Abstract
1. After transplantation, the rat AH-130 Yoshida ascites hepatoma enters a phase of exponential (log) growth, followed by a quasi-stationary (sta) state. Combining measurements made in vivo and in vitro, cessation of protein accumulation (growth) in sta phase has previously been shown to result from convergent reduction of protein synthesis and enhancement of protein breakdown [Tessitore, Bonelli, Cecchini, Amenta & Baccino (1987) Arch. Biochem. Biophys. 255, 372-384]. 2. One day after labelling in the animal with [3H]leucine, AH-130 cells were processed for short-term assays in vitro to measure rates of endogenous protein breakdown. 3. Exposure of AH-130 cells to inhibitors interfering with different steps of the acidic vacuolar pathway (AVP) showed that: (i) in log tumour cells the AVP was extensively suppressed; (ii) in sta tumour cells virtually all of the proteolytic acceleration was accounted for by activation of the AVP. 4. Treating log tumour cells with glucagon, cyclic AMP, or nutritional deprivation failed to elevate substantially the proteolytic rates. Nor could the elevation in proteolysis be explained by changes in free amino acids, which were more concentrated in the ascitic fluid of sta tumours. 5. The enhanced proteolysis in sta tumour cells was not associated with any increase in the intracellular activity levels of lysosomal cathepsins B, D, H, and L. 6. The above growth-related modulation of protein breakdown in AH-130 cells was probably a reflection of the tumour growth state rather than the direct effect of environmental stimuli.
Full text
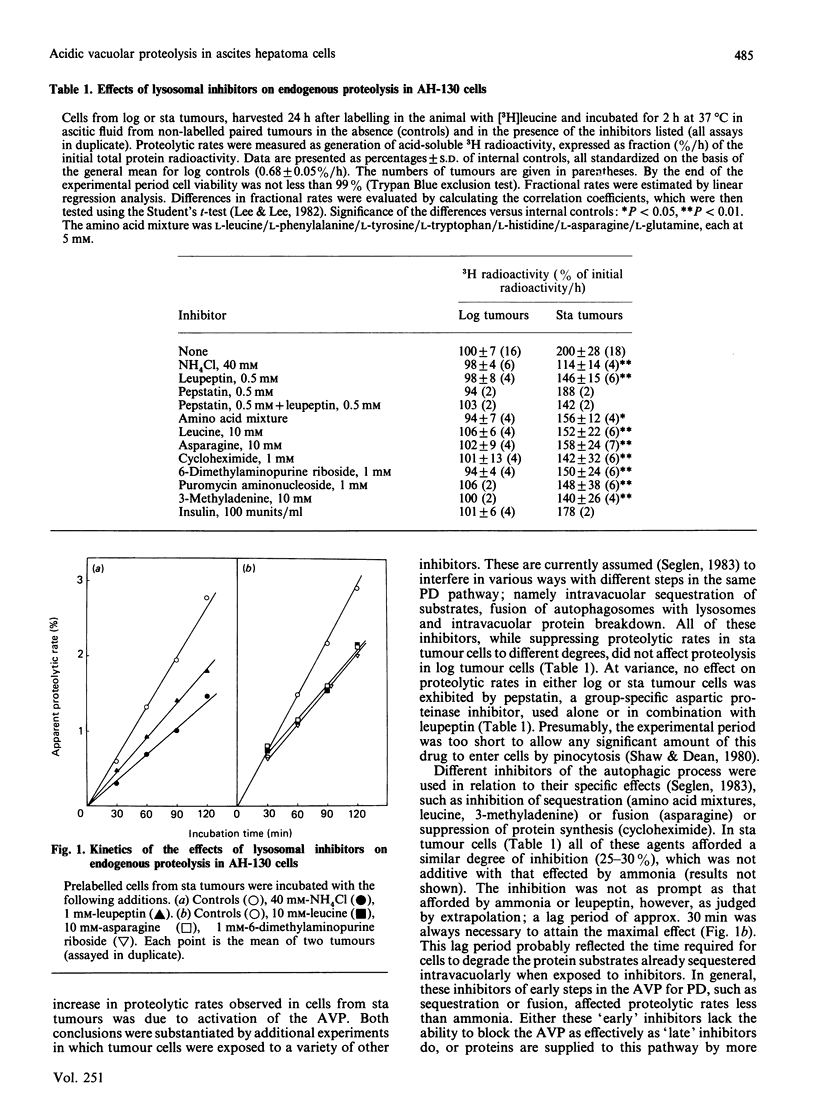
PDF

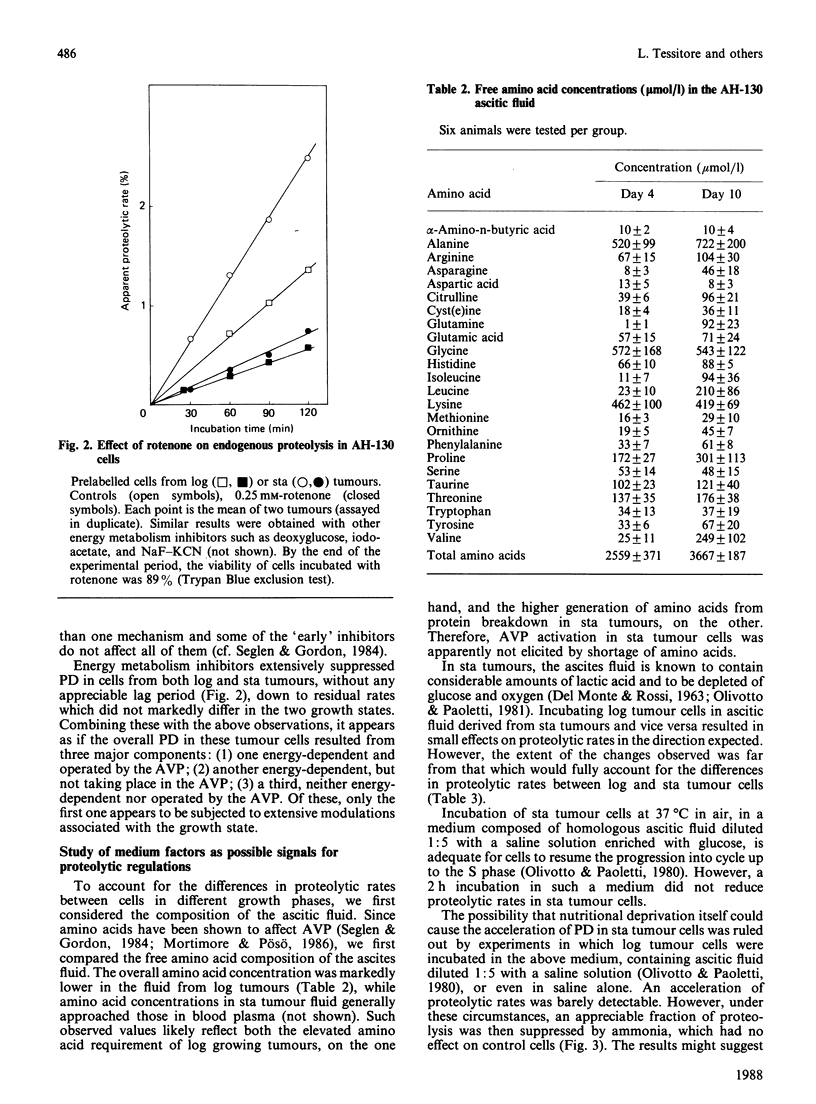


Selected References
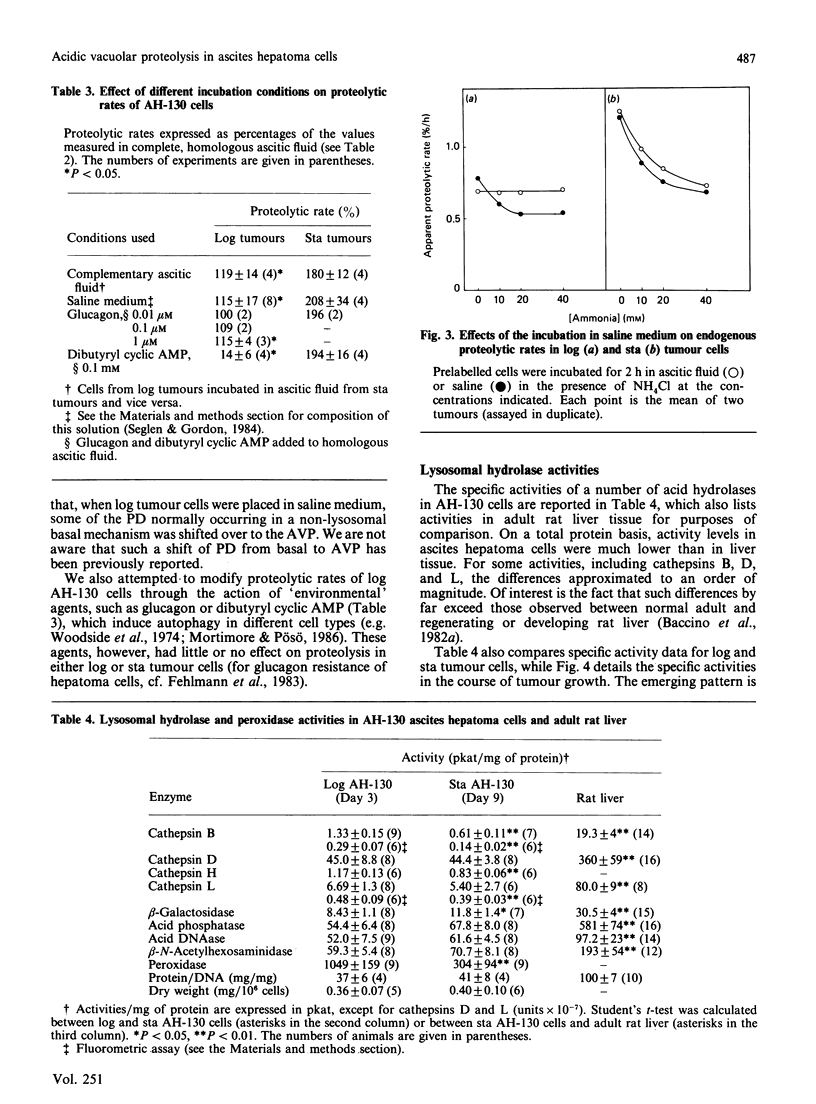
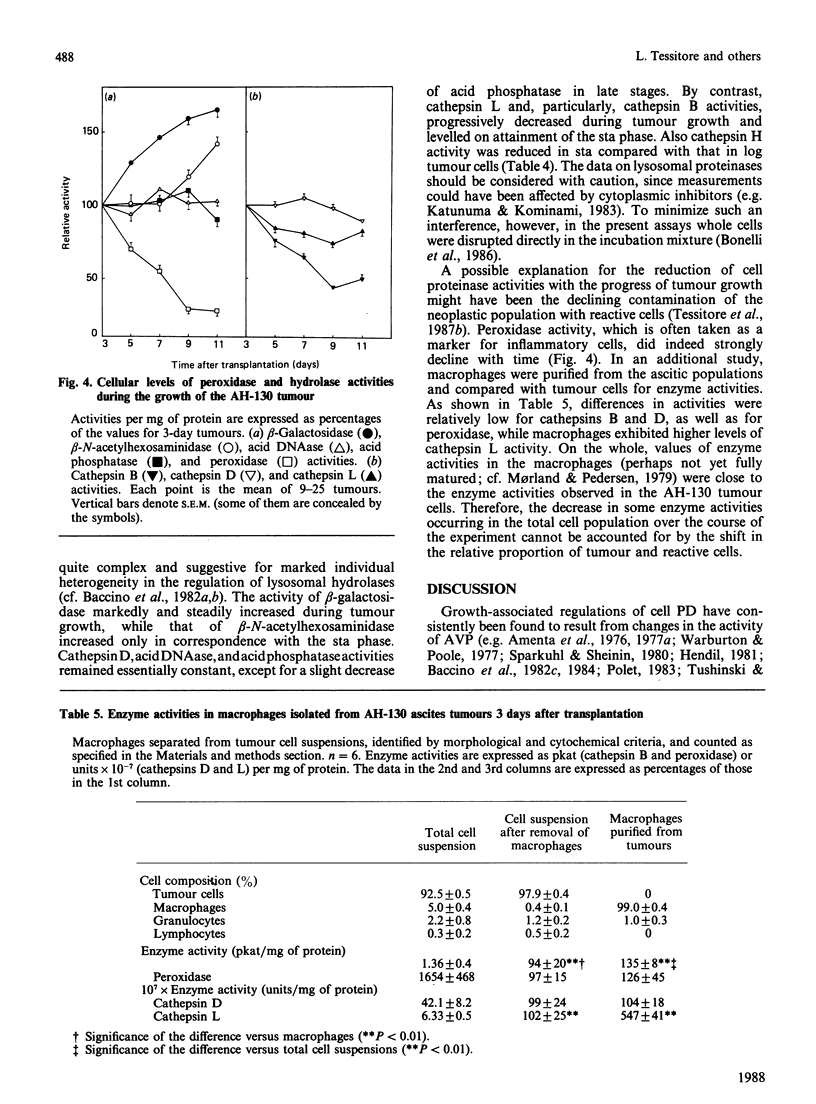
