Abstract
Purpose
Acne is a chronic inflammatory skin condition affecting mainly teenagers and adults as well. Guidelines recommend retinoids as a first-line treatment for mild-to-moderate acne. However, dermocosmetics in adjunct could potentially improve efficacy and tolerability. This study was conducted to determine the effectiveness and safety of a dermocosmetic cream containing salicylic acid, lipohydroxy acid, niacinamide, Aqua posae filiformis, procerad and zinc salt in the treatment of mild-to-moderate acne vulgaris in adjunct to different regimens of adapalene compared to adapalene only.
Patients and Methods
This randomized, controlled, parallel-group, evaluator-blind study was conducted over 8 weeks on male and female acne subjects at five teaching hospitals in Indonesia. A total of 291 participants were enrolled and divided into three treatment groups: Group A adapalene 0.1% cream nightly – Group B dermocosmetic cream daily + adapalene 0.1% cream every two nights – Group C dermocosmetic cream daily + adapalene 0.1% cream nightly. Clinical evaluations of treatment included scoring on Global Evaluation of Acne (GEA) scale, lesion count (Indonesian Acne Expert Meeting scale), treatment tolerability and treatment satisfaction. Evaluations were performed on Day 28 and Day 56 of treatment.
Results
After 28 and 56 days of treatment, all groups exhibited improvements across the various measures. Data analysis, utilizing Anova for repeated measurements, revealed a statistically significant difference between Groups C and A for reduction of GEA scores (p = 0.038) in favor of Group C. On Day 56, percentages of subjects with GEA Scale improvements of at least 1 grade in comparison with baseline were in Group C (61.7%) followed by Group A (47.9%) and Group B (45.3%). Better treatment tolerance and satisfaction scores were noted in Groups B and C.
Conclusion
Combination of the dermocosmetic cream with adapalene showed higher efficacy, tolerability and satisfaction in comparison to adapalene alone.
Keywords: mild and moderate acne vulgaris, adjunct to Adapalene, dermocosmetic cream
Introduction
Acne vulgaris is a skin condition that affects the pilosebaceous units, typically characterized as a non-inflammatory disease (open and closed comedones) or chronic inflammation (papules and pustules).1 According to the Global Burden of Disease study, acne vulgaris affects 85% of the population with the highest age-specific epidemiology between 12 and 25 years and is estimated to impact 9.4% global population, thus making it the eighth most common disease worldwide.1,2 Currently, the increasing incidence of acne vulgaris in late adolescence and young adults is a global issue.2 The prevalence of acne vulgaris in Southeast Asia ranges from 40% to 80% of cases, while according to records from Indonesian Cosmetic Dermatology, there has been a continuous increase, with 60% of acne vulgaris patients in 2006, 80% in 2007, and reaching 90% in 2009.3
Acne consistently represents one of the most common skin conditions in the population, as mentioned in studies conducted in the United Kingdom, France, and the United States.4 Androgen production during puberty can be a reason why acne vulgaris is so common in the late adolescent population, regardless of socioeconomic status, ethnicity and race, or gender.1
Treatment options for acne vulgaris are available in topical and systemic regimens. Acne treatment recommendation is given based on its severity. The international guidelines for management of acne recommend topical retinoids as a first-line treatment of mild-to-moderate acne,5 while systemic (oral) antibiotic therapy is indicated for moderate-to-severe acne.6 The major concern in the current treatment of acne vulgaris with antibiotics is the resistance of Cutibacterium acnes (C. acnes), particularly in monotherapy. Indeed, there is still a high prescription of topical antibiotics, which raises the issue of increasing antibiotic resistance these past years,7 thus making alternatives to antibiotics a key topic in the literature. Accordingly, efforts are being made to reduce the use of antibiotics, including through adjuvant therapy.8,9 Another issue in acne management is the low adherence to treatment, which is partly due to a lack of quick efficacy, complex regimens and adverse events of topical or systemic drugs.10–13 Adjunctive therapy for acne vulgaris may include intralesional steroids, comedo extraction, laser treatment, and dermocosmeceuticals depending on the clinical presentation.6 Dermocosmetics can have an important role in the management of acne, either to improve milder forms of acne, to complement the efficacy of drugs, maintain their benefit when they are stopped, or improve the tolerance of acne medications.14,15 When treating acne, it is important to target the four pathophysiological factors involved such as hyperkeratinization, hyperseborrhea, inflammation and microbiome dysbiosis, including a disbalance between C. acnes phylotypes and Staphylococcus epidermidis, while maintaining a strong skin physical barrier. Aqua posae filiformis is one of the key active ingredients. When cultivated in a medium containing La Roche-Posay (LRP) thermal spring water, Vitreoscilla filiformis transforms into a “super bacteria” called Aqua posae filiformis with important properties. Vitreoscilla filiformis biomass has been widely used in cosmetics and has been shown to modulate the major free radical scavenger, mitochondrial superoxide dismutase, which can be induced in skin cells.16 Aqua posae filiformis has shown increased efficacy in terms of stimulating Beta Defensin 4 and S100 Calcium-Binding Protein A7.16
This clinical study assessed the benefit in acne of a dermocosmetic cream (DC-Eff, Effaclar® Duo+, La Roche-Posay Laboratoire Dermatologique, France) that contains salicylic acid, lipohydroxy acid, niacinamide, 2-oleamido-1,3-octadecanediol, piroctone olamine, zinc gluconate, procerad, the pre- and probiotic aqua posae filiformis, and LRP thermal water (referred to as “combination cream” hereafter).
Materials and Methods
Study Design
This was a randomized, controlled, parallel-group, multicenter, evaluator-blind study. This trial was conducted at five teaching hospitals in Indonesia (Central Public Hospital Dr. Cipto Mangunkusumo Hospital Jakarta, Presidential Gatot Soebroto Central Army Hospital Jakarta, Regional Public Hospital Dr. Moewardi Surakarta, Regional Public Hospital Dr. Saiful Anwar Malang, Central Public Hospital Dr. M Djamil Padang). This study was conducted from September 2022 to December 2022.
