Abstract
The catalytic subunit, Pol, of herpes simplex virus DNA polymerase interacts via its extreme C terminus with the processivity subunit, UL42. This interaction is critical for viral replication and thus a potential target for antiviral drug action. To investigate the Pol-binding region on UL42, we engineered UL42 mutations but also used random peptide display to identify artificial ligands of the Pol C terminus. The latter approach selected ligands with homology to residues 171 to 176 of UL42. Substitution of glutamine 171 with alanine greatly impaired binding to Pol and stimulation of long-chain DNA synthesis by Pol, identifying this residue as crucial for subunit interactions. To study these interactions quantitatively, we used isothermal titration calorimetry and wild-type and mutant forms of Pol-derived peptides and UL42. Each of three peptides corresponding to either the last 36, 27, or 18 residues of Pol bound specifically to UL42 in a 1:1 complex with a dissociation constant of 1 to 2 μM. Thus, the last 18 residues suffice for most of the binding energy, which was due mainly to a change in enthalpy. Substitutions at positions corresponding to Pol residue 1228 or 1229 or at UL42 residue 171 abolished or greatly reduced binding. These residues participate in hydrogen bonds observed in the crystal structure of the C terminus of Pol bound to UL42. Thus, interruption of these few bonds is sufficient to disrupt the interaction, suggesting that small molecules targeting the relevant side chains could interfere with Pol-UL42 binding.
Protein-protein interactions are crucial determinants of biological specificity, and the selective disruption of these interactions has become an important goal of new drug research. A potential target for this type of drug is the DNA polymerase encoded by herpes simplex virus (HSV), which is a heterodimer composed of a catalytic subunit, Pol, and a processivity subunit, UL42. Although UL42 is not required for catalysis by Pol, it is necessary for the synthesis of long-chain DNA products by Pol in vitro and for viral DNA replication in cultured cells (13, 21). In addition to binding Pol, UL42 also binds in a non-sequence-specific manner to double-stranded DNA (ds DNA) (15, 19). UL42 mutants specifically impaired for binding Pol and Pol mutants specifically impaired for binding UL42 are unable to support long-chain DNA synthesis in vitro and are also unable to complement the replication of UL42 and pol null mutant viruses, respectively (5, 6). The importance of the UL42-Pol interaction for viral replication makes it an attractive target for antiviral drug discovery.
The extreme C terminus of Pol is necessary for specific interaction with UL42 (5, 14, 16, 18). Moreover, peptides corresponding to the last 36 (peptide A), 27 (here termed peptide G), and 18 (peptide E) residues of Pol can inhibit the ability of UL42 to stimulate the synthesis of long DNA chains by Pol in vitro (3, 8, 12, 14; unpublished results). Studies of peptide E identified two substitutions that abolish its ability to inhibit long-chain DNA synthesis without disrupting peptide structure — histidine-to-alanine and glutamine-to-alanine substitutions at positions corresponding to Pol residues 1228 and 1229 (H1228A and R1229A), respectively (3). It has been assumed that peptides A, E, and G exert their inhibitory activity by binding to UL42 and thus that the H1228A and R1229A substitutions prevent binding to UL42. In support of this assumption, immobilized fusion proteins consisting of peptide G fused to the B subunit of Escherichia coli heat-labile enterotoxin (11) or peptide A fused to glutathione S-transferase (22) can bind to UL42. Moreover, peptide A forms a complex with UL42 that can be visualized by X-ray crystallography (14). However, in these cases, binding was not shown to be specific to both binding partners, for example, by examining control or mutant proteins. Thus, it has not yet been demonstrated that the extreme C terminus of Pol is sufficient to bind specifically to UL42 in solution. Additionally, studies of the energetics of this interaction, including measurements of binding affinity, have not been reported.
In contrast to our relatively detailed understanding of the UL42-binding surface on Pol, identification of UL42 residues required for Pol binding has lagged. Of more than 50 linker-insertion and deletion mutants of UL42 analyzed, the only one specifically impaired for Pol binding but not DNA binding contains a 4-residue insertion at position 160 (6). Residues 148 to 163 of UL42 are predicted to be hydrophilic, suggesting that they may be surface exposed and accessible to the C terminus of Pol (6). These findings led us to hypothesize that this region formed the Pol-binding surface of UL42. To test this hypothesis, we constructed and characterized mutations in this region. We also undertook a combinatorial approach to identify artificial ligands of the UL42-binding surface of Pol as a starting point for drug discovery. This latter approach unexpectedly led to the identification of UL42 residues crucial for Pol binding.
Then, to further assess the specificity and directly quantify the binding of the C terminus of Pol to UL42 and to examine the contributions to binding energy of specific residues, isothermal titration calorimetry (ITC) assays were performed with wild-type (wt) and mutant forms of UL42 and peptides derived from the C terminus of Pol. These assays yielded binding affinities and stoichiometries, thermodynamic parameters, and the effects of specific substitutions on these values. Interestingly, although numerous hydrophobic interactions are observed in the crystal structure of peptide A bound to UL42 (22), the present results identify specific hydrogen bonds as crucial determinants of binding energy, information that may aid in the design of new antiviral compounds.
MATERIALS AND METHODS
Plasmids.
The UL42 insertion mutation at codon 152 (I-152) was created by linearizing pINGUL42 (6) by partial digestion with NdeI, end-filling with the Klenow fragment of Escherichia coli DNA polymerase I, and insertion of the oligonucleotide linker TGCATCGATGCA, which contains an internal ClaI site. An internal deletion mutant (Δ153–160), which has codons 153 to 160 deleted, was constructed by digesting the I-152 and I-160 mutants with ClaI and HindIII and ligating the 5′ coding fragment from I-152 with the 3′ coding fragment from I-160. Therefore, Δ153–160 contains a 4-amino-acid insertion at the deletion site. Plasmid pGEX-PD3, which encodes the last 36 residues of HSV Pol fused to gluathione S-transferase (GST) was kindly provided by Z. He of this laboratory and has been described previously (22). The pGex6p (Invitrogen) plasmid was used to express GST alone. To express and purify UL42 in E. coli, plasmids encoding UL42 fused to maltose-binding protein (MBP) were used. Because full-length MBP-UL42 aggregates in E. coli (unpublished results), wt and mutant versions of UL42 were truncated either at residue 320 or at residue 340, based on previous studies demonstrating that such truncated proteins retain all known biochemical activities of UL42 (6, 9, 17). Plasmid MBPUL42ΔC340, which contains the N-terminal 340 residues of UL42 cloned into the XbaI and HindIII sites of the pMal-c vector (New England Biolabs), and plasmid MBPUL42ΔC320 (22) were generously provided by D. Wilson and H. Zuccola, respectively, of the Hogle laboratory. The MBPUL42ΔC340/I-160 plasmid was constructed by cutting the PstI fragment from pIngUL42/I-160 (6) and using it to replace the corresponding fragment in MBPUL42ΔC340. The MBPUL42ΔC320/Q171A mutant was constructed by sequential PCR (1) using the mutagenic primers GTGGTGCTGGTTCCCGCGGGAACCCCCGACGTTC and GAACGTCGGGGGTTCCCGCGGGAACCAGCAC and the forward and reverse primers TCGGATCCATGACGGATTCC and CGACCCGGGGAATTCTGCGGC, respectively, with the MBPUL42ΔC320 vector as the template. The resulting products were cloned into the BamHI and SmaI sites of a modified pMal-c2 vector, pMBP-PP (a generous gift of A. Pearson of this laboratory), in which the factor X cleavage site in the pMal-c vector has been replaced with a Prescission protease site. The inserted DNA was sequenced by the Biopolymers Laboratory in the Department of Biological Chemistry and Molecular Pharmacology at Harvard Medical School and shown to contain wt sequences except for the engineered mutation.