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Baccino F. M., Sargus M. J. Cell protein degradation in cultured rat embryo fibroblasts. Suppression by vinblastine of the enhanced proteolysis by serum-deficient media. Biochim Biophys Acta. 1976 Dec 21;451(2):511–516. doi: 10.1016/0304-4165(76)90146-x. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Baccino F. M. Control of cell protein degradation. Changes in activities of lysosomal proteases. Biochim Biophys Acta. 1977 Jun 3;476(3):253–261. doi: 10.1016/0005-2787(77)90008-9. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Baccino F. M. Effect of microtubular or translational inhibitors on general cell protein degradation. Evidence for a dual catabolic pathway. Biochem J. 1977 Nov 15;168(2):223–227. doi: 10.1042/bj1680223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta J. S., Sargus M. J., Baccino F. M. Inhibition of basal protein degradation in rat embryo fibroblasts by cycloheximide: correlation with activities of lysosomal proteases. J Cell Physiol. 1978 Dec;97(3 Pt 1):267–283. doi: 10.1002/jcp.1040970302. [DOI] [PubMed] [Google Scholar]
- Baccino F. M., Messina M., Musi M., Tessitore L. Levels of proteolytic activities and cell protein degradation. Acta Biol Med Ger. 1981;40(10-11):1249–1258. [PubMed] [Google Scholar]
- Baccino F. M., Tessitore L., Bonelli G. Control of protein degradation and growth phase in normal and neoplastic cells. Toxicol Pathol. 1984;12(3):281–287. doi: 10.1177/019262338401200312. [DOI] [PubMed] [Google Scholar]
- Baccino F. M., Tessitore L., Bonelli G., Rubano R., Terzuolo S. Ascites hepatoma: a model system to investigate cell protein degradation in the animal as well as in vitro. Prog Clin Biol Res. 1985;180:595–597. [PubMed] [Google Scholar]