Patients Profile
Inclusion criteria were males and females aged 15–50 years, with mild-to-moderate acne vulgaris (according to Indonesia Acne Expert Meeting scale [IAEM, details of the IAEM guide are provided as Supplementary Data, Data S1] and Global Evaluation Acne [GEA] scale), and willing to participate in the study. Exclusion criteria were patients with a history of systemic diseases that can affect acne course/persistence such as metabolic and hormonal diseases, patients using systemic or topical drugs or cosmeceuticals or aesthetic treatments related with acne vulgaris, the use of other acne therapies (systemic/topical drugs or invasive treatments) over the preceding 30 days, pregnant or breastfeeding women, allergic reaction to dermo-cosmeceutical ingredients.
Ethics
This research has received ethical approval from the University of Indonesia, registered as KET-782/UN2.F1/ETIK/PPM.00.02/2022, with NCT Clinical Trial number NCT05497323. The patients in this manuscript have given written informed consent for the clinical trial and to publication of their case details including images. There were no participants under 18 years of age. This study complied with the Declaration of Helsinki.
Method
The study methodology and study flow diagram are summarized in Figures S1 and S2, respectively.
A total of 300 subjects (60 subjects per center) were to be enrolled and randomly divided into three groups (Group A: subjects only receiving adapalene 0.1% cream nightly, Group B: subjects receiving combination cream daily and adapalene 0.1% cream every two nights, and Group C: subjects receiving combination cream daily and adapalene 0.1% cream nightly), for eight weeks. The study design incorporated these three treatment groups to evaluate the different regimens: The primary comparison between Groups C and A aimed to assess the potential benefit of adding a dermocosmetic to daily adapalene use. The comparison between Groups B and C was designed to evaluate the consistency of the study by confirming the dose effect of adapalene (every night vs every two nights) when combined with a dermocosmetic. Lastly, the comparison between Groups A and B sought to determine if reducing adapalene frequency while adding a dermocosmetic (Group B) could maintain efficacy comparable to daily adapalene use alone (Group A).
Patients were instructed not to take oral or topical daily supplements related to acne vulgaris during the study. Facial skin wash was provided to the patients to minimize bias. All patients received the same standardized neutral facial cleanser during the study, and they were asked not to wear makeup or facial scrubs during the study.
The sample size was determined by comparing the number of lesions (both inflammatory and non-inflammatory) and the global acne score. An 80% power and a confidence rate of 95% were used. The final sample size of 300 participants was determined based on an effect size of 100 lesions for inflammatory lesion count and a standard deviation of 2.75.
Initial examinations were to be carried out on Day 0 (D0) and clinical evaluations of treatment effectiveness were to be carried out every 4 weeks for 8 weeks, ie on Day 28 (D28) and Day 56 (D56):
Investigators assessed treatment effectiveness on acne severity based on GEA scale and lesion count (IAEM scale), and on sebum levels using a JANUS II full facial skin analysis system (One Simple Ver. 2.53 one-click automatic, Bomtech Electronics Co., Ltd., Korea).
Patient’s quality of life (QOL) was assessed using Cardiff acne disability index and acne QOL questionnaires.
Standardized photographs were taken at each time point.
Patients’ satisfaction (graded on a 1 [lowest satisfaction] to 5 scale) and treatment tolerability (graded on a 1 [highest tolerability] to 4 scale) were evaluated separately by the investigators and the patients.
Univariate analysis was performed on numerical and categorical data. Shapiro–Wilk normality test was applied to measure data normality. Numerical data were reported using mean ± standard error. Proportion comparisons among groups were assessed using Chi-square test and its alternative Fisher test. Mean comparisons among groups at each serial timing were assessed using the one-way Anova test, and the value changes from D0-D28-D56 were then compared using the repeated measurements Anova test. When Anova for repeated measurements found significant p-values (p < 0.05), the analysis was continued to post-hoc analysis using Bonferroni tests.
Results
A total of 291 participants were enrolled in the study, with an even distribution across the three treatment groups. For baseline demographic data, see Table S1. The study population consisted entirely of individuals of Asian ethnicity, with the majority being female (60%), having phototype IV skin (79.5%), aged over 25 years (55.3%), of Javanese descent (54%), and possessing a higher level of education (54%).
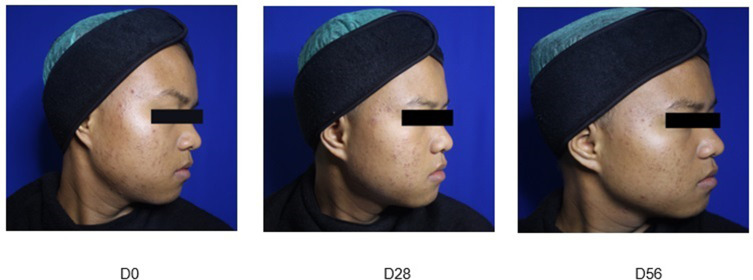
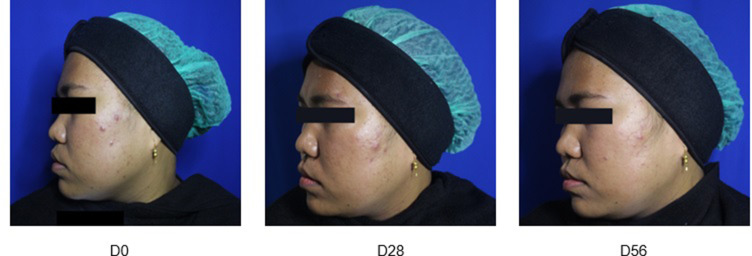
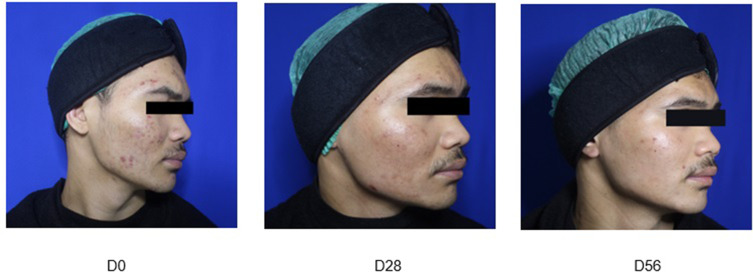
Representative photographs of Group A (Figure 1), Group B (Figure 2) and Group C (Figure 3) patients obtained at baseline and on Day 28 and Day 56 of treatment are presented hereafter (one example per group):
Figure 1.