In vitro transcription-translation and immunoprecipitation.
In vitro transcription-translation and coimmunoprecipitation of radiolabeled UL42 deletion and insertion mutant proteins with unlabeled HSV Pol or E. coli β-galactosidase (β-Gal) were performed as described previously (4). In vitro transcription-translation of full-length Pol was performed using the TNT coupled reticulocyte lysate system from Promega according to the manufacturer's suggestion. The template used in the reactions was a PCR product generated using the forward primer TAATACGACTCACTATAGGGACCGCGATGTTTTCCGGTGGCGGCGC GGCC, which contains the T7 promoter, and the reverse primer TCATGCTA GAGTATCAAAGGCTCTATGCAACATTCGACGAGTTTCCTCCGCCG. Plasmid pINGUL30 (7) was used as the template for PCR. The control luciferase plasmid was provided with the kit. The translation products were labeled with [35S]methionine and [35S]cysteine.
Protein purification.
Purified HSV Pol, prepared as described (20), was kindly provided by K. Kumura-Ishii and K. Weisshart of this laboratory. Full-length UL42 was purified from insect cells infected with the appropriate recombinant baculovirus as described previously (8). Recombinant MBP-UL42 fusion proteins, GST, and GST-peptide A were purified from E. coli BL21(de3)pLysS harboring the appropriate plasmid. For UL42 fusion proteins, after induction, cells were freeze-thawed, resuspended in buffer A (30 mM Tris-Cl [pH 7.5], 2 mM dithiothreitol [DTT], 500 mM NaCl, 0.5 mM EDTA, 5% glycerol, and Complete protease inhibitors [Roche Molecular Biochemicals]), and lysed by sonication with a Branson sonifier until lysis was visibly complete. The proteins were applied to amylose columns (New England Biolabs), washed extensively with buffer B (buffer A minus the Complete tablets and containing only 50 mM NaCl), and eluted with buffer B contaninig 10 mM maltose. Proteins eluted from amylose columns were applied to dsDNA-cellulose (Sigma) columns, washed with buffer B, and eluted with buffer B containing 500 mM NaCl. For GST and GST-peptide A, bacteria were lysed in buffer B containing 5 mM EDTA, and extracts were applied to glutathione-Sepharose columns (Pharmacia) and eluted with 5 mM reduced glutathione in buffer B. The eluted proteins were further purified on anion-exchange columns and eluted with a linear NaCl gradient from 50 to 500 mM in buffer B. Purified proteins were stored in buffer B. Concentrations of UL42 fusion proteins were determined using A280 values and extinction coefficients derived from amino acid analysis of MBP-UL42ΔC320 standards.
Random peptide display.
The GST-peptide A fusion protein and GST were allowed to bind glutathione-coated plates (Pierce) and washed extensively with buffer B. Panning experiments were performed with the FliTrx random peptide display library (Invitrogen) containing approximately 1.7 × 108 primary clones displayed on bacterial flagella, and the Ph.D.-12 peptide library kit (New England Biolabs), containing approximately 1.9 × 109 primary clones displayed on the minor coat protein pIII of M13, as suggested by the manufacturers. The following modifications were made to the FliTrx protocol for 96-well plates: after overnight growth of the primary FliTrx library and a 6-h induction with tryptophan (100 μg/ml), dry milk was added to the bacterial suspension to a final concentration of 1% and NaCl was added to a final concentration of 150 mM. This suspension was then placed on the GST-peptide A-coated plates and incubated for 1 h at 4°C with gentle shaking. The plates were washed six times with 30 mM Tris-Cl (pH 7.5)–0.5 mM EDTA–50 mM NaCl–1 mM DTT. Bound bacteria were eluted with 2 μM UL42, and the remaining bacteria were eluted with vigorous vortexing as described in the manual. The eluted bacteria were allowed to bind GST-coated plates, and only the unbound fractions were amplified. After four biopanning cycles, individual clones were isolated, and their inserts were sequenced. The same modifications in buffer system and temperature were made to the phage display protocol. With this system, phage that did not elute with UL42 were eluted with 1 M NaCl. Five panning cycles were performed with the Ph.D.-12 library.
Binding of radiolabeled Pol to UL42.
The indicated MBP-UL42 wt or mutant fusion protein (0.3 mg) was loaded onto 0.5-ml amylose columns and washed extensively with buffer B, and 100 μl of in vitro transcription-translation reactions was then loaded onto the columns. The columns were washed with 5 ml of buffer B, and bound proteins were eluted with buffer B containing 10 mM maltose. The proteins were visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography.
Polymerase assays.
Purified MBP-UL42 mutant proteins were tested for the ability to stimulate long-chain DNA synthesis using a poly (dA) template and an oligo (dT) primer on which Pol alone adds only a few bases as described previously (9). Reaction mixtures contained 200 fmol of HSV Pol and various amounts of UL42 fusion proteins in a final volume of 25 μl. Reactions were carried out at 37° C for 5 to 10 min. Reactions were stopped by placing the mixtures on ice and adding 5 μl of alkaline loading buffer (2 mM EDTA, 50 mM NaOH, 2.5% glycerol, 0.025% bromcresol green) and then loading them onto a 4% alkaline agarose gel. Gels were dried overnight and used to expose film and phosphoresence screens.
Peptides.
Peptides A, G, and E, which correspond to the last 36, 27, and 18 residues of Pol, respectively were synthesized by the Biopolymers Laboratory in the Department of Biological Chemistry and Molecular Pharmacology at Harvard Medical School. The H1228A and R1229A variants of peptide E (termed H29A and R30A in reference 3) were generously provided by SmithKline Beecham. All peptides were acetylated at the N terminus. Peptides were dissolved in water, and concentrations were determined by quantitative amino acid analysis performed by the Molecular Biology Core Facility, Dana-Farber Cancer Institute. The peptides were then lyophilized prior to use.
ITC.
