Abstract
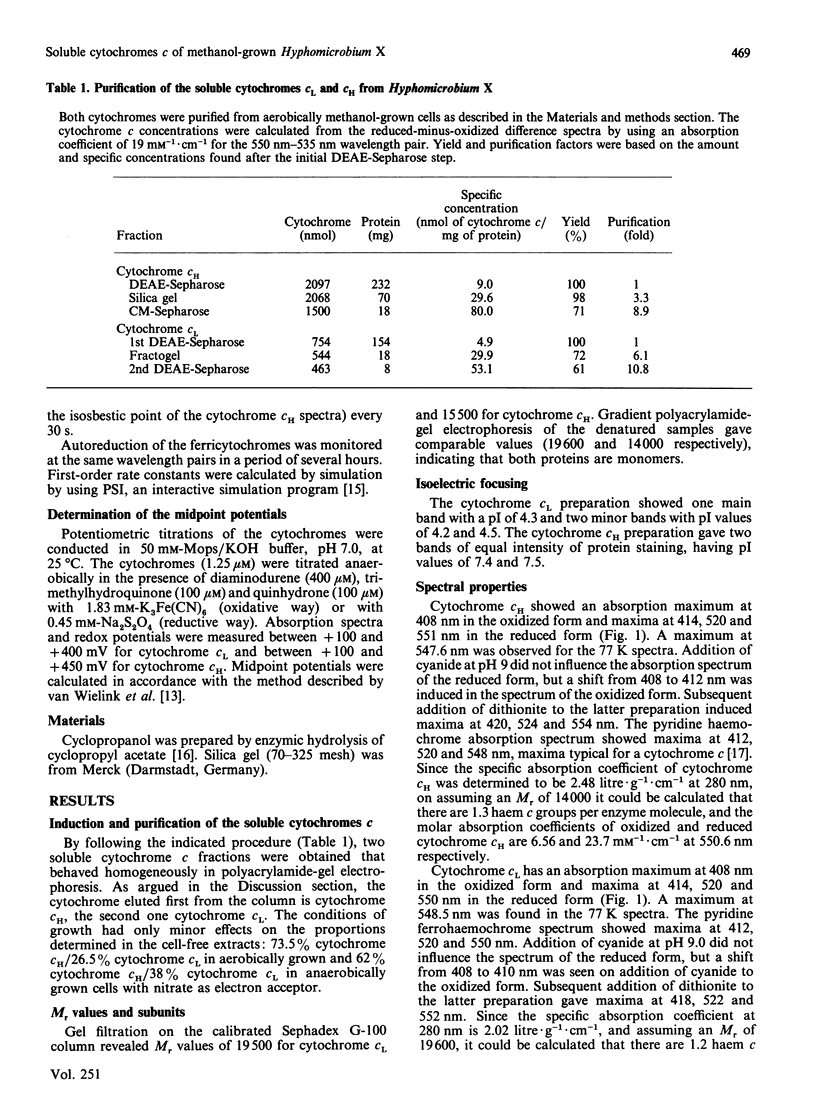
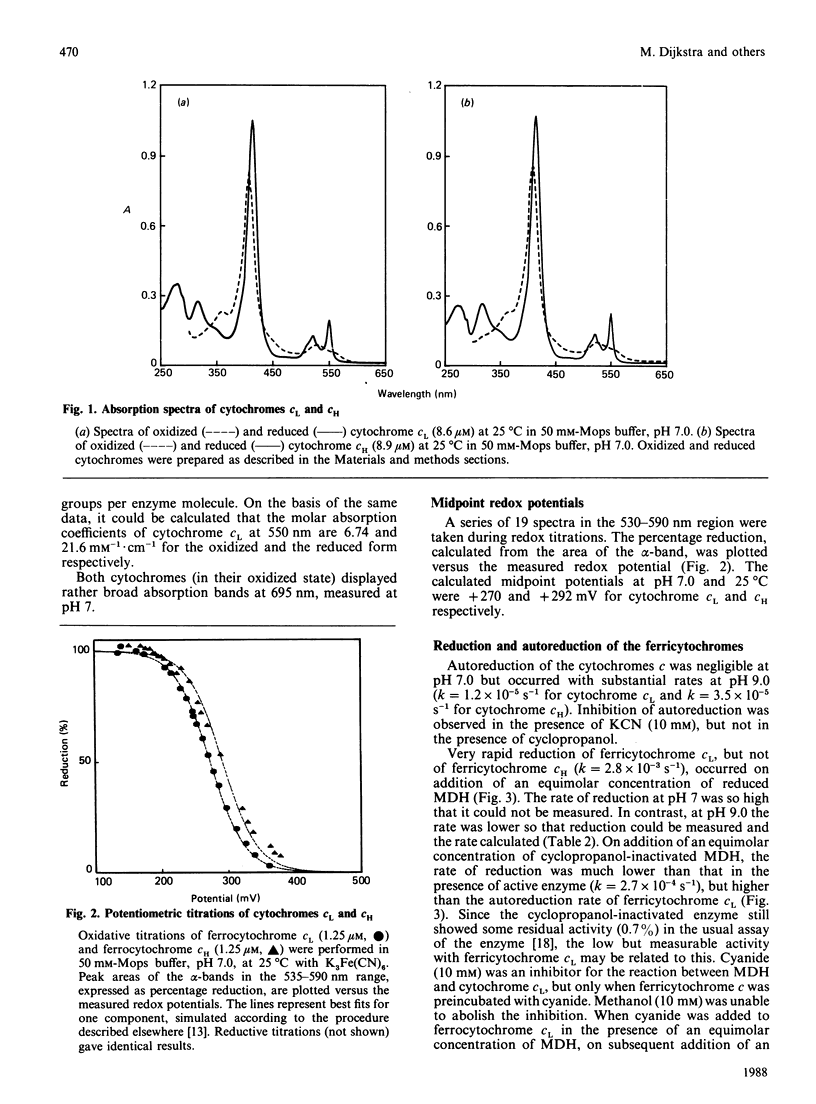
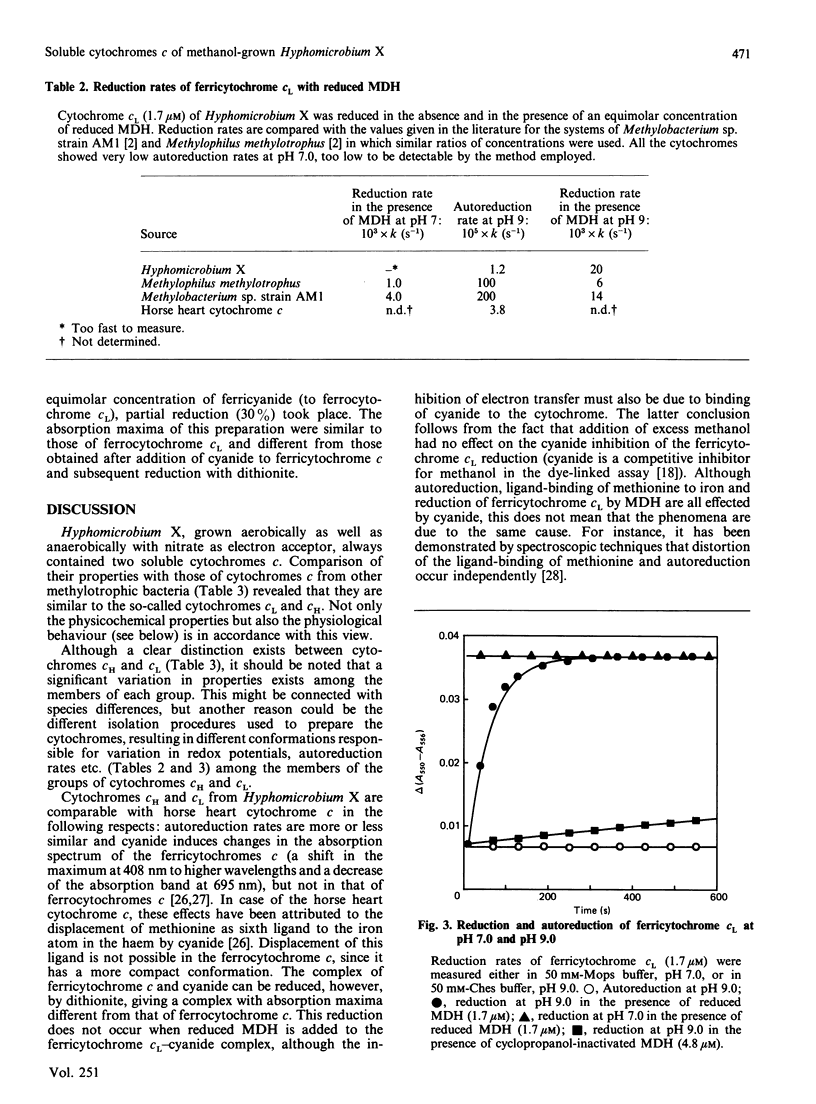
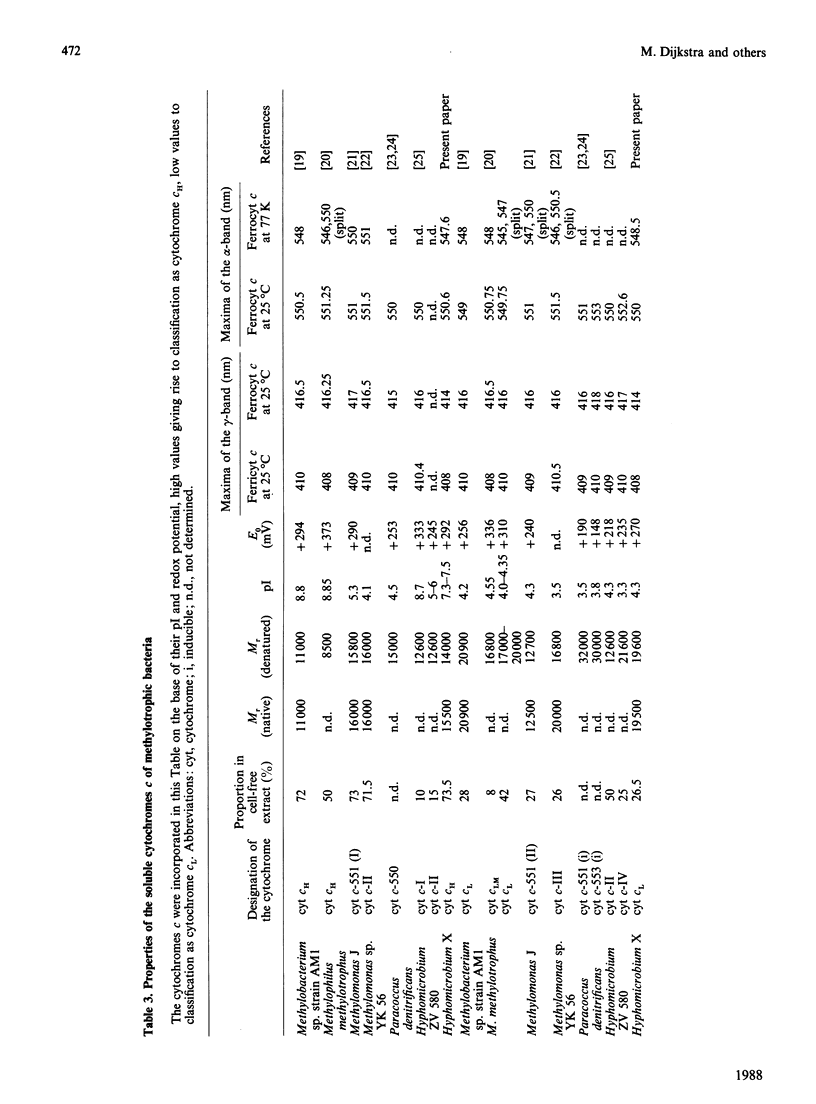
Hyphomicrobium X, grown on methanol with O2 or nitrate as electron acceptor, contains two major soluble cytochromes c. These were isolated in electrophoretically homogeneous form. They are related to cytochromes c already described for other methylotrophic bacteria and designated cytochromes cH and cL (properties indicated in that order) in view of the following characteristics: absorption maxima of the reduced forms (414, 520 and 551 nm and 414, 520 and 550 nm); molar absorption coefficients of the alpha-bands (23,700 M-1.cm-1 and 21,600 M-1.cm-1); maxima of the alpha-bands (no splitting) at 77 K (547.6 nm and 548.5 nm); Mr values of the native proteins (15,000 and 19,500); pI values (7.4 and 7.5, and 4.3); midpoint potentials at pH 7.0 (+292 mV and +270 mV). Both were monomers containing 1 haem c group per protein molecule, the oxidized forms binding cyanide at high pH. Autoreduction also occurred at high pH but at a rate significantly lower than that reported for other ferricytochromes c. On the other hand, the reverse situation applies to the reduction of ferricytochrome cL by reduced methanol dehydrogenase, the reduction occurring instantaneously at pH 7 but much more slowly at pH 9 (ferricytochrome cH was reduced at a 7-fold lower rate, but the rates at pH 7 