- Baccino F. M., Tessitore L., Cecchini G., Messina M., Zuretti M. F., Bonelli G., Gabriel L., Amenta J. S. Control of cell protein catabolism in rat liver. Effects of starvation and administration of cycloheximide. Biochem J. 1982 Aug 15;206(2):395–405. doi: 10.1042/bj2060395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- DEL MONTE U., ROSSI C. B. Glucose supply by the living host and glycolysis of Yoshida ascites hepatoma in vivo. Cancer Res. 1963 Mar;23:363–367. [PubMed] [Google Scholar]
- Fehlmann M., Crettaz M., Kahn C. R. Glucagon resistance of hepatoma cells. Evidence for receptor and post-receptor defects. Biochem J. 1983 Sep 15;214(3):845–850. doi: 10.1042/bj2140845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feo F., Bonelli G. Effect of chemical, physical and enzymic treatments on lysosomes from AH-130 yoshida ascites hepatoma. Experientia. 1971 Mar 15;27(3):317–318. doi: 10.1007/BF02138169. [DOI] [PubMed] [Google Scholar]
- Fiszer-Szafarz B., Szafarz D., Guevara de Murillo A. A general, fast, and sensitive micromethod for DNA determination application to rat and mouse liver, rat hepatoma, human leukocytes, chicken fibroblasts, and yeast cells. Anal Biochem. 1981 Jan 1;110(1):165–170. doi: 10.1016/0003-2697(81)90130-5. [DOI] [PubMed] [Google Scholar]
- Gronostajski R. M., Goldberg A. L., Pardee A. B. The role of increased proteolysis in the atrophy and arrest of proliferation in serum-deprived fibroblasts. J Cell Physiol. 1984 Oct;121(1):189–198. doi: 10.1002/jcp.1041210124. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hendil K. B. Autophagy of metabolically inert substances injected into fibroblasts in culture. Exp Cell Res. 1981 Sep;135(1):157–166. doi: 10.1016/0014-4827(81)90308-6. [DOI] [PubMed] [Google Scholar]
