E, et al. Exploring the Association between Latent Toxoplasma Gondii Infection and COVID-19 in hospitalized patients: First Registry-based study. Acta Parasitol. 2022;67(3):1172–9. 10.1007/s11686-022-00559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib S, Hamza E, El-Gamal R, Nosser NA, Aboukamar WA, Abdelsalam S, et al. Clinical and immunological impacts of latent toxoplasmosis on COVID-19 patients. Cureus. 2023;15(9):e45989. 10.7759/cureus.45989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmoudi MR, Saadat F, Yaghubi Kalurazi T, Ali Verdiloo F, Karanis P. Latent toxoplasmosis in COVID-19 patients and link with higher mortality in COVID-19 male patients. Microb Pathog. 2023;185:106402. 10.1016/j.micpath.2023.106402. [DOI] [PubMed] [Google Scholar]
- 19.Gouda MA, AboShabaan HS, Abdelgawad AS, Abdel Wahed AS, Abd El-Razik A, Elsaadawy K. Association between breakthrough infection with COVID-19 and Toxoplasma Gondii: a cross-sectional study. Sci Rep. 2023;13(1):17636. 10.1038/s41598-023-44616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouda MA, Aboshabaan HS, Abdelgawad AS, Wahed ASA, Ibrahim AF. TOXOPLASMOSIS IN VACCINATED COVID-19 PATIENTS. J Egypt Soc Parasitol. 2023;53(2):381–6. 10.21608/jesp.2023.312186. [Google Scholar]
- 21.Hasanzadeh M, Ahmadpour E, Mahami-Oskouei M, Musavi S, Parsaei M, Sarafraz N, et al. Genetic diversity and seroprevalence of Toxoplasma Gondii in COVID–19 patients; a first case-control study in Iran. BMC Infect Dis. 2024;24(1):42. 10.1186/s12879-023-08964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdeltawab MSA, Fateen M, Saad El-Din S, Elmessiery RM, Mohammady Mohamed O, Marzouk Sadek K, et al. Effect of SARS-CoV-2 and Toxoplasma Gondii co-infection on IFN-γ and TNF-α expression and its impact on disease severity. Cytokine. 2024;177:156545. 10.1016/j.cyto.2024.156545. [DOI] [PubMed] [Google Scholar]
- 23.Nemati Zargaran F, Rostamian M, Kooti S, Madanchi H, Ghadiri K. Co-infection of COVID-19 and parasitic diseases: a systematic review. Parasite Epidemiol Control. 2023;21:e00299. 10.1016/j.parepi.2023.e00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Qushayri AE, Reda A, Shah J. COVID-19 and monkeypox co-infection: a rapid systematic review. Front Immunol. 2022;13:1094346. 10.3389/fimmu.2022.1094346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Morales AJ, León-Figueroa DA, Sah R, Villamil-Gomez WE. Arboviral diseases and monkeypox – an epidemiological overlapping differential diagnosis? Rev Cuerpo Méd Hosp Nac Almanzor Aguinaga Asenjo. 2022;15(3):323–4. 10.35434/rcmhnaaa.2022.153.1678. [Google Scholar]
- 26.Wilairatana P, Masangkay FR, Kotepui KU, Milanez GDJ, Kotepui M. Prevalence and characteristics of malaria among COVID-19 individuals: a systematic review, meta-analysis, and analysis of case reports. PLoS Negl Trop Dis. 2021;15(10):e0009766. 10.1371/journal.pntd.0009766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins-Melo FR, Castro MC, Ribeiro ALP, Heukelbach J, Werneck GL. Deaths related to Chagas Disease and COVID-19 Co-infection, Brazil, March-December 2020. Emerg Infect Dis. 2022;28(11):2285–9. 10.3201/eid2811.212158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Morales AJ, Romaní L, León-Figueroa DA. COVID-19 and Chagas disease in Latin America. Rev Cuerpo Méd Hosp Nac Almanzor Aguinaga Asenjo. 2022;15(2):171–3. 10.35434/rcmhnaaa.2022.152.1499. [Google Scholar]
- 29.Baker RE, Mahmud AS, Miller IF, Rajeev M, Rasambainarivo F, Rice BL, et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022;20(4):193–205. 10.1038/s41579-021-00639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitlik SD. COVID-19 compared to other Pandemic diseases. Rambam Maimonides Med J. 2020;11(3):e0027. 10.5041/RMMJ.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flegr J. Toxoplasmosis is a risk factor for acquiring SARS-CoV-2 infection and a severe course of COVID-19 in the Czech and Slovak population: a preregistered exploratory internet cross-sectional study. Parasit Vectors. 2021;14(1):508. 10.1186/s13071-021-05021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Windle M, Lee HD, Cherng ST, Lesko CR, Hanrahan C, Jackson JW, et al. From epidemiologic knowledge to Improved Health: a vision for translational epidemiology. Am J Epidemiol. 2019;188(12):2049–60. 10.1093/aje/kwz085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayouni I, Maatoug J, Dhouib W, Zammit N, Fredj SB, Ghammam R, et al. Effective public health measures to mitigate the spread of COVID-19: a systematic review. BMC Public Health. 2021;21(1):1015. 10.1186/s12889-021-11111-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ros F, Kush R, Friedman C, Gil Zorzo E, Rivero Corte P, Rubin JC, et al. Addressing the Covid-19 pandemic and future public health challenges through global collaboration and a data-driven systems approach. Learn Health Syst. 2021;5(1):e10253. 10.1002/lrh2.10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haldane V, Jung AS, De Foo C, Bonk M, Jamieson M, Wu S, et al. Strengthening the basics: public health responses to prevent the next pandemic. BMJ. 2021;375:e067510. 10.1136/bmj-2021-067510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguirre AA, Longcore T, Barbieri M, Dabritz H, Hill D, Klein PN, et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. EcoHealth. 2019;16(2):378–90. 10.1007/s10393-019-01405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roe K. The role of polyspecific T-cell exhaustion in severe outcomes for COVID-19 patients having latent pathogen infections such as Toxoplasmagondii. Microb Pathog. 2021;161(Pt B):105299. 10.1016/j.micpath.2021.105299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Table S1: PRISMA Checklist (PRISMA 2020 Main Checklist and PRIMSA Abstract Checklist)
Supplementary Material 2: Table S2: The adjusted search terms as per searched electronic databases
Supplementary Material 3: Table S3: Quality of the included studies
Supplementary Material 4: Table S4: Articles excluded after a thorough review of the complete text
Supplementary Material 5: Figure S1: The funnel plot illustrates the publication bias of the included studies on the frequency of T. gondii exposure by detection of IgG antibodies in patients with COVID-19
Supplementary Material 6: Figure S2. Country-based subgroup analysis of the frequency of T. gondii exposure by IgG antibody detection in patients with COVID-19
Supplementary Material 7: Figure S3. Subgroup analysis by continent of the frequency of exposure to T. gondii by IgG antibody detection in patients with COVID-19
Data Availability Statement
Data is provided within the manuscript or supplementary information files.