and 9 were similar). Insignificant reduction was observed with cyclopropanol-inactivated enzyme or with enzyme in the presence of EDTA. In view of the dissimilarities, it is concluded that different mechanisms operate in the autoreduction of ferricytochrome cL and in its reduction by reduced methanol dehydrogenase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony C. Bacterial oxidation of methane and methanol. Adv Microb Physiol. 1986;27:113–210. doi: 10.1016/s0065-2911(08)60305-7. [DOI] [PubMed] [Google Scholar]
- Beardmore-Gray M., O'Keeffe D. T., Anthony C. The autoreducible cytochromes c of the methylotrophs Methylophilus methylotrophus and Pseudomonas AM1. Biochem J. 1982 Oct 1;207(1):161–165. doi: 10.1042/bj2070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brady R. S., Flatmark T. Autoreduction of horse heart ferricytochrome c. Kinetic and equilibrium studies of the over-all reaction. J Mol Biol. 1971 May 14;57(3):529–539. doi: 10.1016/0022-2836(71)90107-0. [DOI] [PubMed] [Google Scholar]
- Carver M. A., Humphrey K. M., Patchett R. A., Jones C. W. The effect of EDTA and related chelating agents on the oxidation of methanol by the methylotrophic bacterium, Methylophilus methylotrophus. Eur J Biochem. 1984 Feb 1;138(3):611–615. doi: 10.1111/j.1432-1033.1984.tb07958.x. [DOI] [PubMed] [Google Scholar]