Purified UL42 fusion proteins were dialyzed against buffer containing 30 mM Tris-Cl (pH 7.5), 50 mM NaCl, 0.5 mM EDTA, and 0.5 mM DTT immediately before performing the experiments in order to reduce the concentration of DTT, which otherwise would interfere with calorimetric measurements. Lyophilized synthetic peptides were suspended in the dialysate from the UL42 samples. ITC was performed using a VP-ITC calorimeter (MicroCal, Inc.) and protein concentrations of 7 to 15 μM and peptide concentrations of 280 to 350 μM. Peptides were titrated into the UL42-containing sample cell in 10-μl injections at 25°C (unless otherwise indicated), with a mixing speed of 270 rpm. The heats of dilution of both protein and ligand were determined and subtracted prior to analysis, and the data were integrated to generate curves in which the areas under the injection peaks were plotted against the ratio of peptide to protein. Nonlinear least-squares analysis of the data was performed using the software provided with the instrument (Origin 5.0; MicroCal, Inc.), which fits the area curves to the appropriate parametric binding equation using an iterative Marquardt algorithm. This generates experimental values for the change in enthalpy, ΔH, and the association constant, Ka (which is the reciprocal of the dissociation constant, Kd) and the stoichiometry. The change in free energy (ΔG) and the change in entropy (ΔS) were then calculated by the software using the equations ΔG = −RTlnK and ΔG = ΔH − TΔS, respectively, where R is the gas constant, T is absolute temperature, and K is the equilibrium constant.
CD spectroscopy.
For circular dichroism (CD) spectroscopy, lyophilized peptides were resuspended in 10 mM KF and adjusted to pH 8 with KOH. Spectra were recorded with an Aviv 62DS SpectroPolarimeter at 0°C in a 0.1-cm pathlength cuvette. Wavelength scans were recorded at 1-nm intervals with a 1-s averaging time, and 10 to 20 scans were averaged.
RESULTS
Residues immediately upstream of 160 are not critical for Pol binding.
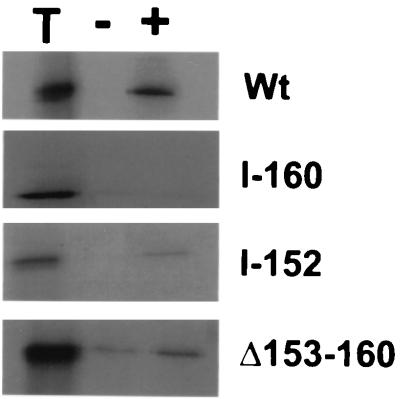
Previous studies of UL42 led to the hypothesis that residues 148 to 163 of UL42, which are predicted to be hydrophilic, might be involved in Pol binding (6). In order to test this hypothesis, a mutant with a UL42 linker insertion at codon 152 and another mutant in which residues 154 to 160 were deleted were constructed and expressed in reticulocyte lysates. These proteins were then incubated with either purified Pol or β-Gal and immunoprecipitated with an antibody against a Pol–β-Gal fusion protein (Fig. 1). As demonstrated previously (6), although the wt protein coimmunoprecipitated with Pol, the I-160 mutant did not. In contrast, the I-152 and Δ154–160 mutants were able to bind Pol more extensively than they did β-Gal, although the Δ154–160 mutant bound less extensively and less specifically (it also bound to β-Gal) than did wt UL42. The I-152 mutant bound to Pol comparably to wt when normalized to input radioactivity. All three UL42 mutant proteins retained wt DNA-binding activity. Unlike the I-160 mutant, the two new mutants were able to stimulate long-chain DNA synthesis by Pol (data not shown). These findings suggest that while mutations in this region may affect the binding of UL42 to Pol, residues 152 to 159 are not crucial for this activity.
FIG. 1.
Effects of mutations on binding UL42 to Pol. In vitro-expressed wt or mutant radiolabeled UL42 was incubated with either Pol (+) or β-Gal (−) and immunoprecipitated with antiserum directed against a Pol–β-Gal fusion, and the precipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Lane T, equivalent amount of input protein. At right are indicated the UL42 proteins assayed.
Random peptides selected for binding to the C terminus of Pol have limited homology to residues 171 to 176 of UL42.
Characterization of the UL42-binding region on Pol has indicated that it is a small discrete surface that may be amenable to antiviral drug action (3). We decided to use a combinatorial approach to identify small peptides that bind to the C terminus of Pol and might be useful starting points for anti-HSV compounds. One library of random dodecapeptides was displayed on the flagella of bacteria, where the peptides were constrained within a loop, and a second library was displayed on a bacteriophage coat protein, where the peptides were unconstrained. Each library was enriched for bacteria or phage that bound to GST fused to the C-terminal 36 residues of Pol (GST-peptide A) but not to GST and that could be eluted with UL42. Additionally, bacteria or phage that bound specifically to GST-peptide A but did not elute with UL42 were eluted separately by vortexing and with high salt, respectively. The peptides identified were aligned in order to identify a consensus Pol C terminus-binding sequence.
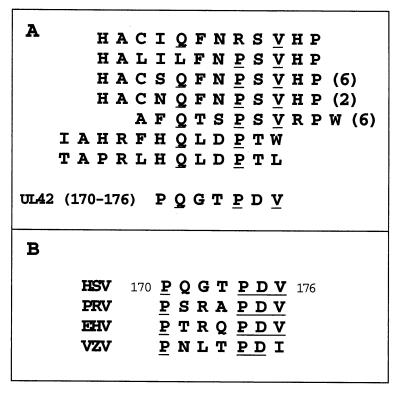
The constrained peptides displayed on bacteria that could be eluted with UL42 showed a high degree of homology with one another (see below). However, all but one of the bacteria that bound to GST-peptide A but did not elute with UL42 (eluted by vortexing) contained stop codons in the peptide-coding sequence; their binding may reflect nonspecific sticking to proteins. Neither of the two classes of unconstrained peptides displayed on phage—those that eluted with UL42 (class A) and those that eluted with high salt (class B)—contained a consensus sequence. However, we then asked whether a consensus could be found when the sequences were aligned with the region of UL42 encompassing residue 160. We focused on sequences downstream of residue 160, given that mutations upstream had relatively little effect on Pol binding. When this alignment was performed, the constrained peptides that eluted with UL42 (which were homologous to each other) and one of the class A and half of the class B unconstrained peptides showed some homology with residues 171 to 176 of UL42, identifying a QxxPxV motif (Fig. 2A). Strikingly, many of the peptides showing this homology with UL42 were represented by multiple isolates having the same amino acid sequence but unique nucleotide sequences. These findings suggested that residues within this small region of UL42 might be involved in Pol binding.
FIG. 2.
(A) Alignment of sequences of bacterial flagellar (top four) and phage (bottom three) display-derived peptides with residues 170 to 176 of UL42. The number of individual isolates is shown in parentheses. (Peptides whose sequences did not align are not shown.) (B) Alignment of residues 170 through 176 of UL42 with homologs from pseudorabies virus (PRV), equine herpesvirus 1 (EHV), and varicella-zoster virus (VZV).