- Herzog V., Fahimi H. D. A new sensitive colorimetric assay for peroxidase using 3,3'-diaminobenzidine as hydrogen donor. Anal Biochem. 1973 Oct;55(2):554–562. doi: 10.1016/0003-2697(73)90144-9. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A., Messmer T. O. Control of growth of benzo(a)pyrene-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3229–3232. doi: 10.1073/pnas.73.9.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunuma N., Kominami E. Structures and functions of lysosomal thiol proteinases and their endogenous inhibitor. Curr Top Cell Regul. 1983;22:71–101. doi: 10.1016/b978-0-12-152822-5.50007-5. [DOI] [PubMed] [Google Scholar]
- Khairallah E. A., Bartolone J., Brown P., Bruno M. K., Makowski G., Wood S. Does glutathione play a role in regulating intracellular proteolysis? Prog Clin Biol Res. 1985;180:373–383. [PubMed] [Google Scholar]
- Kirschke H., Shaw E. Rapid interaction of cathepsin L by Z-Phe-PheCHN12 and Z-Phe-AlaCHN2. Biochem Biophys Res Commun. 1981 Jul 30;101(2):454–458. doi: 10.1016/0006-291x(81)91281-x. [DOI] [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. The role of protein breakdown in growth, quiescence, and starvation of vascular smooth muscle cells. J Cell Physiol. 1984 Mar;118(3):317–323. doi: 10.1002/jcp.1041180315. [DOI] [PubMed] [Google Scholar]
- Mondino A. A new system of automatic amino acid analysis. II. J Chromatogr. 1969 Feb 11;39(3):262–272. doi: 10.1016/s0021-9673(01)98011-2. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Pösö A. R. The lysosomal pathway of intracellular proteolysis in liver: regulation by amino acids. Adv Enzyme Regul. 1986;25:257–276. doi: 10.1016/0065-2571(86)90018-x. [DOI] [PubMed] [Google Scholar]
- Mørland B., Pedersen A. Cathepsin B activity in stimulated mouse peritoneal macrophages. Lab Invest. 1979 Nov;41(5):379–384. [PubMed] [Google Scholar]
