Abstract
In recent years, there has been a notable increase in interest in the use of statins in oral and maxillofacial surgery. The purpose of this literature review was to look into the effectiveness of statins in this area. Using a set of keywords, a thorough search of electronic databases was carried out, including PubMed, Scopus, Web of Science, Excerpta Medica database (EMBASE), and ProQuest. The papers considered were just those published in the English language between January 2012 and January 2024. Only human studies were taken into consideration; those involving animals were not. For the final analysis that assessed the use of statins in dentistry, a total of 30 papers were chosen. The designs, sample sizes, and materials employed in the experiments varied. According to the research, statins improve bone regeneration, have antiviral and antibacterial qualities, and work well as a therapeutic adjuvant for the treatment of periodontal disease. The analysis of the literature indicates that statins may be beneficial for treating periodontal disease, promoting bone regeneration, and improving oral health in the context of oral and maxillofacial surgery. Nevertheless, more investigation is required to completely comprehend the function of statins in this domain.
Keywords: bone regeneration, oral and maxillofacial surgery, oral health, periodontal disease, statins
Introduction and background
Low-density lipoproteins (LDL) are cholesterol particles associated with arteriosclerotic cardiovascular disorders. A class of drugs called statins primarily targets the liver's production of cholesterol [1]. They accomplish this by preventing 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA), an essential stage in the production of cholesterol, from being converted to mevalonate [2]. Furthermore, studies have demonstrated that statins promote osteoblast activity and development by increasing the synthesis of bone morphogenetic protein-2, a chemical that aids in the transformation of cultured osteoblasts into bone-like tissue [3]. Statins may contribute to bone mending because of their capacity to promote bone growth [4].
A new era for statins began in 1976 with the release of lovastatin by Dr. Akira Endo and his colleagues at Sankyo Pharmaceutical (now a part of Daiichi Sankyo) and Merck & Co. [5]. The source of lovastatin was Aspergillus terreus. It was the first statin to lower cholesterol when it was approved by the U.S. Food and Drug Administration (FDA) in 1987 [6]. In the 1990s, the FDA also approved the use of simvastatin (Zocor) and pravastatin(Pravachol), increasing the number of statin alternatives available. Atorvastatin, also marketed as Lipitor, was first made available in the late 1990s and gained rapid popularity due to its strong cholesterol-lowering effects. Third-generation statins, including rosuvastatin (Crestor) and pitavastatin (Livalo), have been created with increased potency and fewer drug interactions [7-9].
An essential component of statins, HMG-CoA reductase inhibitors play a critical role in lowering cholesterol levels [10-11]. The effects of statins on absorption, distribution, metabolism, and excretion are partly attributed to the functional groups they contain, such as lactones and hydroxyl groups, among others. The potency, bioavailability, and pharmacological characteristics of various statins are influenced by the distinct side chains that are attached to their primary structures [12-15].
Statins are commonly recommended to treat familial hypercholesterolemia, manage dyslipidemia, prevent atherosclerosis, improve diabetic management, and avoid heart attacks and strokes [16]. Statins should not be used in certain situations, though, such as when co-occurring medications are being taken, when liver disease is active, when a woman is pregnant or nursing when there has been a history of statin-related muscular complaints, when severe kidney disease is present, and when known hypersensitivity responses have occurred [17].
Considering the intricate nature of oral and maxillofacial surgery and the necessity for practice that is supported by data, it is imperative to conduct a thorough examination of the existing literature in order to clarify the function of statins in this particular area of medicine. Hence, the objective of this literature review is to methodically analyze and condense the current data about the utility of statins in oral and maxillofacial surgery, investigating their possible advantages and consequences for surgical results.
Review
Search strategy
The following keyword searches were used in the titles and abstracts of a methodical electronic search of the PubMed, Scopus, Web of Science, Excerpta Medica database (EMBASE), and ProQuest databases: “Oral" OR "Maxillofacial" OR "Surgery" OR "oral health" OR "oral diseases" OR "dental diseases" OR "oral cancer" AND "simvastatin" OR "statin" OR "rosuvastatin" OR "atorvastatin" OR "mevastatin" OR "lovastatin”.
Inclusion and exclusion criteria
Only English-language papers that were published between January 2012 and January 2024 were included in the search of the literature, to provide a more updated view of the efficacy of statins in oral and maxillofacial surgery. Only studies done on humans were included, and studies done on animals were excluded.