Residue 171 of UL42 is critical for Pol binding.
UL42 homologs from other alphaherpesviruses show limited sequence homology and cannot substitute for one another in vitro (2). However, residues 171 through 176 do lie within a region that is conserved among these homologs (Fig. 2B). While the proline and valine residues of the QxxPxV motif are conserved, the glutamine residue is not, suggesting that this residue might be involved in conferring specificity. To determine if residue 171 is important for Pol binding, a UL42 point mutant in which the codon for Q171 was replaced by one for alanine (Q171A) was constructed. To produce quantities of UL42 sufficient for biochemical studies, proteins were expressed in E. coli as MBP fusions. Because full-length MBP-UL42 aggregates in E. coli (unpublished results), two truncated but otherwise wt proteins were fused to MBP—one truncated at residue 320 (ΔC320) and the other at residue 340 (ΔC340)— based on previous studies demonstrating that the N-terminal 315 residues of UL42 are sufficient for all its known biochemical activities (6, 9, 17). The Q171A mutant was truncated at residue 320. An I-160 mutant (6) truncated at residue 340 was used as a negative control for Pol binding. All of these proteins were able to bind DNA, as determined by column chromatography (they were purified by this property) and ITC (unpublished data).
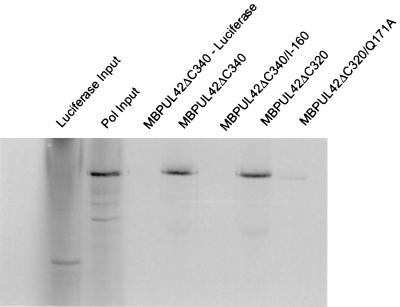
The ability of the UL42 mutants to bind full-length Pol was tested by affinity column chromatography. MBP-UL42 proteins were loaded onto amylose columns and washed, and radiolabeled Pol or, as a negative control, luciferase that had been transcribed and translated in reticulocyte lysates was then loaded onto the columns. The columns were washed, and bound proteins were eluted with 10 mM maltose. The ΔC340 and ΔC320 proteins derived from wt UL42 bound full-length Pol equally well (Fig. 3). As expected (6), no Pol-binding activity could be detected with the ΔC340/I-160 mutant (Fig. 3). Very weak binding of Pol (less than 1/20th that of ΔC320) could be detected with the ΔC320/Q171A mutant (Fig. 3). Very similar results were obtained when radiolabeled Pol expressed in insect cells infected with a recombinant baculovirus was assayed in the same manner (unpublished data). Thus, the Q171A mutation substantially and specifically impaired binding of UL42 to Pol.
FIG. 3.
MBP pulldown experiments. Radiolabeled wt or mutant MBP-UL42 proteins (as indicated at the top of the figure) were allowed to bind to amylose columns before loading in vitro-transcribed and translated, radiolabeled Pol or, where indicated, luciferase as a control. The columns were washed, and the proteins were eluted with 10 mM maltose. The first two lanes show 1/10th the amount of luciferase input and 1/15th the amount of Pol input, respectively. The following lanes show proteins eluted with maltose.
The Q171A mutant is defective for stimulation of long-chain DNA synthesis by Pol.
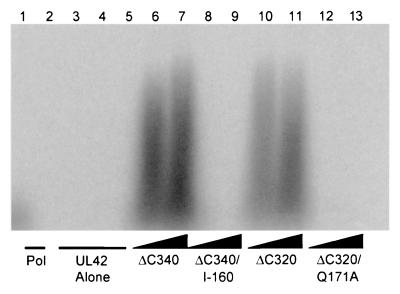
The UL42 fusion proteins were tested for their ability to stimulate long-chain DNA synthesis by Pol. As shown in Fig. 4, only the ΔC340 and ΔC320 proteins derived from wt UL42 were able to increase the length of DNA products synthesized by purified Pol. In contrast, even when a 3:1 (Fig. 4) or 4:1 (data not shown) ratio of UL42 to Pol was used, neither the Q171A nor the I-160 mutant protein stimulated long-chain DNA synthesis. The biochemical properties of each UL42 mutant are summarized in Table 1. These data show that the glutamine residue at position 171 of UL42 is specifically critical for Pol binding and for the stimulation of long-chain DNA synthesis by Pol.
FIG. 4.
Ability of UL42 mutants to stimulate long-chain DNA synthesis by Pol. Experiments were performed using a poly(dA) template with an oligo(dT) primer and labeled TTP. The reaction products were visualized by autoradiography following electrophoresis on a 4% alkaline agarose gel. Lane 1,200 fmol of Pol alone; lanes 2 through 5,600 fmol of ΔC340, ΔC340/I-160, ΔC320, and ΔC320/Q171A alone, respectively. The remaining lanes contain 200 fmol of Pol plus 400 (lane 6) and 600 (lane 7) fmol of ΔC340, 400 (lane 8) and 600 (lane 9) fmol of ΔC340/I-160, 400 (lane 10) and 600 (lane 11) fmol of ΔC320, and 400 (lane 12) and 600 (lane 13) fmol of ΔC320/Q171A.
TABLE 1.
Biochemical properties of UL42 mutantsa
| Protein | Pol binding | DNA binding | Peptide A binding | Long-chain DNA synthesis |
|---|---|---|---|---|
| ΔC340 | ++ | ++ | ++ | ++ |
| ΔC320 | ++ | ++ | ++ | ++ |
| ΔC340/I-160 | − | ++ | − | − |
| ΔC320/Q171A | +/− | ++ | +/− | − |
++, wt levels of activity; +/−, drastically impaired but detectable activity; −, no detectable activity. Binding to full-length Pol was determined using MBP pulldown experiments (Fig. 3). DNA binding was determined by both column chromatography and ITC (unpublished results). Peptide A binding was determined by ITC (Table 2). Long-chain DNA synthesis was determined by using a synthetic primer-template (Fig. 4).
C terminus of Pol is sufficient to bind specifically to UL42 in solution.
