ABSTRACT
Non‐invasive radioablation using stereotactic body radiation therapy with X‐ray has been proposed as a rescue treatment for refractory ventricular tachycardia (VT). However, there are concerns about the occurrence of late valvular or coronary disease. We treated VT originating from the aortic sinus cusp using the Bragg peak principle of a heavy ion beam, minimizing the dose to the aortic valve and coronary artery and providing an anti‐arrhythmic effect and cardiac function recovery due to improved sympathetic nerve heterogeneity. We present a method for targeting sympathetic nerve distribution using 123I‐metaiodobenzylguanidine scintigraphy.
Keywords: 123I‐metaiodobenzylguanidine scintigraphy, arrhythmia radioablation, non‐invasive irradiation technique, sympathetic denervation, targeted heavy ion radiotherapy, ventricular arrhythmia
1. Introduction
Radioablation using stereotactic body radiation therapy (cardiac SBRT) is an innovative and promising non‐invasive treatment for recurrent and refractory ventricular tachycardia (VT) (Cuculich et al. 2017; van der Ree et al. 2020). Although significant reductions in VT burden and implantable cardioverter‐defibrillator (ICD) shock have been demonstrated, consideration should be given to the occurrence of adverse events such as worsening of valvular disease, gastrointestinal perforation, or coronary artery calcification (Balgobind et al. 2023; van der Ree et al. 2023). Currently, X‐rays are the main type of radiation used for treatment, but proton beams and heavy particle beams may be considered as alternatives to prevent complications.
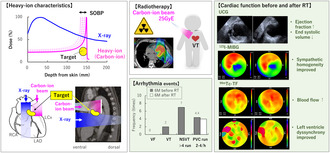
X‐rays and gamma‐rays, frequently used in clinical practice, are classified as electromagnetic waves, whereas those with nuclei heavier than electrons are called particle beams. In Japanese heavy ion medicine, the nucleus of hydrogen is called the proton, and heavy ions refer to nuclei with atomic numbers greater than that of helium (Figure 1A). Owing to a physical property called the Bragg peak, heavy ions and protons scatter less and can irradiate more intensively than X‐rays (Figure 1B). The released energy quickly decays after passing through the target; thus, the dose to the heart can be reduced to microstructures such as the valves, vessels, and surrounding organs such as the trachea and esophagus (Figure 1C). This report is the first case in which heavy ion beams were used to reduce the dose to the periaortic valve.
FIGURE 1.
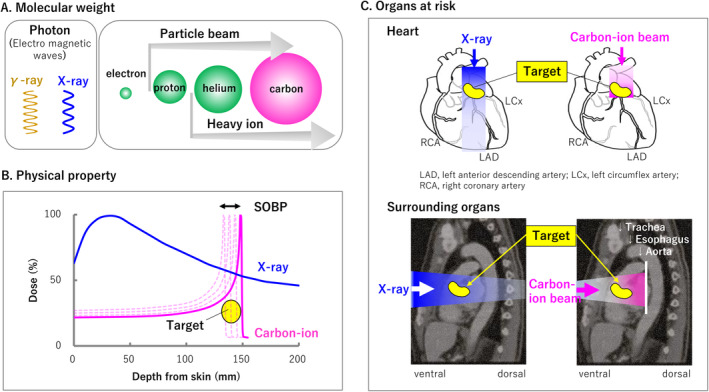
(A) Molecular weight, (B) Physical property, (C) Organs at risk. Bragg peak principle: When a high‐speed charged particle passes through a material, the incident‐heavy ion gradually loses energy and eventually stops. The energy loss peaks immediately before the heavy ion beam stops and quickly approaches zero. This maximum range is named the spread‐out of the Bragg peak after its discoverer. The ionization density of the X‐rays increases until it reaches its maximum value; thus, the energy remains constant after passing through the target material.