- Olivotto M., Paoletti F. Studies on the kinetics of initial cycle progression in vitro of ascites tumour cells subsequent to isolation from ascites fluid. Cell Tissue Kinet. 1980 Nov;13(6):605–612. doi: 10.1111/j.1365-2184.1980.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Olivotto M., Paoletti F. The role of respiration in tumor cell transition from the noncycling to the cycling state. J Cell Physiol. 1981 May;107(2):243–249. doi: 10.1002/jcp.1041070210. [DOI] [PubMed] [Google Scholar]
- Polet H. The effects of concanavalin A and other agents on protein degradation and migration of non-histone proteins (NHP) to the nucleus in lymphocytes. Exp Cell Res. 1983 Oct 15;148(2):345–362. doi: 10.1016/0014-4827(83)90158-1. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B. Amino acid control of autophagic sequestration and protein degradation in isolated rat hepatocytes. J Cell Biol. 1984 Aug;99(2):435–444. doi: 10.1083/jcb.99.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O., Grinde B., Solheim A. E. Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur J Biochem. 1979 Apr 2;95(2):215–225. doi: 10.1111/j.1432-1033.1979.tb12956.x. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Inhibitors of lysosomal function. Methods Enzymol. 1983;96:737–764. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Shaw E., Dean R. T. The inhibition of macrophage protein turnover by a selective inhibitor of thiol proteinases. Biochem J. 1980 Feb 15;186(2):385–390. doi: 10.1042/bj1860385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier A. M., Clark W. A., Jr, Zak R. Replacement perfusion of cultured eucaryotic cells: a method for the accurate measurement of the rates of growth, protein synthesis, and protein turnover. J Cell Biochem. 1984;26(1):47–64. doi: 10.1002/jcb.240260105. [DOI] [PubMed] [Google Scholar]