Results
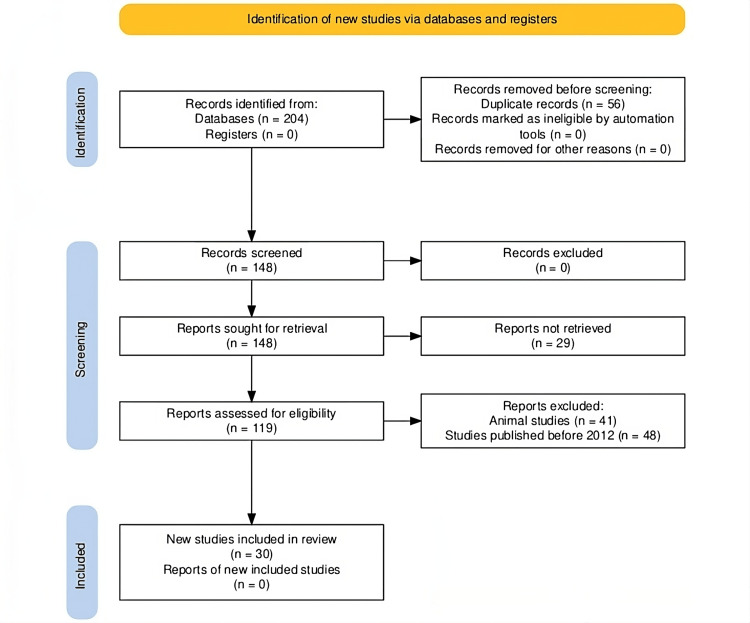
The initial stage of selecting research involved conducting a database search and obtaining 204 entries (Figure 1). After 56 duplicate entries were removed, 148 entries were evaluated. Subsequently, an effort was undertaken to acquire the complete text of every one of the 148 entries. Only 119 records remained for further analysis, though, as 29 reports were not retrievable. Forty-eight articles published before 2012 and 41 research studies involving animals were excluded when specific exclusion criteria were used. Ultimately, 30 papers that satisfied the inclusion requirements were picked for the comprehensive examination.
Figure 1. A PRISMA flowchart outlining the study selection process for this review.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Table 1 presents the included studies and our interpretation of their findings. A variety of statins were utilized in these trials, which were published between 2008 and 2023. They included rosuvastatin, atorvastatin, and simvastatin. A variety of materials were used in these experiments; for instance, in some, statins were used in conjunction with gel foam or polypropylene membrane. These studies examined the effects of statins on bone augmentation and regeneration, as well as the potential uses of statins in the treatment of oral cancer, chronic periodontitis, and other dental health problems. The study's sample sizes ranged from one patient to over 48,000 participants. The research methods also varied; some were in vitro, while others were systematic reviews, retrospective cohort studies, and randomized controlled trials.
Table 1. Statins utilized across different studies till date in the field of oral and maxillofacial surgery.
N/A: not applicable; RCT: randomized controlled trial; PRF: platelet-rich fibrin; RCTs: randomized controlled trials; CBCT-PAI: cone beam computed tomography-periapical index; TCA: tricalcium phosphate; NSAIDs: non-steroidal anti-inflammatory drugs; SACC: salivary gland adenoid cystic carcinoma; TMJ: temporomandibular joint; SIM: simvastatin; LAO-G-gelatin: lactic acid oligomer-G-gelatin; PLGA/HA/b-TCP: poly (d, l-lactide-co-glycolide)/hydroxyapatite/beta-tricalcium phosphate; OSCC: oral squamous cell carcinoma; OAS: overall survival; RFS: recurrence-free survival; PDLCs: periodontal ligament cells
| Study Name | Year | Material Used | Purpose | Parameters Assessed | Study Design | Sample Size | Results Observed | Overall Inference Drawn |
| Abdulrab et al. [18] | 2019 | 1% Simvastatin mouthwash (20 mg tablet dissolved in distilled water) | COVID-19 (as antimicrobial, anti-viral, anti-inflammatory, antioxidant, immunomodulatory) | Anti-viral and anti-bacterial effects of statins | In vitro studies and meta-analysis | N/A | Statins showed promising anti-bacterial effects against periodontal pathogens and potential anti-viral effects against SARS-CoV-2 | Topical statins may minimize the risk of SARS-CoV-2 transmission in dental settings, but clinical trials are needed |
| Abu Sheehah et al. [19] | 2022 | Simvastatin (10 mg) with normal saline | Bone augmentation and socket preservation in extraction socket | Bone regeneration, density, width, height | Comparative study | 20 dental sockets | Significant increase in density, significant difference in width, no significant difference in height | Simvastatin induces bone formation, but cannot preserve alveolar bone height |
| Aljudaibi et al. [20] | 2019 | Simvastatin, rosuvastatin, and atorvastatin | Chronic periodontitis treatment | Efficacy of statins as an adjunct therapy for periodontal treatment | Systematic review and meta-analysis | 15 studies | Statins, particularly simvastatin, improved periodontal parameters, including pocket depth and clinical attachment level gain, when used as adjunct therapy | Statins, especially simvastatin, may be beneficial as an adjunct therapy for periodontal treatment, but more research is needed |
| Ambrósio et al. [21] | 2017 | Simvastatin, Rrosuvastatin, atorvastatin | Adjuvant local delivery of statins | Adjunctive local delivery of statins, periodontal therapy | Systematic review and meta-analysis | 10 RCTs | Local delivery of statins improved pocket depth and clinical attachment gain | Statins may be a promising adjunct to periodontal therapy |
| Bahrami-Hessari et al. [22] | 2021 | Systemic statins | Peri-implant bone loss | Peri-implantitis, bone loss, statin use | Retrospective cohort pilot study | 60 exposed, 196 non-exposed | Statin use correlated with peri-implant bone loss | Statins may have a beneficial effect on peri-implant bone loss |
| Baliga et al. [23] | 2018 | Statin + PRF | Osteoradionecrosis of the lower jaw | Management of osteoradionecrosis with simvastatin and PRF | Case report | One patient | Remarkable healing of the non-healing socket with adequate gain in alveolar height. | A combination of simvastatin and PRF may be effective in managing Stage 1 osteoradionecrosis. |
| Cai et al. [24] | 2018 | Simvastatin (10, 20, 30, 40, 50 micromol/L | Human salivary | Proliferation, invasion, apoptosis, survivin expression | In vitro study | - | Simvastatin inhibited proliferation and invasion, induced apoptosis, and downregulated survivin expression | Simvastatin may be a novel target for SACC therapy |