2. Case Presentation
The patient is a 60‐year‐old man with multiple premature ventricular contractions (PVCs) since his 40s. His left ventricle ejection fraction (EF) gradually declined over 10 years since PVC emerged. He had pre‐existing bronchial asthma and was treated with a steroid/β2‐agonist single inhaler and xanthine‐bronchodilator. Electrocardiography (ECG), coronary catheter angiography, and magnetic resonance imaging studies ruled out myocardial infarction or cardiomyopathy. Finally, he was diagnosed with PVC‐dependent dilated cardiomyopathy in 2014 (Table S1). Although EF temporarily improved with oral medications such as selective beta‐blockers and angiotensin‐converting enzyme (ACE) inhibitors (27%–60%), PVCs did not decrease; radiofrequency catheter ablation (RFCA) was performed on the right ventricular outflow tract (RVOT). Amiodarone was added due to inefficiency, but the patient developed persistent monomorphic VTs. In 2017, a second RFCA was performed on the aortic sinus cusp, immediately below the pulmonary valve and RVOT septum. After 6 months, the patient presented to the hospital via ambulance due to recurrent VTs, wherein an ICD was inserted. Although ICD shocks were recognized in 2021/2022, the patient refused to consent to undergo epicardial ablation owing to its invasiveness. Informed consent for cardiac SBRT was obtained on paper according to the Clinical Research Protocol (Japan Registry of Clinical Trials: CRB 3180004).
The information required to determine the radiation target was classified into three categories (Cuculich et al. 2017). (1) Electrophysiological information: The VT origin was the aortic sinus of Valsalva or the junction between the left and right coronary cusps, according to Yamada et al.'s (Yamada 2019) classification of 3‐documented 12‐lead ECG localization; an electrophysiological study identified the origin as the aortic sinus cusp. According to the ECG classification by Andreu et al. (2018), VT ECGs showed the highest voltage magnitude in either II/III/aVF; the polarity of the QRS axis in V3 and V4 was equipotential, indicating an AHA17 segment#7 (anterior wall) origin (Figure 2A). The pseudo‐12‐lead ECG of high‐resolution ambulatory recordings also showed that > 80% of PVCs originated from segments#1 and #7 (Figure 2B). (2) Structural information: The patient's most recent echocardiography showed diffuse hypokinesis and severe posteroinferior hypokinesis but no wall thinning or calcification on cardiac computed tomography. (3) Functional information: (99 m) Technetium‐tetrofosmin (99mTc‐TF) showed decreased posteroinferior blood flow, mild cardiac enlargement, and left ventricular asynchrony. 123I‐metaiodobenzylguanidine (123I‐MIBG) indicated denervation posteroinferior to the apex. These results suggested that PVC/VT might be generated from the anterior wall due to regional sympathetic hyperinnervation contralateral to the posteroinferior cardiac dysfunction (Inoue and Zipes 1987; Yoshioka et al. 2000). Therefore, we targeted the basal‐, mid‐, and apex‐anterior regions (segments: #1, #7, and #13), anticipating an adequate denervation effect from the carbon‐ion beam (Amino et al. 2023).
FIGURE 2.
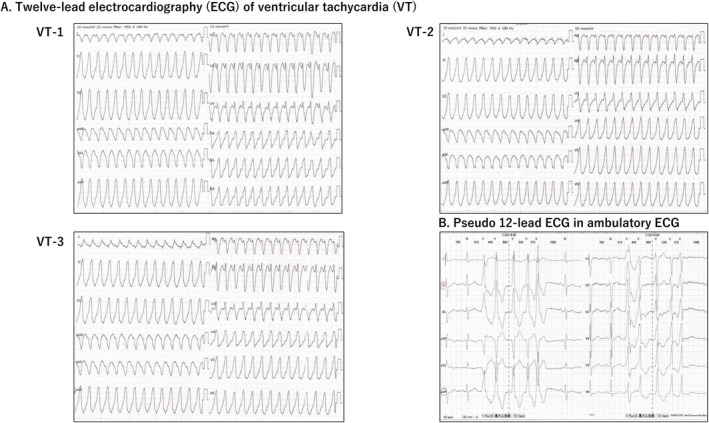
(A) Twelve‐lead electrocardiography (ECG), (B) Pseudo‐12‐lead ECG in ambulatory ECG. Sustained ventricular tachycardia (VT)‐1, VT‐2, and VT‐3 shows 176–190 bpm, left bundle branch block, inferior axis, and transition zone of V3.
According to this treatment plan, the scanned carbon‐ion beam treatment planning system (XiO, Elekta AB, Sweden) created the target volume from two beam directions (12.5 Gy × 2) (Figure 3). A dose‐volume distribution was created by extending a 5‐mm circumference around the coronary artery, considering the movement of cardiac contraction, and was checked against the dose convention to determine whether carbon beams provide maximum coverage of the treatment volume while minimizing the organ at risk (Table S2). The prescribed doses were set at 2.5 relative biological effectiveness and 25 GyE for single irradiation, based on the past clinical safety and experimental data (Text S1). The patient was awake and immobilized in the supine position on a vacuum‐fixed cushion covered with a shell; he used a therapy device (CI‐1000S; Toshiba Energy Systems Corporation) with respiratory synchronization system (AZ‐733V; Anzai Medical Co., Tokyo, Japan) (Text S2). There were no abnormalities in vital signs, ECG monitoring, or ICD during irradiation. The total time from entering to leaving the treatment room was 30 min, and the beam‐on times were 4 min and 6 s.