Group A: Adapalene 0.1% cream nightly.
Figure 2.
Group B: combination cream daily and Adapalene 0.1% cream every two nights.
Figure 3.
Group C: combination cream daily and Adapalene 0.1% cream nightly.
Treatment Effectiveness
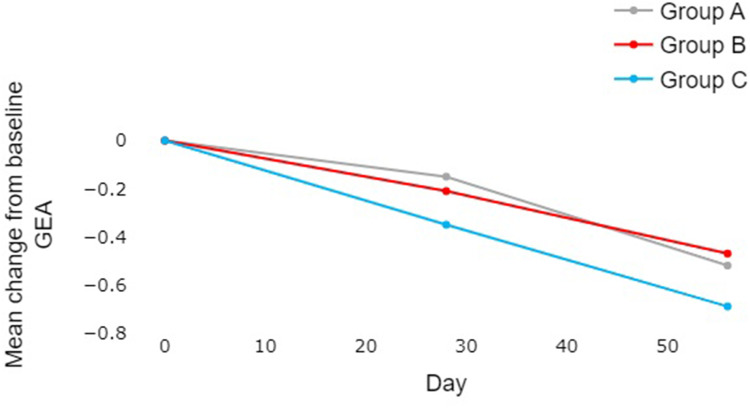
After 56 days of treatment, all three treatment groups exhibited improvements across the various measures, especially in the GEA scale (Table 1, Figure 4). There was a statistically significant difference between Group C and Group A in terms of reduction of GEA scores (p = 0.038, Table 1). Data analysis, utilizing ANOVA for repeated measurements, revealed several key findings. In the primary comparison between Groups C and A, Group C demonstrated significantly better reduction in GEA scores at Day 28 (p = 0.007) (Table 2) and a strong tendency towards significance at Day 56 (p = 0.052). The comparison between Groups B and C, designed to evaluate the consistency of the study by evaluating the already demonstrated dose effect of adapalene, showed a tendency towards significance at Day 28 (p = 0.072) and reached statistical significance at Day 56 (p = 0.024), supporting a dose-dependent response. Finally, the comparison between Groups A and B suggested similar efficacy in GEA score reduction throughout the study period.
Table 1.
Comparison of GEA, IAEM, and Janus Scores Between the Treatment Groups
| Variable | Treatment (Group) |
p Anova for repeated measurements |
|||||
| A (n=97) | B (n=99) | C (n=97) | |||||
| Mean | (SE) | Mean | (SE) | Mean | (SE) | ||
| GEA Scale | |||||||
| D0 | 2.14 | (0.06) | 2.21 | (0.07) | 2.33 | (0.07) | 0.038* |
| D28 | 2.00 | (0.06) | 2.00 | (0.07) | 1.98 | (0.08) | |
| D56 | 1.63 | (0.07) | 1.74 | (0.07) | 1.64 | (0.07) | |
| IAEM Scale Comedone | |||||||
| D0 | 27.03 | (2.14) | 26.14 | (1.94) | 25.64 | (1.96) | 0.350 |
| D28 | 24.78 | (2.88) | 22.23 | (2.13) | 21.00 | (1.76) | |
| D56 | 13.31 | (0.97) | 13.98 | (1.45) | 13.97 | (1.21) | |
| IAEM Scale Papule | |||||||
| D0 | 6.76 | (0.67) | 7.70 | (0.73) | 8.66 | (0.78) | 0.789 |
| D28 | 4.95 | (0.43) | 5.48 | (0.51) | 5.72 | (0.62) | |
| D56 | 3.86 | (0.39) | 4.64 | (0.42) | 4.93 | (0.55) | |
| IAEM Scale Pustule | |||||||
| D0 | 2.04 | (0.31) | 1.97 | (0.30) | 1.97 | (0.32) | 0.493 |
| D28 | 1.09 | (0.17) | 1.49 | (0.25) | 1.78 | (0.43) | |
| D56 | 0.73 | (0.16) | 0.78 | (0.16) | 0.77 | (0.14) | |
| IAEM Scale Nodule | |||||||
| D0 | 0.26 | (0.09) | 0.42 | (0.12) | 0.22 | (0.08) | 0.443 |
| D28 | 0.19 | (0.07) | 0.20 | (0.08) | 0.18 | (0.06) | |
| D56 | 0.04 | (0.03) | 0.06 | (0.04) | 0.02 | (0.01) | |
| JANUS U ZONE | |||||||
| D0 | 482.89 | (61.79) | 369.03 | (43.07) | 597.06 | (76.81) | 0.416 |
| D28 | 531.29 | (54.92) | 490.39 | (47.20) | 669.66 | (84.57) | |
| D56 | 539.02 | (49.45) | 485.94 | (45.36) | 564.22 | (54.60) | |
| JANUS T ZONE | |||||||
| D0 | 1500.98 | (209.05) | 1086.75 | (138.78) | 1902.25 | (268.36) | 0.263 |
| D28 | 1162.15 | (148.90) | 1073.18 | (139.04) | 1641.66 | (225.31) | |
| D56 | 907.14 | (96.84) | 998.05 | (121.06) | 1482.74 | (199.44) | |
Notes: *statistically significant (p<0.05).
Abbreviations: n=number of subjects at baseline; SE=standard error.
Figure 4.
Comparative analysis of changes in GEA scores from baseline between the three groups over the 56-day experimental period. The reduction of GEA scores was numerically higher in Group C compared to A and B, and the statistical analysis showed a significant difference between Groups A and C on Day 28.
Table 2.