- Cross A. R., Anthony C. The purification and properties of the soluble cytochromes c of the obligate methylotroph Methylophilus methylotrophus. Biochem J. 1980 Nov 15;192(2):421–427. doi: 10.1042/bj1920421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra M., Frank J., Jongejan J. A., Duine J. A. Inactivation of quinoprotein alcohol dehydrogenases with cyclopropane-derived suicide substrates. . Eur J Biochem. 1984 Apr 16;140(2):369–373. doi: 10.1111/j.1432-1033.1984.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jr Studies on methanol dehydrogenase from Hyphomicrobium X. Isolation of an oxidized form of the enzyme. Biochem J. 1980 Apr 1;187(1):213–219. doi: 10.1042/bj1870213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Westerling J. Purification and properties of methanol dehydrogenase from Hyphomicrobium x. Biochim Biophys Acta. 1978 Jun 9;524(2):277–287. doi: 10.1016/0005-2744(78)90164-x. [DOI] [PubMed] [Google Scholar]
- Gray K. A., Knaff D. B., Husain M., Davidson V. L. Measurement of the oxidation-reduction potentials of amicyanin and c-type cytochromes from Paracoccus denitrificans. FEBS Lett. 1986 Oct 27;207(2):239–242. doi: 10.1016/0014-5793(86)81496-x. [DOI] [PubMed] [Google Scholar]
- Husain M., Davidson V. L. Characterization of two inducible periplasmic c-type cytochromes from Paracoccus denitrificans. J Biol Chem. 1986 Jul 5;261(19):8577–8580. [PubMed] [Google Scholar]
- Ilan Y., Shafferman A. Effects of alcohol/water mixtures on the structure and reactivity of cytochrome c. Biochim Biophys Acta. 1978 Jan 11;501(1):127–135. doi: 10.1016/0005-2728(78)90101-9. [DOI] [PubMed] [Google Scholar]