FIGURE 3.
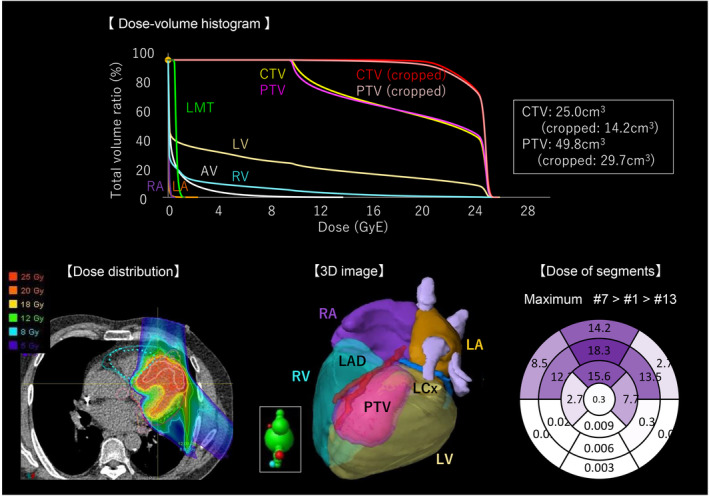
DVH: The graph displays the dose (GyE) on the horizontal axis and the percentage of exposed intracardiac structures on the vertical axis. The upper right of the target area and the lower left of the normal organs may be a better treatment plan. Heavy ion's Bragg peak creates a steep dose gradient near the target. Cropped ‐CTV, ‐PTV is the volume created by considering organs at risk. AV, aortic valve; CTV, clinical target volume; LA, left atrium; LAD, left anterior descending artery; LCx, left circumflex artery; LMT, left main trunk; LV, left ventricle; PTV, planning target volume; RA, right ventricle; RV, right ventricle.
No adverse events or abnormal blood findings (Table S3) were observed at the regular follow‐up visits 6 months after treatment. The ICD recording showed no VT recurrence and a 50% reduction in PVCs (Figure 4). Non sustained VT (NSVT) was 160–170 bpm, without other remarkable findings. There was no change in the beta‐blocker and amiodarone dosage or ICD setting 6 months before or after cardiac‐SBRT. The echocardiography showed no occurrence of valvular disease, unchanged diastolic dysfunction, ventricular end‐systolic volume reduction, and 10% EF improvement (Figure 5). Pre‐ and post‐treatment nuclear medicine studies suggested decreased uptake of 123I‐MIBG at the anterior irradiated wall, indicating mild denervation (Figure 6A); 99mTc‐TF showed slightly increased uptake in the anterior area (Figure 6B) and significant narrowing of phase dispersion in the assessment of left ventricular dyssynchrony (Figure 6C). Detailed analysis showed improvements in EF and ischemia scores and no deterioration in the diastolic index (Table S4).
FIGURE 4.
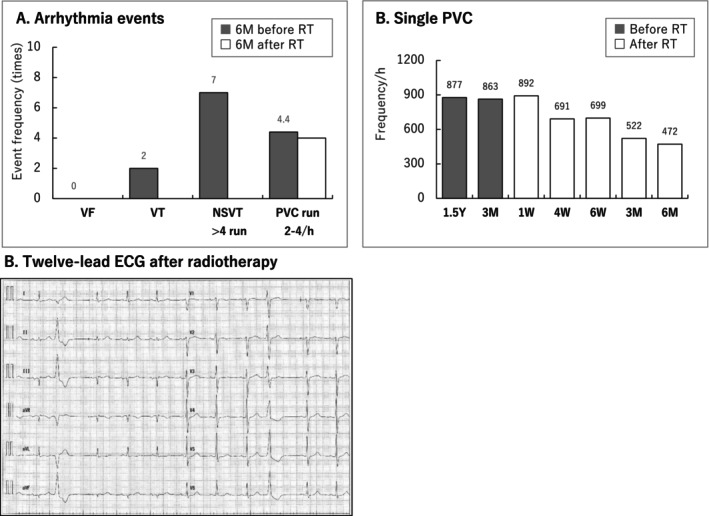
(A) Arrhythmia events, (B) Single PVC, (C) Twelve‐lead electrocardiography (ECG) after radiotherapy. ICD, implantable cardioverter‐defibrillator; NSVT, non‐sustained VT; PVC, premature ventricular contraction; RT, radiotherapy; VF, ventricular fibrillation; VT, ventricular tachycardia.