Change of GEA and Total, Inflammatory and Non-Inflammatory Lesion Counts from Baseline Across Treatment Groups
| Change from baseline | Group | p-value | |||||||||
| A | B | C | |||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |||
| GEA | D28 | 96 | 0.15 | 0.52 | 95 | 0.21 | 0.48 | 94 | 0.35 | 0.50 | A-B=0.325 A-C=0.007* B-C=0.072@ |
| D56 | 96 | 0.52 | 0.62 | 95 | 0.47 | 0.70 | 94 | 0.69 | 0.61 | A-B=0.681 A-C=0.052§ B-C=0.024** |
|
| Total lesion count | D28 | 96 | 5.40 | 20.49 | 95 | 7.72 | 14.50 | 94 | 8.69 | 15.99 | A-B=0.257 A-C=0.453 B-C=0.502 |
| D56 | 96 | 18.33 | 17.95 | 95 | 17.37 | 19.76 | 94 | 17.41 | 19.85 | A-B=0.775 A-C=0.695 B-C=0.950 |
|
| Inflammatory lesion count | D28 | 96 | 2.90 | 6.12 | 95 | 3.13 | 6.86 | 94 | 3.40 | 7.24 | A-B=0.648 A-C=0.257 B-C=0.505 |
| D56 | 96 | 4.47 | 6.13 | 95 | 4.78 | 8.09 | 94 | 5.31 | 8.74 | A-B=0.638 A-C=0.389 B-C=0.674 |
|
| Non-Inflammatory lesion count | D28 | 96 | 2.51 | 18.20 | 95 | 4.59 | 11.68 | 94 | 5.29 | 12.75 | A-B=0.581 A-C=0.578 B-C=0.891 |
| D56 | 96 | 13.86 | 15.81 | 95 | 12.59 | 15.31 | 94 | 12.10 | 14.65 | A-B=0.967 A-C=0.734 B-C=0.895 |
|
Notes: For Group C (values in bold): *statistically significant (p<0.05) compared to Group A; @tendency towards significance (p=0.072) compared to Group B; §tendency towards significance (p=0.052) compared to Group A; **statistically significant (p<0.05) compared to Group B.
This is further supported by the data presented in Table 3, showing percentages of subjects presenting with GEA scale improvements of at least 1 grade between study visits for each group. The highest GEA improvement percentage observed in the final evaluation (Day 56) in comparison with baseline was found in Group C (61.7%), followed by Group A (47.9%) and Group B (45.3%).
Table 3.
Percentage of Subjects with at Least 1 grade Improvement of the GEA Scores Between Study Visits
| Day | Group A | Group B | Group C |
|---|---|---|---|
| D0 to D28 | 19.8% | 24.2% | 34%*@ |
| D28 to D56 | 37.5% | 32.6% | 37.2% |
| D0 to D56 (Final Evaluation) | 47.9% | 45.3% | 61.7%**§ |
Notes: *statistically significant (p<0.05) compared to Group A; @tendency towards significance (p=0.072) compared to Group B; **statistically significant (p<0.05) compared to Group B; §tendency towards significance (p=0.052) compared to Group A.
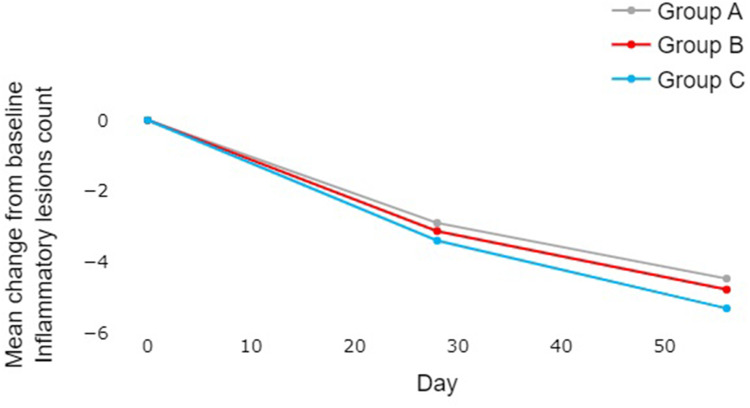
Subjects in all three groups showed a non-statistically significant reduction in the IAEM scale for Inflammatory Lesions, IAEM scale for Non-Inflammatory Lesions, and IAEM scale for Total Lesions over time (Table 1). There was a trend towards a numerically higher reduction of inflammatory lesion count from baseline in Group C compared to A and B (Table 2 and Figure 5). There were no noticeable differences between groups for the reduction of non-inflammatory and total lesions after 56 days of treatment.
Figure 5.
Comparative analysis of changes in inflammatory lesion count from baseline between the three groups over the 56-day experimental period. There was a trend towards a numerically higher reduction in Group C compared to A and B, although no statistically significant difference could be demonstrated (Mann–Whitney test) between the groups.
No significant difference was found on sebum levels (Janus scores) over time and among the three groups (Table 1).
Treatment Tolerability and Satisfaction
The study included an assessment of treatment satisfaction and tolerability, which were evaluated separately by both the investigators and the subjects themselves.
Treatment Tolerability Evaluation
Significant differences were observed in the investigators’ tolerance scores among the three treatment groups (p < 0.001 on Day 28, p = 0.005 on Day 56) (Table 4). Group A, which received adapalene cream alone, had a higher score, indicating lower tolerance compared to Groups B and C on both Day 28 and Day 56. On the other hand, when assessing the patients’ tolerance score, no significant difference was observed between Groups B and C. The majority of subjects in both groups scored 1–2, indicating good tolerance (92–94%).
Table 4.