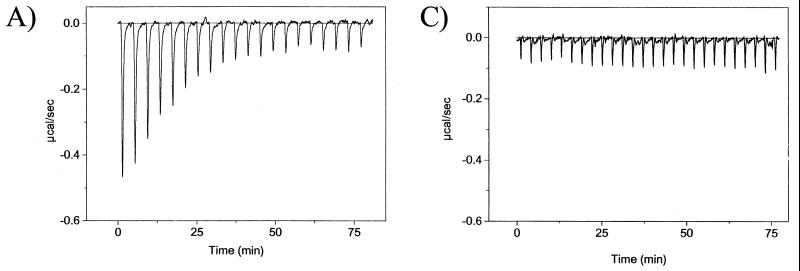
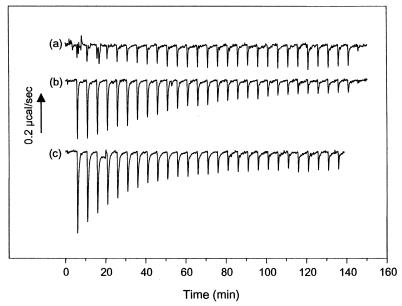
To examine the Pol-UL42 interaction more quantitatively, we investigated the interaction of peptides corresponding to the C-terminal 18 to 36 residues of Pol with UL42. Although previous studies have suggested that such peptides are sufficient for UL42 binding (3, 8, 11, 22), specific binding in solution had not yet been demonstrated. To demonstrate specific binding and obtain affinities and thermodynamic parameters, we used ITC. ITC directly measures heat generated or absorbed upon binding. A typical titration experiment for binding of peptide A to an MBP-UL42 fusion protein, ΔC340, derived from wt UL42 is shown in Fig. 5. The raw data, which are shown in Fig. 5A, indicate an exothermic interaction, based on the negative values observed for the peaks, each of which corresponds to a fixed amount of peptide injected into a solution containing the UL42 fusion protein. With each injection of peptide, less and less heat was released until constant values were obtained (corresponding to the heat released due to dilution), reflecting a saturable process. The area under each injection peak was integrated, resulting in the curve shown in Fig. 5B, in which the molar ratio of peptide to protein is plotted against the kilocalories per mole of injected peptide.
FIG. 5.
ITC of peptide A binding to UL42ΔC340. (A) Raw data for the titration of peptide A with ΔC340, in which the power output in microcalories per second is measured as a function of time in minutes. (B) The heats of dilution of both protein and ligand in A were subtracted, and the area under each injection curve was integrated to generate the points, which represent heat exchange in kilocalories per mole, which are plotted against the cumulative peptide-to-protein ratio for each injection. The solid line is the best-fit curve for the data. (C) Raw data for titration of peptide A with the I-160 mutant of UL42.
Evidence for the specificity of this interaction comes from its being a saturable process with a stoichiometry of about 1 molecule of peptide per molecule of UL42 fusion protein (Table 2), which can be approximated from the peptide-protein ratio at half-saturation (Fig. 5B). To further address specificity, the I-160 and Q171A mutants were tested. Although the wt ΔC340 and ΔC320 fusion proteins were able to bind peptide A robustly (Fig. 5; see also below), no release or absorption of heat was detected when either ΔC340/I-160 (Fig. 5C) or ΔC320/Q171A (not shown) was titrated with peptide A. A very small release of heat (Table 2) was detected when a large excess of peptide A was added to the ΔC320/Q171A mutant in a single injection, indicative of a very weak interaction. No release of heat was detected with the I-160 mutant in a similar single-injection experiment (not shown). Also, substitutions in Pol-derived peptides drastically reduced the heat released (see below). Thus, the interaction measured by ITC is affected by mutations in the binding partners previously shown to specifically affect the Pol-UL42 interaction and is thereby specific.
TABLE 2.
Parameters for peptide A binding to wt and mutant UL42 fusion proteinsa
| Protein | Stoichiometry | ΔH (kcal/mol) | Kd (μM) | ΔG (kcal/mol) | TΔS (kcal/mol) |
|---|---|---|---|---|---|
| ΔC340 | 0.89 ± 0.17 | −6.2 ± 1.4 | 1.4 ± 0.8 | −8.4 ± 1.8 | −2.2 ± 1.2 |
| ΔC340/I-160 | —b | NDc | — | — | — |
| ΔC320 | 0.99 ± 0.14 | −6.6 ± 0.7 | 0.9 ± 0.1 | −8.5 ± 0.9 | −1.9 ± 0.6 |
| ΔC320/Q171A | — | <0.4 | — | — | — |
Titrations were performed with 10-μl peptide injections. Heats of dilution of both peptide and protein were subtracted from the raw data prior to analysis. Nonlinear least-squares analysis of the raw data was performed with the fitting algorithm provided with the calorimeter. Values are means of three to five determinations ± standard deviation.
—, not quantifiable.
ND, not detected.
Energetics of binding.
Titration curves such as those shown in Fig. 5 were analyzed by curve-fitting algorithms to provide values for the stoichiometry, the change in enthalpy (ΔH), and the dissociation constant (Kd) of the interaction, a measure of affinity. The Kd value permits calculation of the change in free energy (ΔG), which then permits calculation of the entropic term TΔS. These parameters for the binding of peptide A to the two wt-derived UL42 fusion proteins are summarized in Table 2. For both fusion proteins, stoichiometries were ∼1 and Kds were ca ∼1 μM. A similar value for Kd has been obtained by using immobilized peptide A and baculovirus-expressed full-length UL42 in surface plasmon resonance measurements (S. Mahdiyoun and D. M. Coen, unpublished results). The Kd values translate to ΔG values of about −8.5 kcal/mol (Table 2). Of this energy, ∼75% was derived from enthalpic changes (ΔH ≅ −6.4 kcal/mol; Table 2).
Binding of Pol-derived peptides of different lengths to UL42.
Pol-derived peptides of three different lengths—36 (peptide A), 27 (here termed peptide G), and 18 residues (peptide E)—have previously been used in studies of the Pol-UL42 interaction (3, 8, 11, 12, 14, 22). Nuclear magnetic resonance and X-ray crystallographic studies of peptide A indicate that it contains a short N-terminal helix and a longer C-terminal helix separated by a less ordered region (3, 22); peptide E corresponds to the C-terminal helix and peptide G to peptide A minus the N-terminal helix. To examine the contribution of different regions of the Pol C terminus to binding energy, ITC experiments were performed by titrating peptide A, E, or G with ΔC340. The parameters for binding of these peptides are shown in Table 3. Although the mean Kd values for peptides E and G were slightly higher and the mean ΔG values were thus slightly lower than that for peptide A, these differences were not statistically significant. These data demonstrate that most of the binding energy of peptides A and G is due to interactions of UL42 with their C-terminal 18 residues, which correspond to peptide E.
TABLE 3.
Parameters for binding of Pol-derived peptides to UL42a
| Peptide | Stoichiometry | ΔH (kcal/mol) | Kd (μM) | ΔG (kcal/mol) | TΔS (kcal/mol) |
|---|---|---|---|---|---|
| A | 0.89 ± 0.17 | −6.2 ± 1.4 | 1.4 ± 0.8 | −8.4 ± 1.8 | −2.2 ± 1.2 |
| E | 1.06 ± 0.16 | −5.7 ± 1.2 | 1.7 ± 0.7 | −8.1 ± 1.9 | −2.4 ± 1.5 |
| G | 0.81 ± 0.11 | −2.2 ± 0.7 | 1.9 ± 0.4 | −7.9 ± 0.4 | −5.7 ± 0.2 |
Titrations were performed with 10-μl injections of peptide into a sample cell containing ΔC340. Heats of dilution of both peptide and protein were subtracted from the raw data prior to analysis. Nonlinear least-squares analysis of the raw data was performed with the fitting algorithm provided with the calorimeter. Values are the means of at least three determinations ± standard deviation.