- O'Keeffe D. T., Anthony C. The interaction between methanol dehydrogenase and the autoreducible cytochromes c of the facultative methylotroph Pseudomonas AM1. Biochem J. 1980 Aug 15;190(2):481–484. doi: 10.1042/bj1900481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe D. T., Anthony C. The two cytochromes c in the facultative methylotroph Pseudomonas am1. Biochem J. 1980 Nov 15;192(2):411–419. doi: 10.1042/bj1920411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta S., Tobari J. Two cytochromes c of Methylomonas J. J Biochem. 1981 Jul;90(1):215–224. doi: 10.1093/oxfordjournals.jbchem.a133452. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Van Wielink J. E., Oltmann L. F., Leeuwerik F. J., De Hollander J. A., Stouthamer A. H. A method for in situ characterization of b- and c-type cytochromes in Escherichia coli and in complex III from beef heart mitochondria by combined spectrum deconvolution and potentiometric analysis. Biochim Biophys Acta. 1982 Aug 20;681(2):177–190. doi: 10.1016/0005-2728(82)90021-4. [DOI] [PubMed] [Google Scholar]
- van Iersel J., Jzn J. F., Duine J. A. Determination of absorption coefficients of purified proteins by conventional ultraviolet spectrophotometry and chromatography combined with multiwavelength detection. Anal Biochem. 1985 Nov 15;151(1):196–204. doi: 10.1016/0003-2697(85)90072-7. [DOI] [PubMed] [Google Scholar]