Comparison of Investigators’ and Patients’ Tolerability Evaluation Among the Treatment Groups
| Evaluation | Treatment (Group) | p-value between groupsa | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| n | % | n | % | n | % | |||
| Investigators’ Tolerability score | ||||||||
| D28 | 1–2 | 58 | 60.40% | 91 | 95.80% | 91 | 96.90% | <0.001* |
| 3–4 | 38 | 39.50% | 4 | 4.30% | 3 | 3.20% | ||
| D56 | 1–2 | 62 | 64.50% | 84 | 89.40% | 81 | 86.20% | 0.005* |
| 3–4 | 34 | 35.40% | 10 | 10.70% | 13 | 13.90% | ||
| p-value D28-D56 within groupb | 0.904 | 0.135 | 0.212 | |||||
| Patients’ Tolerability score | ||||||||
| D28 | 0 | 96 | 100.00% | 0 | 0.00% | 0 | 0.00% | 0.814c |
| 1–2 | 0 | 0.00% | 90 | 94.70% | 87 | 92.60% | ||
| 3–4 | 0 | 0.00% | 5 | 5.30% | 7 | 7.50% | ||
| D56 | 0 | 96 | 100.00% | 0 | 0.00% | 0 | 0.00% | 0.314c |
| 1–2 | 0 | 0.00% | 84 | 89.30% | 81 | 86.20% | ||
| 3–4 | 0 | 0.00% | 10 | 10.70% | 13 | 13.90% | ||
| p-value D28-D56 within groupb | 1 | 0.040* | 0.785 | |||||
Notes: *statistically significant with p<0.005; aChi square test, 1–2 and 3–4 levels were combined; bMcNemar test; ccomparison between B and C Groups only, 1–2 and 3–4 levels were combined.
Treatment Satisfaction Evaluation
There was a significant difference in the investigators’ evaluation of patient satisfaction among the three groups (p < 0.001) (Table 5). Both Groups B and C had higher satisfaction scores, with the majority scoring 4–5, compared to Group A on both Day 28 and Day 56.
Table 5.
Comparison of Investigators’ and Patients’ Satisfaction Evaluation Among the Treatment Groups
| Evaluation | Treatment (Group) | p-value between groups a | ||||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| n | % | n | % | n | % | |||
| Investigators’ Satisfaction Score | ||||||||
| D28 | 1 | 1 | 1.00% | 0 | 0.00% | 0 | 0.00% | <0.001* |
| 2 | 21 | 21.90% | 1 | 1.10% | 1 | 1.10% | ||
| 3 | 47 | 49.00% | 18 | 18.90% | 17 | 18.10% | ||
| 4 | 27 | 28.10% | 71 | 74.70% | 71 | 75.50% | ||
| 5 | 0 | 0.00% | 5 | 5.30% | 5 | 5.30% | ||
| D56 | 1 | 1 | 1.00% | 1 | 1.10% | 0 | 0.00% | <0.001* |
| 2 | 17 | 17.70% | 1 | 1.10% | 3 | 3.20% | ||
| 3 | 39 | 40.60% | 13 | 13.80% | 14 | 14.90% | ||
| 4 | 39 | 40.60% | 72 | 76.60% | 64 | 68.10% | ||
| 5 | 0 | 0.00% | 7 | 7.40% | 13 | 13.80% | ||
| p-value D28 - D56 within groupb | 0.021 | 0.503 | 0.276 | |||||
| Patients’ Satisfaction score | ||||||||
| D28 | 0 | 96 | 100.00% | 0 | 0.00% | 0 | 0.00% | 0.100c |
| 2 | 0 | 0.00% | 2 | 2.10% | 1 | 1.10% | ||
| 3 | 0 | 0.00% | 27 | 28.40% | 19 | 20.20% | ||
| 4 | 0 | 0.00% | 57 | 60.00% | 71 | 75.50% | ||
| 5 | 0 | 0.00% | 9 | 9.50% | 3 | 3.20% | ||
| D56 | 0 | 96 | 100.00% | 0 | 0.00% | 0 | 0.00% | 0.267c |
| 1 | 0 | 0.00% | 1 | 1.10% | 0 | 0.00% | ||
| 2 | 0 | 0.00% | 2 | 2.10% | 3 | 3.20% | ||
| 3 | 0 | 0.00% | 10 | 10.60% | 20 | 21.30% | ||
| 4 | 0 | 0.00% | 68 | 72.30% | 60 | 63.80% | ||
| 5 | 0 | 0.00% | 13 | 13.80% | 11 | 11.70% | ||
| p-value D28 - D56 within groupb | 1 | 0.005 | 0.623 | |||||
Notes: *statistically significant with p<0.005; aChi square test, 1–2 and 3–4 levels were combined; bMcNemar test; ccomparison between B and C Groups only, 1–2 and 3–4 levels were combined.
In terms of patients’ evaluation of satisfaction, there was no significant difference between Groups B and C. The majority of subjects in both groups scored 4–5, indicating high satisfaction. In Group B, there was a significant increase in the number of subjects scoring 4–5 between Day 28 and Day 56, suggesting an improvement in satisfaction over time.
Quality of Life
Both acne QOL and Cardiff Acne Disability Index questionnaires revealed significant improvements over time in each group (p < 0.001, Table 6). However, no significant difference was found among the three groups. Group C had the highest Acne QOL score improvement (53.2%), followed by Group A (43.6%), and Group B (40.7%). As for Cardiff scores, Group A had the highest improvement (21.1%), followed by Group C (17%) and Group B (13.7%).
Table 6.