FIGURE 5.
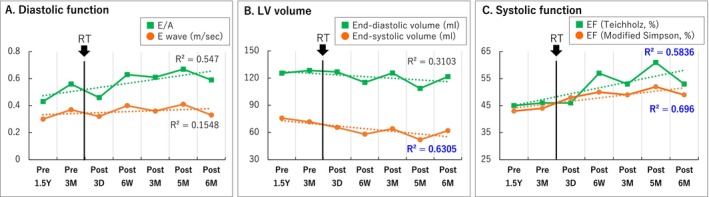
(A) Diastolic function, (B) LV volume, (C) Systolic function. E/A, early peak filling rate to atrial‐peak filling rate; EF, ejection fraction.
FIGURE 6.
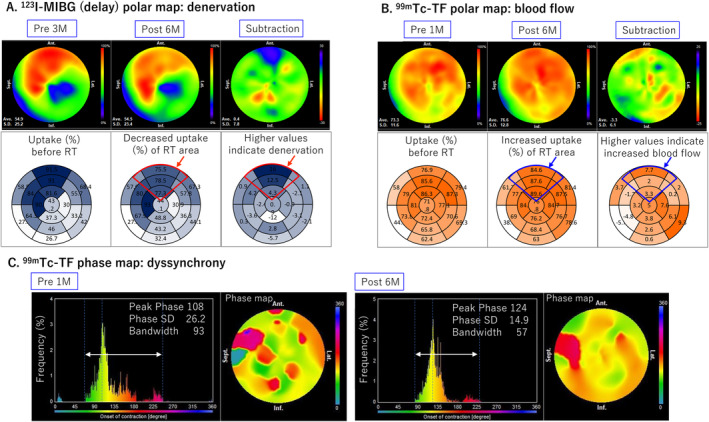
(A) 123I‐MIBG polar map (delay), (B) 99mTc‐TF polar map, (C) 99mTc‐TF phase map. 123I‐MIBG, 123I‐metaiodobenzylguanidine scintigraphy; 99mTc‐TF, (99m)Technetium‐tetrofosmin.
3. Discussion
Although short‐term VT suppression exceeds 85% by cardiac SBRT (van der Ree et al. 2020), the target determination process and technical procedure have not been clearly defined (Lydiard et al. 2021). A multicenter survey by the Euro‐STOPSTORM consortium revealed that the current emphasis is on estimating VT‐exit and VT‐substrate using catheter ablation mapping as the target area for irradiation (Grehn et al. 2023). Idiopathic PVC/VT lacks anatomical scarring and organic changes, unlike structural cardiac diseases such as myocardial infarction and cardiomyopathy. Although RVOT origins are the most common, they can also be found in other locations, including the aortic sinus cusp, pulmonary artery, aortic mitral valve connection, left ventricle summit, and mitral valve ring (Yamada 2019). The challenge with cardiac‐SBRT is setting an alternative target within the myocardium when the PVC origin is in complex microstructures.
As the underlying mechanism of anti‐arrhythmic early effects after irradiation is not necessarily due to transmural fibrosis‐based blockade of the re‐entry circuit, it is preferable to set targets considering radiobiological effects related to cardiac reprogramming (Zhang et al. 2021). We previously reported that carbon‐ion beams suppressed nerve sprouting and reduced VT inducibility in aging rabbits (Amino et al. 2023). Denervation hypersensitivity in intact regions leads to arrhythmogenicity owing to repolarization instability in ischemia‐independent denervation hearts with phenol application (Inoue and Zipes 1987; Yoshioka et al. 2000). Thus, the sympathetic hyperinnervation area may be a new target for irradiation.