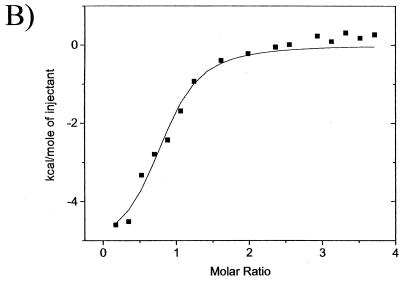
Interestingly, although the ΔG of peptide G binding to UL42 was only slightly lower than those of peptides A and E, the ΔH for binding of peptide G was significantly lower. The less favorable binding enthalpy was compensated for by an increase in binding entropy, suggesting that peptide G binds UL42 differently than do peptides A and E. This led us to suspect that it might be altered structurally. Previous studies with peptides corresponding to the Pol C terminus have demonstrated that CD spectroscopy can identify structural changes associated with decreased inhibitory activity of these peptides (3, 8). Therefore, we compared the CD spectrum of peptide G with that of peptide A (Fig. 6). Both spectra were typical of helical peptides, with minima at around 222 and 205 nm and a maximum at 190. However, the spectrum of peptide G was distorted, with the 205-nm minimum being both much larger and shifted to 203 nm. This type of distortion was previously seen in a peptide E variant that had severely impaired ability to inhibit long-chain DNA synthesis by Pol and UL42 (3).
FIG. 6.
CD spectra of peptides A and G. Wavelength scans of peptides in 10 mM KF were recorded at 1-nm intervals with a 1-s averaging time, and 10 to 15 scans were averaged. ⧫, peptide A; ■, peptide G.
Importance of Pol residues H1228 and R1229.
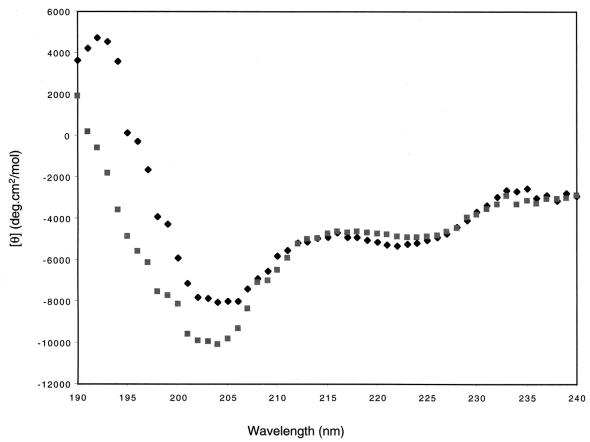
Previous studies of peptide E identified two substitutions that abolish the inhibitory activity of this peptide without disrupting peptide structure—histidine-to-alanine and glutamine-to-alanine substitutions at residues corresponding to Pol residues 1228 and 1229 (H1228A and R1229), respectively (3). Titrations for binding of each of these mutant peptides to ΔC340 are shown in Fig. 7. No binding of the H1228A variant was detected. The R1229A variant had lower values for binding energy than peptide E, and more injections were required to reach saturation. Analysis of the binding data indicated that the Kd of the R1229 variant was about 10-fold higher than that of peptide E (Kd = 17 μM). Thus, each of these single-amino-acid substitutions had a dramatic impact on binding to UL42.
FIG. 7.
ITC of H1228 and R1229 variants of peptide E. Raw data are shown as in Fig. 1A for the interaction of (a) H1228A, (b) R1229A, and (c) wt peptide E with UL42ΔC340. Experiments were performed using 7.2 μM UL42ΔC340 and 335 μM peptide. The arrow indicates the scale for heat exchange in microcalories per second.
DISCUSSION
The Pol-binding site of UL42.
Although UL42 has been well characterized biochemically, identification of specific residues involved in Pol binding has lagged. Previous mutational analyses led us to hypothesize that UL42 residues immediately upstream of residue 160 are involved in Pol binding (6). However, we have shown here that deleting these residues does not significantly impair UL42 function. By using random peptide display coupled with additional mutational studies, we have identified the glutamine residue at position 171 of UL42 as being critical for Pol binding. This residue lies within a segment that is conserved among other alphaherpesvirus UL42 homologs.
The mutational analysis presented here, which was conducted independently of and prior to the recent solution of the crystal structure of UL42 bound to peptide A, is strikingly consistent with that structure (22). The structure indicates that the Q171 side chain of UL42, which is within a portion of UL42 encompassing residues 160 to 175, known as the connector loop, is hydrogen bonded to the side chain of Pol residue R1229. The data presented here demonstrate the crucial importance of this interaction. Interestingly, Pol residue 1229 is also important for the inhibitory activity of peptide E (5) and for its binding affinity (Fig. 7).
The crystal structure also provides an explanation of the Pol-binding phenotypes of the I-160, I-152, and Δ154–160 mutants. Because residue 160 is located at the beginning of the connector loop, an insertion of 4 residues at this position may force the critical Q171 residue out of register. The residues immediately upstream of 160 lie within a flexible loop which could likely accommodate the addition (I-152) or deletion (Δ154–160) of several residues without much change in the register of Q171. In addition, the 4-amino-acid insertion at the deletion site in the Δ153–160 mutant may have lessened its effect on the connector loop region of UL42. The other UL42 residues identified in the alignment of the peptide display-derived peptides were P174 and V176 of UL42. Based on the crystal structure, neither of these residues is directly involved in binding to the Pol peptide, and mutating them would be unlikely to have a large effect on Pol binding. The conservation of these two residues among alphaherpesvirus homologs suggests that their role may be in maintaining the structure of UL42.
The finding that the peptides identified by peptide display were homologous to residues 171 to 176 of UL42 leads to the hypothesis that this region, which lies partially within a constrained segment of UL42 known as the connector loop, is sufficient for Pol binding, much as residues 1218 to 1235 of Pol are sufficient for UL42 binding (see below). However, UL42 residues 160 to 190 fused to GST did not bind full-length Pol in a pull-down assay, nor did this fusion protein bind peptide A in ITC experiments (K. G. Bridges, B. Appleton, and D. M. Coen, unpublished results). The failure of residues 160 to 190 to interact with Pol may indicate a requirement to be in a constrained conformation, as they are in UL42. This also might explain why a good consensus sequence was obtained from the library of constrained peptides but not from the library of unconstrained peptides. Conversely, our data may indicate that residues in other regions of UL42 are essential for Pol binding. The importance of Pol residue H1228 (Fig. 7), which in the crystal structure interacts with UL42 residue R64 (22), is consistent with the latter alternative. These issues are currently under investigation.
The UL42 binding site of Pol.