Comparative Evolution of Patients’ QOL Over Time Between the Treatment Groups
| Variable | Treatment (Group) | p (one-way Anova) | p (Anova repeated measurement) | |||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | ||||||
| Mean | (SE) | Mean | (SE) | Mean | (SE) | |||
| ACNE QUALITY OF LIFE Score | ||||||||
| D0 | 66.37 | (2.62) | 66.10 | (2.54) | 60.36 | (2.74) | 0.213 | 0.963 |
| D56 | 81.14 | (2.40) | 80.82 | (2.32) | 76.38 | (2.52) | 0.299 | |
| p-value D0-D56 within group | <0.001 | <0.001 | <0.001 | |||||
| QOL change (%) | 43.6% | (10.4%) | 40.7% | (6.7%) | 53.2% | (12.9%) | 0.674 | |
| CARDIFF ACNE DISABILITY INDEX Score | ||||||||
| D0 | 4.98 | (0.28) | 4.89 | (0.27) | 5.67 | (0.34) | 0.224 | 0.784 |
| D56 | 3.57 | (0.27) | 3.69 | (0.28) | 4.19 | (0.31) | 0.344 | |
| p-value D0-D56 within group | <0.001 | <0.001 | <0.001 | |||||
| Cardiff score change (%) | 21.1% | (6.3%) | 13.7% | (6.9%) | 17.0 | (7.7%) | 0.755 | |
Discussion
Acne vulgaris requires long-term therapy.17 Treatment options can be considered based on the severity, ranging from mild, moderate, to severe acne. In mild acne, oral antibiotics are not needed, whereas for moderate-to-severe acne, they can be prescribed as needed. Oral isotretinoin may also be used in severe acne.18 The use of long-term oral antibiotics can induce bacterial resistance. A study about C. acnes sensitivity to antibiotics in acne vulgaris has been done in Indonesia.19 In this study, Sitohang et al identified Staphylococcus epidermidis (50.5%), Propionibacterium acnes - now called Cutibacterium acnes - (11.0%), and Staphylococcus aureus (7.7%) in acne lesions. Of note, C. acnes was 100% sensitive to doxycycline and to minocycline, but 10% was resistant to erythromycin, clindamycin, and tetracycline. It should also be noted that Malassezia spp might be involved in the development of acne lesions.20,21
Patient adherence to treatment is essential to achieve optimal therapeutic outcomes. Approximately 48% of acne patients in Asia exhibit low adherence to regular acne treatment.17 Adjuvant therapy may be used to shorten the duration of acne vulgaris therapy, eg dermocosmetics,8,22 acne lesion extraction,23 and vitamin D might also have a role.24
Active ingredients in dermocosmetics that are well established include niacinamide, zinc, salicylic acid, panthenol, ceramides, and glycerin, targeting various aspects of acne pathogenesis. They improve skin barrier function by controlling hyperkeratinization, increasing epidermal thickness, and reducing irritation. With advancing research in acne therapy, including studies on dermocosmetics use, some new active ingredients in dermocosmetics have been found to provide significant effects in adjunctive acne therapy. One such active ingredient is Aqua posae filiformis, which is a lysate of Vitreoscilla filiformis, a microorganism that thrives in thermal spring water. These active ingredients, for example, keratolytic agents, also play a role in stabilizing the microbiome through exfoliation, thereby reducing inflammation through immunomodulation.14,25
This study aimed to assess the effectiveness and safety of a multitargeted dermocosmetic cream as adjunctive therapy for mild and moderate acne vulgaris. The effectiveness and safety of adapalene have been well established in numerous studies, both as monotherapy and in combination with antibiotics.9 In the present study, there was a statistically significant difference between Group C and Groups A and B in terms of reduction of GEA scores. Overall, Group C demonstrated the greatest reduction in GEA scores, indicating a more pronounced and statistically significant improvement in acne severity at Day 28 versus Group A, and a strong tendency towards significance at Day 56. The comparison between Groups B and C, designed to evaluate the consistency of the study by evaluating the already demonstrated dose effect of adapalene, showed a tendency towards significance at Day 28 and reached statistical significance at Day 56, supporting a dose-dependent response. Subjects in all three groups showed a trend (non-statistically significant) for reduction in the IAEM scale for acne lesions, and there was also a trend towards a numerically higher reduction of inflammatory lesion count from baseline in Group C compared to A and B.
As per investigators’ evaluation, subjects in Groups B and C exhibited better tolerance than those in Group A (p ≤ 0.005). Therefore, it can be concluded that the addition of adjunctive therapy with dermocosmetics containing active ingredients acting on the main targets of acne lesions while protecting the skin barrier significantly enhances the effectiveness and safety of first-line therapy. Indeed, investigators’ satisfaction was higher in Groups B and C compared to Group A (p < 0.001).
One limitation of this study is the lack of homogeneity among participants, such as hormonal imbalances or lifestyle habits. Additionally, there are no available data on global satisfaction and tolerance reported by subjects in Group A. These limitations suggest that a more comprehensive evaluation, including subjective measurements of satisfaction and tolerance, can provide a more comprehensive understanding of the effectiveness of this treatment.
Another limitation of this study is the use of IAEM (Indonesian Acne Expert Meeting) which is an Indonesian guideline on acne vulgaris severity. The IAEM scale might not be applicable to some other (non-Indonesian) countries.
Previous studies have highlighted the potential benefits of using active dermocosmetics for managing acne:
The efficacy and tolerability of a nightly application of adapalene 0.1% used with a twice-daily (BID) non-antibiotic combination of niacinamide, an antibacterial adhesive agent and zinc-pyrrolidone carboxylic acid were compared to those of adapalene used in combination with the corresponding placebo cream for the reduction of acne lesions in patients with moderate acne.22 This randomized study was conducted over a limited period of 6 weeks on a total of 140 subjects recruited in five teaching hospitals in Indonesia. Overall, there was a significantly higher decrease in the non-inflammatory lesion counts in the combination cream group compared to placebo, but only in the first two weeks of therapy. Regarding inflammatory lesions, there was no difference between the groups.
In a randomized study of 150 Caucasian subjects with mild-to-moderate acne, a multitargeted dermocosmetic cream containing salicylic acid, lipohydroxy acid, niacinamide, 2-oleamido-1,3-octadecanediol, piroctone olamine, zinc, Aqua posae filiformis, and thermal spring water applied BID for up to 56 days was shown to be as beneficial as benzoyl peroxide (BPO) 5% gel and was better tolerated.26
The efficacy and safety of a cream containing octyl salicylic acid, salicylic acid, linoleic acid, niacinamide, and piroctone olamine alone or combined with 5% BPO was investigated in a trial conducted over up to 56 days in a total of 67 Chinese subjects with mild-to-moderate acne randomized into 3 groups: treatment with BID cosmetic cream alone; treatment with BID cosmetic cream combined with once-daily (QD) BPO; and treatment with QD BPO alone.27 This study revealed that after 56 days of treatment, the combination group had the highest rate of skin lesion clearance.