| Cruz et al. [25] | 2021 | Simvastatin 1.2% with Polypropylene membrane | Healing of extraction socket | Dimensional changes, soft tissue healing, and pain perception in post-extraction sockets with simvastatin gel | Randomized controlled trial | 26 patients | Simvastatin gel reduced dimensional changes, but not soft tissue healing and pain perception | Simvastatin gel is effective in reducing dimensional changes in post-extraction sockets |
| Degala et al. [26] | 2018 | Simvastatin (10 mg) | Regeneration of bone after surgical removal of Impacted third molars | Bone regeneration, pain, postoperative swelling, bone density | Randomized, split-mouth, single-blinded, single-center trial | 30 patients | Significantly higher mean grey-level histographic values for study sockets at one, four, eight, and 12 weeks | Local application of simvastatin can stimulate and hasten osseous regeneration |
| Fu JH et al. [4] | 2012 | Different concentrations of statins | Implant healing and Osseointegration | Systemic medications, peri-implant bone healing | Literature review | - | Statins improve implant osseointegration, conflicting results for glucocorticoids and NSAIDs | Statins may improve peri-implant bone healing, but more research is needed |
| Gouda et al. [27] | 2017 | Beta-TCP and simvastatin (7.21 mg) | Sinus lift and osteoinductive capacity | Simvastatin, sinus augmentation, bone quality and quantity | Clinical trial | Six patients, eight sinus lift procedures | The simvastatin group showed higher newly formed bone, but a higher resorption rate | Simvastatin is safe and has promising osteoinductive capacity, but a larger sample size is needed |
| Gupta et al. [28] | 2019 | Simvastatin | Bone, soft tissue, and TMJ cartilage healing | Osteopromotive potential, soft tissue and TMJ cartilage healing properties of simvastatin | Systematic review | 10 animal studies, six clinical studies | Simvastatin administration displays positive treatment outcomes in various oral therapies, including periodontal infection control and bone regeneration | Simvastatin is beneficial for the healing of oral bone and cartilage |
| Gupta S et al. [29] | 2020 | Simvastatin | Bone regeneration | Effectiveness of simvastatin, hydroxyapatite, and platelet-rich fibrin in bone regeneration of periapical defects | Randomized controlled trial | 39 patients | Significant change in CBCT-PAI scores in the SIM group, indicating a faster rate of bone regeneration | Simvastatin is more effective in promoting bone regeneration compared to hydroxyapatite and platelet-rich fibrin |
| Harsha et al. [30] | 2021 | Simvastatin powder (10 mg) with gel foam | Third molar extraction socket for bone regeneration | Bone regeneration, density, pain, swelling | Randomized controlled trial | 50 patients (100 extraction sockets) | Significant increase in bone density at first, fourth, eighth, and 12th weeks, reduced pain and swelling | Local application of simvastatin promotes and enhances bone formation in extraction sockets |
| Hayder et al. [31] | 2021 | Simvastatin powder (10 mg) with gel foam | Bone density after third molar removal | Bone regeneration, density | Prospective comparative randomized clinical study | 24 patients (32 cases) | Significant increase in bone density three months postoperatively | Local application of simvastatin increases bone density in sockets of surgically extracted mandibular third molars |
| Jin et al. [32] | 2021 | Simvastatin | Bone regeneration | Molecular mechanisms of simvastatin on bone metabolism and angiogenesis, osteogenic differentiation, drug delivery systems | Review | - | Simvastatin promotes osteogenesis and angiogenesis, enhances bone regeneration | Simvastatin-loaded drug delivery systems have the potential for bone regeneration |
| Kabra et al. [33] | 2023 | Simvastatin | Different aspects of dental and oral health | Role of statins in oral health applications, including dentin regeneration, bone health, and wound healing | Review | - | Statins have promising effects on oral health, including dental pulp cells, chronic periodontitis, and alveolar bone loss | Statins, including simvastatin, have significant impact on enhancing oral health |
| Miyazawa et al. [34] | 2015 | A mixture of LAO- G-gelatin solution and Simvastatin solution | Odontoblastic differentiation | Odontoblastic differentiation, simvastatin, dental pulp stem cells | In vitro and in vivo study | - | Simvastatin enhanced odontoblastic differentiation and bone formation | Statins can enhance odontoblastic differentiation and bone formation |
| Noronha et al. [35] | 2017 | Poly (d, l-lactide-co-glycolide) with hydroxyapatite/b-TCP (PLGA/HA/b-TCP) scaffolds, 2.0% simvastatin scaffold | Extraction sockets of upper Third molars | Healing of maxillary third molars postextraction sockets with different ridge preservation techniques | Randomized controlled trial | 26 sockets (13 patients) | Simvastatin-loaded scaffolds showed fewer clinical complications and graft loss | Simvastatin-loaded scaffolds are superior to others in ridge preservation |