Previous studies have shown that the extreme C terminus of Pol is necessary for its interaction with UL42 (5, 14, 16, 18). The data presented here demonstrate that peptides corresponding to the C terminus of Pol bind in solution to a UL42 fusion protein with 1:1 stoichiometry and in a manner dependent upon specific Pol or UL42 residues. We conclude that this region of Pol is sufficient to bind specifically to UL42 in solution.
The 18-residue peptide (peptide E) bound with an affinity slightly but not significantly lower than that of the 36-residue peptide (peptide A). This is consistent with the crystal structure of peptide A bound to UL42, in which the vast majority of interactions involve the region of peptide A corresponding to peptide E (22). In studies of these peptides as inhibitors of long-chain DNA synthesis by Pol and UL42, peptide E was only 3- to 15-fold less potent an inhibitor than peptide A, while peptides corresponding to other portions of peptide A had no specific inhibitory activity (3, 8). Why peptide E exhibits similar affinity to that of peptide A but lower potency as an inhibitor is not clear. Perhaps this reflects less stable binding to UL42 at the higher temperatures of the polymerase inhibition assay (37°C) than the ITC assay (25°C).
The 27-residue peptide (peptide G) also bound with an affinity slightly but not significantly lower than those of peptides A and E. More surprisingly, its thermodynamics of binding differed from those of the other two peptides in that a change in entropy dominated rather than a change in enthalpy. This was accompanied by a distorted CD spectrum, suggestive of an altered secondary structure. It is not clear what accounts for these findings. One speculation is that the N-terminal portion of peptide G, which is predicted to be disordered, interacts with and perhaps stabilizes the C-terminal portion that corresponds to peptide E in a manner prevented in peptide A by its N-terminal helix. Upon binding to UL42, this interaction would be lost, permitting free movement of the N-terminal portion and a gain in entropy.
The data presented here do not address possible contributions by regions upstream of the Pol C terminus to UL42 binding. Deletions within the region upstream of the C-terminal 36 residues of Pol affected binding to UL42 in coimmunoprecipitation experiments, but not drastically (5). Additionally, Pol lacking the C-terminal 27 residues (peptide G) competed fourfold less well than full-length Pol in a competition enzyme-linked immunosorbent assay (14). These observations argue that if there is a contribution of upstream residues of Pol to binding UL42, it is small relative to that of the C terminus. On the other hand, based on a titration of UL42 for stimulation of long-chain DNA synthesis by Pol, Hamatake et al. (9) reported an association constant corresponding to a Kd of 80 nM. This value, whose validity depends on several assumptions about the enzymatic assay used, is substantially lower than the affinity of the C terminus measured here, implying major contributions to affinity by the upstream region. Thus, contributions by this region to UL42 binding have yet to be quantified directly.
Importance of hydrogen-bonding interactions.
The crystal structure of peptide A bound to UL42 (22) reveals numerous interactions that bury nearly half of the surface area of the peptide. In particular, the C-terminal 18 residues of the peptide (corresponding to peptide E) form a helix that binds in a deep groove of UL42. The residues lying beneath the peptide are mainly hydrophobic, as is the portion of the helix facing the groove (e.g., residues M1226, L1227, A1230, F1231, L1234, and A1235); with residues F1231 and L1234 being completely buried by the interaction. At first glance, one would predict that the energy of the interaction would depend mainly on this large hydrophobic interface. However, while X-ray crystallography can identify points of contact between two proteins, it does not provide information on the importance of each contact to binding energy. Indeed, the data presented here show that, despite the large hydrophobic interface, a few specific hydrogen bonds are crucial for the interaction. In particular, the role of a hydrogen-bonding network which connects the side chain of Pol residue R1229 to UL42 residue Q171, which in turn is hydrogen bonded to the main chain of Pol residue F1211, is emphasized by the results here. The Q171A substitution of UL42 drastically reduced both binding to and long-chain DNA synthesis by Pol. It reduced ΔH >16-fold and affinity for peptide A to unquantifiable levels. The R1229A variant of Pol peptide E exhibited a 10-fold reduction in affinity for UL42, representing ∼2 kcal/mol less binding energy. The hydrogen bond of UL42 residue Q171 to the main chain of Pol residue F1211 may explain why the Q171A substitution had a greater effect on binding than the R1229A substitution. Additionally, the H1228A variant of peptide E exhibited no detectable binding in this study and no detectable inhibitory activity against long-chain DNA synthesis by Pol and UL42. H1228 of Pol hydrogen bonds with R64 of UL42. Interestingly, R64 of UL42 is also hydrogen bonded to the side chain of Pol residue D1232. Substitution of D1232 with an alanine residue in peptide E resulted in a 4.5-fold decrease in potency in the inhibition assay (3). Thus, the data suggest that this hydrogen bond plays a role in the Pol-UL42 interaction, but not as crucial a one.
Consistent with the idea that hydrogen bonds play a more important role in the strength of Pol-UL42 binding than do hydrophobic interactions are the thermodynamic parameters. Often, binding reactions driven by hydrophobic interactions are dominated by positive ΔS values rather than by negative ΔH values (reviewed in reference 10). The ΔS values for the binding of peptides A and E to UL42 were positive but small, and the negative ΔG was due mainly to a negative ΔH. Preliminary studies of the heat capacity of the binding of UL42 and peptide A (data not shown) support this idea.
Implications for drug discovery.
Because the interaction of Pol with UL42 is specific and essential, it is a promising target for antiviral drugs. A study showing that the C-terminal 27 residues of Pol fused to enterotoxin B could enter cells and inhibit viral replication further supports the validity of the Pol-UL42 interface as a drug target (12). Our initial goal in adopting the peptide display approach was to identify novel inhibitors of this interaction. Indeed, a consensus peptide derived from peptide A-binding sequences identified with the constrained library was able to inhibit the ability of UL42 to stimulate long-chain DNA synthesis by Pol in vitro, albeit weakly (data not shown). Optimization of this and/or other sequences that bind to the C terminus of Pol, perhaps aided by structure-based design, might ultimately lead to new anti-HSV drugs.
More generally, one obstacle to the discovery of small-molecule inhibitors of protein-protein interactions is that these interactions often involve a large surface area and multiple contacts. Another obstacle to the development of small-molecule inhibitors of protein-protein interactions is that they often involve less specific hydrophobic contacts. We have demonstrated here, however, that despite extensive hydrophobic contacts observed in the crystal structure, much of the binding energy for the association of the Pol C terminus with UL42 comes from only a few residues that are involved in specific hydrogen bonds. These results suggest that a small molecule that could interfere with one or two of these hydrogen bonds would effectively disrupt the Pol-UL42 interaction and thus viral replication.
ACKNOWLEDGMENTS
We thank the following: A. Pearson for the MBP-PP vector, K. Kumura-Ishii and K. Weisshart for purified Pol, Z. He for the GST-peptide A vector, H. Zuccola for the MBPUL42ΔC320 plasmid, and D. Wilson for the MBPUL42ΔC340 vector; J. Martin and P. Hensley of SmithKline Beecham for provision of peptide E variants; D. Wiley and S. Harrison for use of a CD spectropolarimeter; and L. Lin and J. Brandts of MicroCal, Inc., for advice on ITC. We also thank H. Zuccola, A. Griffiths, and J. Randell for helpful discussions and assistance, especially with graphics.