In an exploratory study conducted in 55 subjects with acne, the characteristics of the microbiota on the surface of both skin areas without and with acne lesions was investigated, and changes in the microbiota profile after 28 days of a QD application of either erythromycin 4% or a dermocosmetic containing lipohydroxy acid, salicylic acid, linoleic acid, niacinamide, piroctone olamine, a ceramide and thermal spring water (Effaclar® Duo+) were determined.28 The study showed that prior to the application of the products, the skin surface microbiota of the different sampled areas was dominated by Staphylococcus, while Propionibacteria represented less than 2% of the population. The study demonstrated that erythromycin reduced the number of Actinobacteria (including Corynebacterium and Propionibacterium) while it only had a limited antibacterial effect on Staphylococci, potentially confirming the increased resistance of the bacterium to macrolides. By contrast, the tested dermocosmetic not only reduced the number of Actinobacteria—it also reduced the number of Staphylococcus spp, thus confirming that the tested dermocosmetic may be a potential alternative to topical macrolides in the management of acne that bears the advantage of not causing antibacterial resistance of the targeted bacteria. Moreover, this study showed a similar kinetic and level of efficacy in the improvement of both acne severity and acne lesions, including inflammatory lesions, over time.
Conclusion
The present study was designed (sample size, study duration) to enable a solid comparison between the treatment groups. The findings of this study demonstrated a progressive improvement in acne symptoms across all three treatment groups. Notably, the combination of the dermocosmetic cream and QD application of adapalene showed statistically significant superior efficacy when assessed using the GEA scale. Consistency of the study was also shown through the observation of the dose-dependency of adapalene effects. Additionally, the regimens that incorporated both the dermocosmetic cream and adapalene exhibited higher tolerability and satisfaction compared to using adapalene alone.
Overall, previous investigations and the present results underscore the potential of integrating dermocosmetics into acne treatment approaches, offering enhanced effectiveness and improved patient experiences.
Acknowledgments
This study was supported by La Roche-Posay Laboratoire Dermatologique.
The authors wish to thank assistants in the clinical study: Cyntia Yulyana, MD; Mufqi Handaru Priyanto, MD; Anindya Latona Sidarta, MD; Inasa Amalia, MD; Arsy Indrafatina, MD; Faya Nuralda Sitompul, MD; Kartika Purnamasari, MD; Dwi Sabtika Julia, MD; Alamanda Murasmita, MD.
This work was presented as “Effectiveness and Safety of a dermocosmetic cream containing Salicylic Acid, Lipohydroxy Acid, Niacinamide, Aqua-Posae-Filiformis, Procerad and Zinc-PCA as an adjuvant treatment for Mild and Moderate Acne in Indonesia” at the 32nd European Academy and Venereology (EADV) congress of October 11–14, 2023, as a poster presentation with interim findings. The poster’s abstract was published in the Abstract Book released by EADV.org (eadv.org › scientific › abstract-books) for the EADV Congress 2023, sub-section “Acne and related disorders, hidradenitis suppurativa”, abstract N° 4136, page 191–192 (https://eadv.org/wp-content/uploads/scientific-abstracts/EADV-congress-2023/Acne-and-related-disorders-hidradenitis-suppurativa.pdf).
Funding Statement
This study was supported by La Roche-Posay Laboratoire Dermatologique.
Abbreviations
BID, twice-daily; BPO, benzoyl peroxide; C. acnes, Cutibacterium acnes; GEA scale, Global Evaluation of Acne scale; IAEM scale, Indonesian Acne Expert Meeting scale; LRP, La Roche-Posay; n, number of subjects at baseline; QD, once-daily; QOL, quality of life; SE, standard error.
Data Sharing Statement
The authors will, upon reasonable request, share the study protocol and all data collected and statistically analyzed and in relationship with this study, except de-identified participant data, for 1 year after publication of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Delphine Kerob is a full-time employee of La Roche Posay Laboratoire Dermatologique. The authors report no other conflicts of interest in this work.
References
- 1.Lynn DD, Umari T, Dunnick CA, Dellavalle RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25. PMID: 26955297; PMCID: PMC4769025. doi: 10.2147/AHMT.S55832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Layton AM, Thiboutot D, Tan J. Reviewing the global burden of acne: how could we improve care to reduce the burden? Br J Dermatol. 2021;184(2):219–225. PMID: 32770673. doi: 10.1111/bjd.19477 [DOI] [PubMed] [Google Scholar]
- 3.Sibero HT, Sirajudin A, Anggraini DI. Prevalensi dan gambaran epidemiologi akne vulgaris di Provinsi Lampung. JK Unila. 2019;3(2):308–312. Indonesian. [Google Scholar]
- 4.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485. PMID: 23210645. doi: 10.1111/bjd.12149 [DOI] [PubMed] [Google Scholar]
- 5.Thiboutot DM, Dreno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the global alliance to improve outcomes in acne. J Am Acad Dermatol. 2018;78(2):S1–S23. doi: 10.1016/j.jaad.2017.09.078 [DOI] [PubMed] [Google Scholar]
- 6.PERDOSKI. Panduan Praktik Klinis bagi Dokter Spesialis Kulit dan Kelamin di Indonesia. 2017. Indonesian.