| Park et al. [36] | 2009 | Simvastatin | Bone regeneration | Effects of simvastatin on bone formation, osteoblastic and osteoclastic activity, anti-inflammatory effects | Review | - | Simvastatin promotes bone formation, inhibits osteoclastic activity, has anti-inflammatory effects | Local application of simvastatin with carriers promotes bone formation |
| Saifi et al. [3] | 2017 | Simvastatin 10 mg mixed with gelatin sponge | Healing of extraction socket | Bone formation, density, pain, swelling | Prospective study | 15 patients (30 extraction sites) | Significant increase in bone density at eighth and 16th weeks reduced pain and swelling | Local application of simvastatin induces bone formation in extraction sockets |
| Saka-Harran et al. [37] | 2022 | Different doses of statins | Oral squamous cell carcinoma | Statin use, head and neck cancer risk | Hospital-based case-control study | 101 cases, 101 controls | No association between prior statin use and head and neck cancer risk | Statins do not have a beneficial effect on head and neck cancer risk |
| Sezavar et al. [38] | 2018 | Simvastatin 20 mg | Healing of extraction socket | Application of simvastatin in alveolar ridge preservation | Split-mouth study | 10 dental sockets | The simvastatin group showed higher percentages of vital bone, amorphous, and trabecular bone, but no significant difference compared to the control group. | Simvastatin may improve the quality of osteogenesis in the jaw bone, but further studies are necessary. |
| Shah et al. [39] | 2015 | Statin (not specified) | Bone regeneration | Effects of statins on bone regeneration, bone turnover, and regeneration via effects on cell types | Review article | N/A | Statins have pleiotropic effects, including anti-inflammatory and antimicrobial properties, and can affect bone turnover and regeneration | Statins are promising for bone regeneration and tissue engineering |
| Spoerl et al. [40] | 2023 | Statin (not specified) | OSCC patients (overall survival and recurrence-free survival) | Prognostic role of statins in OSCC | Retrospective cohort study | 602 | Statin use correlated with improved OAS and RFS in OSCC patients | Statin use may improve oncological outcomes in OSCC patients, but prospective clinical trials are needed |
| Tahamtan et al. [2] | 2020 | Statin (not specified) | Different aspects of dental and oral health | Effects of statins on dental and oral health | Literature review | N/A | Statins possess remarkable effects on various aspects of dental and oral health, including periodontitis, alveolar bone loss, osseointegration, and tissue healing. | Statins can be considered as novel therapeutic agents to improve dental and oral health. |
| Wu et al. [41] | 2008 | Simvastatin | Alveolar bone remodelling | Residual ridge resorption, bone turnover, bone formation, bone mineral density | Randomized controlled trial | 60 male Wistar rats (30 in each group) | Significant increase in relative height of residual alveolar ridge, bone mineral density, and bone formation rate and quality in simvastatin group compared to the control group at various time points | Local application of simvastatin effectively preserves residual alveolar bone by promoting bone formation in extraction sockets |
| Wuster et al. [42] | 2023 | Rosuvastatin, simvastatin, fluvastatin, pravastatin, lovastatin, atorvastatin | Head and neck carcinoma | Influence of statin medication on overall survival of head and neck cancer patients | Retrospective clinical data analysis | 48,626 | Statin medication was associated with significantly improved five-year survival in head and neck cancer patients | Statin use may improve survival outcomes in head and neck cancer patients, but retrospective study design limits conclusions |
| Xu et al. [43] | 2020 | Six concentrations (0, 0.01, 0.05, 0.1, 0.5, 1) | Tooth augmentation during orthodontic tooth movement | Effects of simvastatin on orthodontic tooth movement | In vitro and in vivo study | Rat periodontal ligament cells (PDLCs) | Simvastatin triggered osteogenic differentiation of PDLCs, attenuated inflammation, and decreased osteoclastogenesis. | Simvastatin can promote bone formation and attenuate inflammation during orthodontic tooth movement. |
| Yaghobee et al. [44] | 2020 | Bovine bone material + simvastatin (1.6 gm ) | Augmentation of the maxillary sinus | Efficacy of simvastatin in maxillary sinus augmentation | Randomized clinical trial with a split-mouth design | 24 maxillary sinuses in 12 patients | No significant differences in newly formed bone and residual particles between the simvastatin and control groups. | Simvastatin may not have a significant positive effect on maxillary sinus augmentation. |
The results show that statins have promising antiviral and antibacterial qualities, particularly against periodontal diseases and SARS-CoV-2. The results of Abdulrab et al. [18] were similar. In terms of bone regeneration, statins, especially simvastatin, have been demonstrated to dramatically enhance bone density, width, and height. Ambrósio et al. [21], Baliga et al. [23], Cai et al. [24], Degala et al. [26], Fu JH et al. [4], Gouda et al. [27], Gupta et al. [28], Hayder et al. [31], Jin et al. [32], Kabra et al. [33], Miyazawa et al. [34], Park et al. [36], Spoerl et al. [40], Tahamtan et al. [2], Wu et al. [41], and Wuster et al. all came to similar conclusions.