This work was supported by NIH grants RO1 AI19838 and RO1 AI26077 to D.M.C. and F32 AI10111 to K.G.B.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1987. [Google Scholar]
- 2.Berthomme H, Monahan S J, Parris D S, Jacquemont B, Epstein A L. Cloning, sequencing, and functional characterization of the two subunits of the pseudorabies virus DNA polymerase holoenzyme: evidence for specificity of interaction. J Virol. 1995;69:2811–2818. doi: 10.1128/jvi.69.5.2811-2818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridges K G, Hua Q, Brigham-Burke M R, Martin J D, Hensley P, Dahl C E, Weiss M A, Coen D M. Secondary structure and structure-activity relationships of peptides corresponding to the subunit interface of herpes simplex virus DNA polymerase. J Biol Chem. 2000;275:472–478. doi: 10.1074/jbc.275.1.472. [DOI] [PubMed] [Google Scholar]
- 4.Chow C S, Coen D M. Mutations that specifically impair the DNA binding activity of the herpes simplex virus protein UL42. J Virol. 1995;69:6965–6971. doi: 10.1128/jvi.69.11.6965-6971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Digard P, Bebrin W R, Weisshart K, Coen D M. The extreme C terminus of herpes simplex virus DNA polymerase is crucial for functional interaction with processivity factor UL42 and for viral replication. J Virol. 1993;67:398–406. doi: 10.1128/jvi.67.1.398-406.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Digard P, Chow C S, Pirrit L, Coen D M. Functional analysis of the herpes simplex virus UL42 protein. J Virol. 1993;67:1159–1168. doi: 10.1128/jvi.67.3.1159-1168.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Digard P, Coen D M. A novel functional domain of an α-like DNA polymerase: the binding site on the herpes simplex virus polymerase for the viral UL42 protein. J Biol Chem. 1990;265:17393–17396. [PubMed] [Google Scholar]
- 8.Digard P, Williams K P, Hensley P, Brooks I S, Dahl C E, Coen D M. Specific inhibition of herpes simplex virus DNA polymerase by helical peptides corresponding to the subunit interface. Proc Natl Acad Sci USA. 1995;92:1456–1460. doi: 10.1073/pnas.92.5.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamatake R K, Bifano M, Tenney D J, Hurlburt W W, Cordingley M G. The herpes simplex virus type 1 DNA polymerase accessory protein, UL42, contains a functional protease-resistant domain. J Gen Virol. 1993;74:2181–2189. doi: 10.1099/0022-1317-74-10-2181. [DOI] [PubMed] [Google Scholar]
- 10.Ladbury J E, Chowdhry B Z. Sensing the heat: the application of isothermal titration calorimetry to thermodynamic studies of biomolecular interactions. Chem Biol. 1996;3:791–801. doi: 10.1016/s1074-5521(96)90063-0. [DOI] [PubMed] [Google Scholar]
- 11.Loregian A, Hirst T R, Marsden H S, Palù G. Use of Vibrio spp. for expression of Escherichia coli enterotoxin B subunit fusion proteins: purification and characterization of a chimera containing a C-terminal fragment of DNA polymerase from herpes simplex virus type 1. Protein Expr Purif. 1996;8:381–389. doi: 10.1006/prep.1996.0114. [DOI] [PubMed] [Google Scholar]
- 12.Loregian A, Papini E, Satin B, Marsden H S, Hirst T R, Palù G. Intranuclear delivery of an antiviral peptide mediated by the B subunit of Escherichia coli heat-labile enterotoxin. Proc Natl Acad Sci USA. 1999;96:5221–5226. doi: 10.1073/pnas.96.9.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchetti M E, Smith C A, Schaffer P A. A temperature-sensitive mutation in a herpes simplex virus type 1 gene required for viral DNA synthesis maps to coordinates 0.609 through 0.614 in UL. J Virol. 1988;62:715–721. doi: 10.1128/jvi.62.3.715-721.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsden H S, Murphy M, McVey G L, MacEachran K A, Owisanka A M, Stow N D. Role of the carboxy terminus of herpes simplex virus type 1 DNA polymerase in its interaction with UL42. J Gen Virol. 1994;75:3127–3135. doi: 10.1099/0022-1317-75-11-3127. [DOI] [PubMed] [Google Scholar]
- 15.Parris D S, Cross A, Haarr L, Orr A, Frame M C, Murphy M, McGeoch D J, Marsden H. Identification of the gene encoding the 65-kilodalton DNA binding protein of herpes simplex virus type 1. J Virol. 1988;62:818–825. doi: 10.1128/jvi.62.3.818-825.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stow N D. Sequences at the C-terminus of the herpes simplex virus type 1 UL30 protein are dispensable for DNA polymerase activity but not for viral origin-dependent DNA replication. Nucleic Acids Res. 1993;21:87–92. doi: 10.1093/nar/21.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenney D J, Hurlburt W W, Bifano M, Stevens J T, Micheletti P A, Hamatake R K, Cordingley M G. Deletions of the carboxy terminus of herpes simplex virus type 1 UL42 define a conserved amino-terminal functional domain. J Virol. 1993;67:1959–1966. doi: 10.1128/jvi.67.4.1959-1966.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenney D J, Micheletti P A, Stevens J T, Hamatake R K, Matthews J T, Sanchez A R, Hurlburt W W, Bifano M, Cordingley M G. Mutations in the C terminus of herpes simplex virus type 1 DNA polymerase can affect binding and stimulation by its accessory protein UL42 without affecting basal polymerase activity. J Virol. 1993;67:543–547. doi: 10.1128/jvi.67.1.543-547.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weisshart K, Chow C S, Coen D M. The herpes simplex virus processivity factor, UL42, imparts increased DNA-binding specificity on viral DNA polymerase and decreased dissociation from primer-template without reducing elongation rate. J Virol. 1999;73:55–66. doi: 10.1128/jvi.73.1.55-66.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisshart K, Kuo A A, Hwang C B C, Kumura K, Coen D M. Structural and functional organization of herpes simplex virus DNA polymerase investigated by limited proteolysis. J Biol Chem. 1994;269:22788–22796. [PubMed] [Google Scholar]
- 21.Wu C A, Nelson N J, McGeoch D J, Challberg M D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988;62:435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuccola H Z, Filman D J, Coen D M, Hogle J M. The crystal structure of an unusual processivity factor, herpes simplex virus UL42, bound to the C-terminus of its cognate polymerase. Mol Cell. 2000;5:267–278. doi: 10.1016/s1097-2765(00)80422-0. [DOI] [PubMed] [Google Scholar]