Although the improvement in contractility in this case is consistent with a reduction in arrhythmic events, the resulting improvement in left ventricular synchrony despite incomplete suppression of PVCs has the potential to prevent heart failure (HF) independently. Similar improvements have been reported in mouse HF models and patients with VT who received low‐dose X‐ray irradiation (Pedersen et al. 2023). Nevertheless, because HF worsening was the main cause of death (52%) after cardiac SBRT (Benali et al. 2024), treatment may be desirable before end‐stage HF or using doses below 25 Gy to achieve functional recovery. Case reports using proton therapy have revealed a slight improvement in cardiac strain function (Dusi et al. 2021); robust optimization techniques can help reduce the mean dose to the heart and left lung (Widesott et al. 2020). No adverse events in humans related to heavy ion or proton beams have been reported. However, the studies have demonstrated that the aortic valve may be affected by X‐rays (Balgobind et al. 2023; van der Ree et al. 2023); therefore, its monitoring should be continued in patients.
4. Limitations
The limitations of this study are presented in Text S3.
5. Conclusions
Cardiac‐SBRT was performed in a patient with idiopathic VT using the unique physical properties of targeted heavy ion radiation, with a reduced risk of exposure to surrounding organs. Modification of the heterogeneous sympathetic distribution with appropriate denervation results in anti‐arrhythmic effects, restoration of cardiac contractility, and left ventricular synchrony.
Author Contributions
Conceptualization: K.Y. Data curation: S.K., S.M., A.Y., Y.I. Formal analysis: S.K. Funding acquisition: M.A., T.S., K.Y. Investigation: M.A., S.K. Methodology: E.K., M.W., T.S. Project administration: K.Y. Resources: J.H. Software: T.Y. Supervision: E.K., K.Y. Validation: S.K., S.M. Visualization: M.A., K.S. Writing: M.A. Review and Editing: M.A., M.W., S.M., S.K., Y.I., K.Y.
Ethics Statement
The authors attest that they comply with the Human Study Committees of the authors' institutions, including providing patient written consent.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Tables S1–S4
Text S1–S3
Acknowledgments
Tokai University: Cardiology, Kyong Hee Lee, Susumu Sakama; Clinical Engineering Technology, Ami Owaki, Akari Takahashi, Misako Shirasu, Yu Kojima; Radiology, Tomoyuki Hiroki, Hideharu Todaka; Radiation Oncology, Toshihisa Kuroki, Tsuyoshi Fukuzawa, Akitomo Sugahara; Medical Information and Systems Unit, Tsubasa Yamaguchi, Masatomo Suzuki; Clinical Research Coordinator, Yukiko Fujiwara; Emergency Care Medicine, Seiji Morita, Yoshihide Nakagawa; Baseline and Yamato Electrocardiography Analysis: Keiko Yamaguchi, Hiromichi Fukushi; Fukuda Denshi: Takanao Fujii; QST Hospital: Miho Fujishima, Mari Motomura, Makoto Shinoto, Sumitaka Hasegawa, Hitoshi Ishikawa, Shigeru Yamada; QST Clinical Research Coordinator: Kazuko Suzuki, Kyoko Tsuyuki, Yuka Nishimura; QST: Toshiyuki Shirai, Yukio Uchihori, Toshio Hirano, Shigeo Koyasu; Ex‐QST: Yoshiya Furusawa, Toshio Hirano.
Funding: This work was supported by Japan Society for the Promotion of Science KAKENHI (Grant No. 20K08459), the Tokai University School of Medical Research Grant 2023, and the QST President's Strategic Grant (Creative Research 2019–2022). Consulting fee/honorarium: None. Patent royalties/licensing fees: None. Employment/Leadership position/Advisory role: None. Others: None.
Data Availability Statement
The data supporting this study's findings are available on request from the corresponding author. The data are not publicly available due to restrictions e.g. their containing information that could compromise the privacy of research participants.