Furthermore, statins improved clinical attachment level rise and pocket depth, demonstrating their potential as a therapeutic adjuvant in the management of periodontal disease. Cruz et al. [25] and Aljudaibi et al. [20] came to similar conclusions. These studies also looked into the effects of statin use on osteoradionecrosis, peri-implantitis, and bone loss. The findings indicated a link between statin use and implant-related bone loss. Bahrami-Hessari et al. [22] showed similar results, although Gouda et al. [27] found that non-healing sockets could recover dramatically with an increase in alveolar height.
Additionally, it was demonstrated that statins cause apoptosis, inhibit invasion and proliferation, and reduce the production of survivin. Cai et al. [24] had similar results. Given their potential in treating dental pulp cells, alveolar bone loss, and chronic periodontitis, statins may be advantageous for oral health. Gupta S. et al. [29], Harsha et al. [30], Jin et al. [32], Kabra et al. [33], Noronha et al. [35], Saka-Harran et al. [37], Sezavar et al. [38], Shah et al. [39], Xu et al. [43], and Yaghobee et al. [44] all came to similar conclusions.
The effect of statins on the risk of head and neck cancer was also examined in the studies; the findings revealed no association between the disease's risk and previous statin use. Sezavar et al. reported similar results [38]. On the other hand, statin use was linked to improved overall and recurrence-free survival in patients with oral squamous cell carcinoma. Saifi et al. [3] obtained similar results.
Discussion
The LDL-cholesterol levels and liver cholesterol production can both be safely and effectively controlled with the statin drug family, which is a safe and effective treatment for arteriosclerotic cardiovascular disease. In addition to their strong ability to lower cholesterol, which lowers mortality and cardiovascular risk, statins are said to have a number of positive health impacts on people [1-3]. Improved endothelial function, and anti-inflammatory, antioxidant, immunomodulatory, and anti-thrombotic actions are some of these pleiotropic benefits. More and more research in recent years indicates that statins may benefit dental and oral health via many pathways [2].
Statins, particularly simvastatin, were observed to have a major impact on improving oral health and bone regeneration across the articles that we analyzed in this review. Our assessment is consistent with the study conducted by Abdulrab et al. [18], which demonstrated that topical statins may reduce the incidence of SARS-CoV-2 transmission in dentistry settings. In a similar vein, simvastatin may not retain alveolar bone height, according to the study conducted by Abu Sheehah et al. [19]. This is in line with the results of studies conducted by Aljudaibi et al. [20] and Ambrósio et al. [21].
Additionally, our research showed that the results of Baliga et al. [23] and Cai et al. [24] complement the study by Bahrami-Hessari et al. [22], which found that statins may have a favorable effect on peri-implant bone loss. In addition, Cruz et al.'s study [25] supported the findings of Degala et al. [26] and Fu et al. [4] by indicating that statins might be a promising addition to periodontal therapy.
Furthermore, we discovered that Gouda et al.'s study [27] supported the findings of Gupta et al. [28] and Gupta et al. [29] by concluding that simvastatin is safe and has the potential capacity to induce osteoinduction. Furthermore, research by Hayder et al. [31] and Jin et al. [32] supports the work by Harsha et al. [30], which hypothesized that simvastatin would help oral bone and cartilage recover.
In line with the findings of Miyazawa et al. [34] and Noronha et al. [35], our research also showed that the study by Kabra et al. [33] discovered that simvastatin is more effective in stimulating bone regeneration compared to hydroxyapatite and platelet-rich fibrin. Furthermore, although additional research is required, the Park et al. study [36] revealed that statins may enhance peri-implant bone repair.
We also contrasted the results of Saka-Harran et al. [37] and Saifi et al. [3], which revealed that statins significantly improve bone regeneration and dental health. Additionally, our data showed that simvastatin may enhance the quality of osteogenesis in the jaw bone, as reported by Sezavar et al. [38]. These findings are corroborated by those of Shah et al. [39] and Spoerl et al. [40].
Additionally, we noted that Tahamtan et al.'s work [2] supported the findings of Wu et al. [41] and Wuster et al. [42] by concluding that statins show promise for bone regeneration and tissue engineering. Furthermore, simvastatin may help to stimulate bone formation and reduce inflammation during orthodontic tooth movement, according to Xu et al.'s study [43] and Yaghobee et al.'s findings [44].