- Sparkuhl J., Sheinin R. Protein synthesis and degradation during expression of the temperature-sensitive defect in ts A1S9 mouse L-cells. J Cell Physiol. 1980 Nov;105(2):247–258. doi: 10.1002/jcp.1041050208. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ikegaki N., Ichihara A. Purification and characterization of hemoglobin-hydrolyzing acidic thiol protease induced by leupeptin in rat liver. J Biol Chem. 1984 May 10;259(9):5937–5944. [PubMed] [Google Scholar]
- Tessitore L., Bonelli G., Cecchini G., Amenta J. S., Baccino F. M. Regulation of protein turnover versus growth state: ascites hepatoma as a model for studies both in the animal and in vitro. Arch Biochem Biophys. 1987 Jun;255(2):372–384. doi: 10.1016/0003-9861(87)90405-x. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of macrophage protein turnover by a colony stimulating factor (CSF-1). J Cell Physiol. 1983 Jul;116(1):67–75. doi: 10.1002/jcp.1041160111. [DOI] [PubMed] [Google Scholar]
- Vidrich A., Airhart J., Bruno M. K., Khairallah E. A. Compartmentation of free amino acids for protein biosynthesis. Influence of diurnal changes in hepatic amino acid concentrations of the composition of the precursor pool charging aminoacyl-transfer ribonucleic acid. Biochem J. 1977 Feb 15;162(2):257–266. doi: 10.1042/bj1620257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton M. J., Poole B. Effect of medium composition on protein degradation and DNA synthesis in rat embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2427–2431. doi: 10.1073/pnas.74.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside K. H., Ward W. F., Mortimore G. E. Effects of glucagon on general protein degradation and synthesis in perfused rat liver. J Biol Chem. 1974 Sep 10;249(17):5458–5463. [PubMed] [Google Scholar]