References
- Amino, M. , Yamazaki M., Yoshioka K., et al. 2023. “Heavy Ion Irradiation Reduces Vulnerability to Atrial Tachyarrhythmias―Gap Junction and Sympathetic Neural Remodeling.” Circulation Journal 87: 1016–1026. 10.1253/circj.CJ-22-0527. [DOI] [PubMed] [Google Scholar]
- Andreu, D. , Fernández‐Armenta J., Acosta J., et al. 2018. “A QRS Axis‐Based Algorithm to Identify the Origin of Scar‐Related Ventricular Tachycardia in the 17‐Segment American Heart Association Model.” Heart Rhythm 15: 1491–1497. 10.1016/j.hrthm.2018.06.013. [DOI] [PubMed] [Google Scholar]
- Balgobind, B. V. , Visser J., Grehn M., et al. 2023. “Refining Critical Structure Contouring in STereotactic Arrhythmia Radioablation (STAR): Benchmark Results and Consensus Guidelines From the STOPSTORM.Eu Consortium.” Radiotherapy and Oncology 189: 109949. 10.1016/j.radonc.2023.109949. [DOI] [PubMed] [Google Scholar]
- Benali, K. , Zei P. C., Lloyd M., et al. 2024. “One‐Year Mortality and Causes of Death After Stereotactic Radiation Therapy for Refractory Ventricular Arrhythmias: A Systematic Review and Pooled Analysis.” Trends in Cardiovascular Medicine 34: 488–496. 10.1016/j.tcm.2023.12.008. [DOI] [PubMed] [Google Scholar]
- Cuculich, P. S. , Schill M. R., Kashani R., et al. 2017. “Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia.” New England Journal of Medicine 377: 2325–2336. 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusi, V. , Vitolo V., Frigerio L., et al. 2021. “First‐In‐Man Case of Non‐Invasive Proton Radiotherapy for the Treatment of Refractory Ventricular Tachycardia in Advanced Heart Failure.” European Journal of Heart Failure 23: 195–196. 10.1002/ejhf.2056. [DOI] [PubMed] [Google Scholar]
- Grehn, M. , Mandija S., Miszczyk M., et al. 2023. “STereotactic Arrhythmia Radioablation (STAR): The Standardized Treatment and Outcome Platform for Stereotactic Therapy of Re‐Entrant Tachycardia by a Multidisciplinary Consortium (STOPSTORM.Eu) and Review of Current Patterns of STAR Practice in Europe.” Europace 25: 1284–1295. 10.1093/europace/euac238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue, H. , and Zipes D. P.. 1987. “Results of Sympathetic Denervation in the Canine Heart: Supersensitivity That May Be Arrhythmogenic.” Circulation 75: 877–887. 10.1161/01.cir.75.4.877. [DOI] [PubMed] [Google Scholar]
- Lydiard, S. , Blanck O., Hugo G., O'Brien R., and Keall P.. 2021. “A Review of Cardiac Radioablation (CR) for Arrhythmias: Procedures, Technology, and Future Opportunities.” International Journal of Radiation Oncology, Biology, Physics 109: 783–800. 10.1016/j.ijrobp.2020.10.036. [DOI] [PubMed] [Google Scholar]
- Pedersen, L. N. , Valenzuela Ripoll C., Ozcan M., et al. 2023. “Cardiac Radiation Improves Ventricular Function in Mice and Humans With Cardiomyopathy.” Med 4: 928–943.e5. 10.1016/j.medj.2023.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ree, M. H. , Blanck O., Limpens J., et al. 2020. “Cardiac Radioablation—A Systematic Review.” Heart Rhythm 17: 1381–1392. 10.1016/j.hrthm.2020.03.013. [DOI] [PubMed] [Google Scholar]
- van der Ree, M. H. , Luca A., Herrera Siklody C., et al. 2023. “Effects of Stereotactic Arrhythmia Radioablation on Left Ventricular Ejection Fraction and Valve Function Over Time.” Heart Rhythm 20: 1206–1207. 10.1016/j.hrthm.2023.05.022. [DOI] [PubMed] [Google Scholar]
- Widesott, L. , Dionisi F., Fracchiolla F., et al. 2020. “Proton or Photon Radiosurgery for Cardiac Ablation of Ventricular Tachycardia? Breath and ECG Gated Robust Optimization.” Physica Medica 78: 15–31. 10.1016/j.ejmp.2020.08.021. [DOI] [PubMed] [Google Scholar]
- Yamada, T. 2019. “Twelve‐Lead Electrocardiographic Localization of Idiopathic Premature Ventricular Contraction Origins.” Journal of Cardiovascular Electrophysiology 30: 2603–2617. 10.1111/jce.14152. [DOI] [PubMed] [Google Scholar]
- Yoshioka, K. , Gao D., Chin M., et al. 2000. “Heterogeneous Sympathetic Innervation Influences Local Myocardial Repolarization in Normally Perfused Rabbit Hearts.” Circulation 101: 1060–1066. 10.1161/01.cir.101.9.1060. [DOI] [PubMed] [Google Scholar]
- Zhang, D. M. , Navara R., Yin T., et al. 2021. “Cardiac Radiotherapy Induces Electrical Conduction Reprogramming in the Absence of Transmural Fibrosis.” Nature Communications 12: 5558. 10.1038/s41467-021-25730-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
Text S1–S3
Data Availability Statement
The data supporting this study's findings are available on request from the corresponding author. The data are not publicly available due to restrictions e.g. their containing information that could compromise the privacy of research participants.