A considerable proportion of the populace is afflicted with coronary heart disease (CHD), a common medical condition. One of the leading causes of death in the US is CHD. For those 65 years of age and older, it is the main cause of stroke or death. Among young people (those between the ages of 20 and 45), the prevalence of CHD is almost 65% [1-3]. Hyperlipidaemia, also known as hypercholesterolaemia, is the most modifiable risk factor for CHD and is very common in the US population. This is the reason statins, or drugs that lower cholesterol, are often prescribed. Their main method of action is inhibiting HMG-CoA reductase activity, which impedes the synthesis of cholesterol. New evidence suggests that statins may affect bone metabolism [3, 45].
Although many strategies have been proposed, there is currently no evidence to support an optimal strategy or biomaterial for the maintenance of alveolar ridges [28-29]. Therefore, more information on biomaterials and procedures is required to increase the processes' predictability and reproducibility.
One advantage of applying osteoinductive drugs locally is the reduction of toxicity and side effects. Two further advantages are improved patient compliance and postponed medication release [2, 7]. Compared to systemic statin use, local osteoinduction appears to be 50 times more linked with statin use [9].
A semi-synthetic equivalent of lovastatin, simvastatin, has been demonstrated to enhance bone metabolism. Elevated bone mineral density (BMD) was found to be associated with increases in osteocalcin, bone alkaline phosphatase, and the type I collagen C-terminal telopeptide [46]. Women with type II diabetes who had experienced menopause also showed comparable results [46]. Hydrophilic statins had no discernible effects on serum osteocalcin, alkaline phosphatase, atorvastatin, fluvastatin, or the C-terminal telopeptide of type I collagen [47]. It was therefore anticipated that lipophilic statins would influence bone remodeling more than other statins [3].
Nyan et al. [48] provided an explanation for the simvastatin burst release event that occurred from the graft particles on the first day, which was followed by a delayed release. This is advantageous since the proper dosage of the drug promotes BMP-2 production in surrounding cells without inducing an inflammatory reaction. Because of its regenerative properties, simvastatin may be used as a medication for soft tissue regeneration and repair [49], and due to the fact that it can reduce inflammatory mediators and accelerate soft tissue regeneration, this is especially advantageous in cases of periapical infections and extraction sockets [50-51].
We admit that the accuracy of our conclusions may have been hampered by the variability of the included studies. The generalisability of our findings may have been impacted by biases and confounding variables introduced by the variations in study designs, sample sizes, and materials used. Moreover, our review was limited to publications written in English, which might have excluded pertinent works written in other languages. Furthermore, because of the precise keywords and databases we utilized, it's possible that our search method overlooked pertinent studies.
In light of our findings, we suggest that more rigorous study designs, bigger sample sizes, and standardized materials be used in future research examining the use of statins in oral and maxillofacial surgery. To further comprehend the therapeutic potential of statins, we further recommend that researchers investigate the underlying processes of these drugs' effects on periodontal disease and bone regeneration. Furthermore, we suggest that physicians think about using statins as an additional treatment for patients having oral and maxillofacial surgery or periodontal disease.
Conclusions
The cumulative evidence analyzed in our review indicates that statins have a significant effect on enhancing bone regeneration, a crucial factor in the field of oral and maxillofacial surgery, where achieving the best possible tissue repair is vital for achieving favorable results. Moreover, statins have been shown to have antiviral and antibacterial characteristics, rendering them a useful supplementary therapy in the management of periodontal disease, a widespread phenomenon that presents substantial obstacles to oral health. The literature analysis emphasizes the possible advantages of statins as a supplementary treatment in the management of periodontal disease. It highlights their ability to regulate the biological response of the host, decrease inflammation, and stimulate the regeneration of tissues. Although the current body of evidence strongly supports the use of statins in oral and maxillofacial surgery, more research is required to completely understand the mechanisms responsible for their effects and to determine their effectiveness in different clinical situations. This will ultimately help to develop evidence-based recommendations for their integration into daily clinical practice.