- 7.Koyanagi S, Koizumi J, Nakase K, et al. Increased frequency of clindamycin-resistant Cutibacterium acnes strains isolated from Japanese patients with acne vulgaris caused by the prevalence of exogenous resistance genes. J Dermatol. 2023;50(6):793–799. doi: 10.1111/1346-8138.16757 [DOI] [PubMed] [Google Scholar]
- 8.Goh CL, Noppakun N, Micali G, et al. Meeting the challenges of acne treatment in Asian patients: a review of the role of dermocosmetics as adjunctive therapy. J Cutan Aesthet Surg. 2016;9(2):85–92. PMID: 27398008; PMCID: PMC4924420. doi: 10.4103/0974-2077.184043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gollnick HP. From new findings in acne pathogenesis to new approaches in treatment. J Eur Acad Dermatol Venereol. 2015;29(Suppl 5):1–7. PMID: 26059819. doi: 10.1111/jdv.13186 [DOI] [PubMed] [Google Scholar]
- 10.Dreno B, Thiboutot D, Gollnick H, et al. Large-scale worldwide observational study of adherence with acne therapy. Int J Dermatol. 2010;49(4):448–456. doi: 10.1111/j.1365-4632.2010.04416.x [DOI] [PubMed] [Google Scholar]
- 11.Moradi Tuchayi S, Alexander TM, Nadkami A, Feldman SR. Interventions to increase adherence to acne treatment. Patient Prefer Adherence. 2016;10:2091–2096. doi: 10.2147/PPA.S117437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sevimli Dikicier B. Topical treatment of acne vulgaris: efficiency, side effects, and adherence rate. J Int Med Res. 2019;47(7):2987–2992. doi: 10.1177/0300060519847367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park C, Kim G, Patel I, Chang J, Tan X. Improving adherence to acne treatment: the emerging role of application software. Clin Cosmet Invest Dermatol. 2014;7:65–72. doi: 10.2147/CCID.S46051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araviiskaia E, Dreno B. The role of topical dermocosmetics in acne vulgaris. J Eur Acad Dermatol Venereol. 2016;30(6):926–935. doi: 10.1111/jdv.13579 [DOI] [PubMed] [Google Scholar]
- 15.Poli F, Auffret N, Claudel JP, Leccia MT, Dreno B. AFAST: an adult female acne treatment algorithm for daily clinical practice. Eur J Dermatol. 2018;28(1):101–103. doi: 10.1684/ejd.2017.3157 [DOI] [PubMed] [Google Scholar]
- 16.Mahe YF, Perez MJ, Tacheau C, et al. A new Vitreoscilla filiformis extract grown on spa water-enriched medium activates endogenous cutaneous antioxidant and antimicrobial defenses through a potential Toll-like receptor 2/protein kinase C, zeta transduction pathway. Clin Cosmet Invest Dermatol. 2013;6:191–196. PMID: 24039440; PMCID: PMC3770492. doi: 10.2147/CCID.S47324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh CL, Abad-Casintahan F, Aw DC, et al. South-East Asia study alliance guidelines on the management of acne vulgaris in South-East Asian patients. J Dermatol. 2015;42:945–953. doi: 10.1111/1346-8138.12993 [DOI] [PubMed] [Google Scholar]
- 18.Sitohang IBS. Isotretinoin for treating acne vulgaris. Int J Appl Pharm. 2021;13(2):20–25. doi: 10.22159/ijap.2021v13i2.40045 [DOI] [Google Scholar]
- 19.Sitohang IBS, Fathan H, Effendi E, Wahid M. The susceptibility of pathogens associated with acne vulgaris to antibiotics. Med J Indones. 2019;28(1):21–27. doi: 10.13181/mji.v28i1.2735 [DOI] [Google Scholar]
- 20.Sutarjo AS, Sitohang IBS, Wahid MH, Widaty S. Comparison of Malassezia spp. Proportions in inflammatory and non-inflammatory facial acne vulgaris lesions. Int J Appl Pharm. 2020;12(3):7–11. doi: 10.22159/ijap.2020.v12s3.39454 [DOI] [Google Scholar]
- 21.Paichitrojjana A, Chalermchai T. The prevalence, associated factors, and clinical characterization of Malassezia folliculitis in patients clinically diagnosed with acne vulgaris. Clin Cosmet Invest Dermatol. 2022;15:2647–2654. doi: 10.2147/CCID.S395654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sitohang IBS, Yahya YF, Simanungkalit R, Winarni DRA, Madjid A. Efficacy and tolerability of topical nicotinamide plus antibacterial adhesive agents and zinc-pyrrolidone carboxylic acid versus placebo as an adjuvant treatment for moderate acne vulgaris in Indonesia: a multicenter, double-blind, randomized, controlled trial. J Clin Aesthet Dermatol. 2020;13(7):27–31. [PMC free article] [PubMed] [Google Scholar]
- 23.Sitohang IBS, Soebaryo RW, Kanoko M. Acne lesion extraction versus oral doxycycline for moderate acne vulgaris: a randomized clinical trial. J Clin Aesthet Dermatol. 2021;14(6):E61–E65. PMID:34804358. [PMC free article] [PubMed] [Google Scholar]
- 24.Saptarini D, Witjaksono F, Sitohang IBS. Correlation study of acne vulgaris and serum Vitamin D levels in adolescents. Int J Appl Pharm. 2020;12(3):19–21. doi: 10.22159/ijap.2020.v12s3.39462 [DOI] [Google Scholar]
- 25.Kurokawa I, Kobayashi M, Nomura Y, Abe M, Kerob D, Dreno B. The role and benefits of dermocosmetics in acne management in Japan. Dermatol Ther. 2023;13:1423–1433. doi: 10.1007/s13555-023-00943-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dal Belo SE, Kanoun-Copy L, Lambert C, et al. Efficacy of a multitargeted, salicylic acid-based dermocosmetic cream compared to benzoyl peroxide 5% in Acne vulgaris: results from a randomized study. J Cosmet Dermatol. 2023:1–7. doi: 10.1111/jocd.16052 [DOI] [PubMed] [Google Scholar]
- 27.Li W, Yu Q, Shen Z, Zhang L, Zhang W, Li C. Efficacy and safety of a cream containing octyl salicylic acid, salicylic acid, linoleic acid, nicotinamide, and piroctone olamine combined with 5% benzoyl peroxide in the treatment of acne vulgaris: a randomized controlled study. Chin Med J. 2022;135(11):1381–1382. doi: 10.1097/CM9.0000000000002191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreno B, Martin R, Moyal D, Henley JB, Khammari A, Seité S. Skin microbiome and acne vulgaris: staphylococcus, a new actor in acne. Exp Dermatol. 2017;26(9):798–803. doi: 10.1111/exd.13296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors will, upon reasonable request, share the study protocol and all data collected and statistically analyzed and in relationship with this study, except de-identified participant data, for 1 year after publication of this manuscript.