Disclosures
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Saloni J. Kanabar, Deepankar Shukla, Nitin Bhola, Anchal Agarwal
Acquisition, analysis, or interpretation of data: Saloni J. Kanabar, Nitin Bhola, Anchal Agarwal
Drafting of the manuscript: Saloni J. Kanabar, Deepankar Shukla, Nitin Bhola, Anchal Agarwal
Critical review of the manuscript for important intellectual content: Saloni J. Kanabar, Deepankar Shukla, Nitin Bhola, Anchal Agarwal
Supervision: Deepankar Shukla, Nitin Bhola, Anchal Agarwal
References
- 1.Statins: 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitors demonstrate anti-atherosclerotic character due to their antioxidant capacity. Puttananjaiah MK, Dhale MA, Gaonkar V, Keni S. Appl Biochem Biotechnol. 2011;163:215–222. doi: 10.1007/s12010-010-9031-z. [DOI] [PubMed] [Google Scholar]
- 2.The effects of statins on dental and oral health: a review of preclinical and clinical studies. Tahamtan S, Shirban F, Bagherniya M, Johnston TP, Sahebkar A. J Transl Med. 2020;18:155. doi: 10.1186/s12967-020-02326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healing of extraction socket following local application of simvastatin: a split mouth prospective study. Saifi AM, Giraddi GB, Ahmed N. J Oral Biol Craniofac Res. 2017;7:106–112. doi: 10.1016/j.jobcr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Statins, glucocorticoids, and nonsteroidal anti-inflammatory drugs: their influence on implant healing. Fu JH, Bashutski JD, Al-Hezaimi K, Wang HL. Implant Dent. 2012;21:362–367. doi: 10.1097/ID.0b013e3182611ff6. [DOI] [PubMed] [Google Scholar]
- 5.Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Alberts AW, Chen J, Kuron G, et al. Proc Natl Acad Sci U S A. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Lennernäs H, Fager G. Clin Pharmacokinet. 1997;32:403–425. doi: 10.2165/00003088-199732050-00005. [DOI] [PubMed] [Google Scholar]
- 7.Overcoming patient reluctance to statin intolerance. Katamesh BE, Mickow AA, Huang L, Dougan BM, Ratrout BM, Nanda S, Vincent A. Kardiol Pol. 2024;82:485–491. doi: 10.33963/v.phj.100526. [DOI] [PubMed] [Google Scholar]
- 8.Statins: past and present. Hajar R. Heart Views. 2011;12:121–127. doi: 10.4103/1995-705X.95070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effects of statins on mitochondrial pathways. Mollazadeh H, Tavana E, Fanni G, et al. J Cachexia Sarcopenia Muscle. 2021;12:237–251. doi: 10.1002/jcsm.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pregnancy-related adverse events associated with statins: a real-world pharmacovigilance study of the FDA Adverse Event Reporting System (FAERS) Wu T, Shi Y, Zhu B, Li D, Li Z, Zhao Z, Zhang Y. Expert Opin Drug Saf. 2024;23:313–321. doi: 10.1080/14740338.2023.2251888. [DOI] [PubMed] [Google Scholar]
- 11.Correction: statin drugs enhance responses to immune checkpoint blockade in head and neck cancer models. J Immunother Cancer. 2023;11:0. doi: 10.1136/jitc-2022-005940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Effect of monoclonal antibodies to PCSK9 on high-sensitivity C-reactive protein levels: a meta-analysis of 16 randomized controlled treatment arms. Sahebkar A, Di Giosia P, Stamerra CA, et al. Br J Clin Pharmacol. 2016;81:1175–1190. doi: 10.1111/bcp.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Baigent C, Keech A, Kearney PM, et al. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 14.Stimulation of bone formation in vitro and in rodents by statins. Mundy G, Garrett R, Harris S, et al. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 15.Autotransplantation of immature third molars and orthodontic treatment after en bloc resection of conventional ameloblastoma. Osterne RL, Moreira Neto JJ, de Araújo Lima AD, Nogueira RL. J Oral Maxillofac Surg. 2015;73:1686–1694. doi: 10.1016/j.joms.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Effects of statins on skeletal muscle: a perspective for physical therapists. Di Stasi SL, MacLeod TD, Winters JD, Binder-Macleod SA. Phys Ther. 2010;90:1530–1542. doi: 10.2522/ptj.20090251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simvastatin inhibits oral squamous cell carcinoma by targeting TMEM16A Ca(2+)-activated chloride channel. Wang H, Wang T, Zhang Z, et al. J Cancer Res Clin Oncol. 2021;147:1699–1711. doi: 10.1007/s00432-021-03575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statins-based prophylactic mouthwash and nasal spray may protect against coronavirus disease 2019. Abdulrab S, Alkadasi B, Al-Maweri S, Halboub E, Alhadainy H, Geerts G. New Microbes New Infect. 2020;37:100751. doi: 10.1016/j.nmni.2020.100751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evaluation of simvastatin efficacy on bone regeneration for socket preservation. Abu Sheehah HA, Hosny A, El Mohandes W. Al-Azhar J Dent Sci. 2022;1:43–48. [Google Scholar]
- 20.Do adjunctive statins improve periodontal treatment outcomes in patients with chronic periodontitis? Aljudaibi S, Duane B. Evid Based Dent. 2019;20:18–19. doi: 10.1038/s41432-019-0009-6. [DOI] [PubMed] [Google Scholar]
- 21.Does the adjunctive use of statins provide additional benefits to nonsurgical periodontal treatment? A systematic review and meta-analysis. Ambrósio LM, Rovai ES, Sendyk DI, Holzhausen M, Pannuti CM. J Periodontal Res. 2018;53:12–21. doi: 10.1111/jre.12480. [DOI] [PubMed] [Google Scholar]
- 22.Peri-implant marginal bone loss and systemic statin use: a retrospective cohort pilot study. Bahrami-Hessari B, Jansson L. Clin Exp Dent Res. 2022;8:20–27. doi: 10.1002/cre2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Is there a role for PRF with simvastatin in stage I osteoradionecrosis? Baliga M, Chakraborty S, Kumari T, Tusharbhai DM, Sarkar S. Oral Oncol. 2018;87:177–178. doi: 10.1016/j.oraloncology.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Effect of survivin downregulation by simvastatin on the growth and invasion of salivary adenoid cystic carcinoma. Cai WY, Zhuang Y, Yan F, Li T, Song WT, Sun JH. Mol Med Rep. 2018;18:1939–1946. doi: 10.3892/mmr.2018.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clinical efficacy of simvastatin gel combined with polypropylene membrane on the healing of extraction sockets: a triple-blind, randomized clinical trial. Cruz R, Moraschini V, Calasans-Maia MD, de Almeida DC, Sartoretto SC, Granjeiro JM. Clin Oral Implants Res. 2021;32:711–720. doi: 10.1111/clr.13740. [DOI] [PubMed] [Google Scholar]
- 26.Evaluation of the efficacy of simvastatin in bone regeneration after surgical removal of bilaterally impacted third molars-a split-mouth randomized clinical trial. Degala S, Bathija NA. J Oral Maxillofac Surg. 2018;76:1847–1858. doi: 10.1016/j.joms.2018.04.035. [DOI] [PubMed] [Google Scholar]
- 27.Maxillary sinus lift using osteoinductive simvastatin combined with β-TCP versus β-TCP - a comparative pilot study to evaluate simvastatin enhanced and accelerated bone formation. Gouda A, Helal E, Ali S, Bakry S, Yassin S. Acta Odontol Scand. 2018;76:39–47. doi: 10.1080/00016357.2017.1381345. [DOI] [PubMed] [Google Scholar]
- 28.The impact of simvastatin intervention on the healing of bone, soft tissue, and TMJ cartilage in dentistry: a systematic review and meta-analysis. Gupta S, Del Fabbro M, Chang J. Int J Implant Dent. 2019;5:17. doi: 10.1186/s40729-019-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Effect of local application of simvastatin in bone regeneration of peri-apical defects-a clinico-radiographic study. Gupta S, Verma P, Tikku AP, Chandra A, Yadav RK, Bharti R, Bains R. J Oral Biol Craniofac Res. 2020;10:583–591. doi: 10.1016/j.jobcr.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evaluation of efficacy of simvastatin in bone regeneration following local application in third molar extraction socket: a randomized control trial. Harsha G, Madhavi S, Arthi S, Haritha S. Natl J Maxillofac Surg. 2023;14:286–293. doi: 10.4103/njms.njms_317_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efficacy of simvastatin in bone regeneration after surgical removal of mandibular third molars. Deepanjali M, Prasad TS, Manodh P. Oral Maxillofac Surg. 2023;27:427–432. doi: 10.1007/s10006-022-01081-y. [DOI] [PubMed] [Google Scholar]
- 32.Simvastatin-incorporated drug delivery systems for bone regeneration. Jin H, Ji Y, Cui Y, Xu L, Liu H, Wang J. ACS Biomater Sci Eng. 2021;7:2177–2191. doi: 10.1021/acsbiomaterials.1c00462. [DOI] [PubMed] [Google Scholar]
- 33.Exploring the synergistic effect of simvastatin in oral health applications: a literature review. Kabra S, Thosar NR, Malviya NS. Cureus. 2023;15:0. doi: 10.7759/cureus.44411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Controlled release of simvastatin from biodegradable hydrogels promotes odontoblastic differentiation. Miyazawa A, Matsuno T, Asano K, Tabata Y, Satoh T. Dent Mater J. 2015;34:466–474. doi: 10.4012/dmj.2014-272. [DOI] [PubMed] [Google Scholar]
- 35.Ridge preservation after maxillary third molar extraction using 30% porosity plga/ha/β-tcp scaffolds with and without simvastatin: a pilot randomized controlled clinical trial. Noronha Oliveira M, Rau LH, Marodin A, Corrêa M, Corrêa LR, Aragones A, Magini RS. Implant Dent. 2017;26:832–840. doi: 10.1097/ID.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 36.The use of simvastatin in bone regeneration. Park JB. http://www.medicinaoral.com/pubmed/medoralv14_i9_pe485.pdf. Med Oral Patol Oral Cir Bucal. 2009;14:0–8. [PubMed] [Google Scholar]
- 37.Effects of the prior use of statins on head and neck cancer risk: a hospital-based case-control study. Saka-Herrán C, Jané-Salas E, Mano-Azul A, Torrejón-Moya A, Estrugo-Devesa A, López-López J. Pharmaceuticals (Basel) 2022;15:579. doi: 10.3390/ph15050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simvastatin effects on dental socket quality: a comparative study. Sezavar M, Bohlouli B, Farhadi S, Tabatabaee S, Latifi R. Contemp Clin Dent. 2018;9:55–59. doi: 10.4103/ccd.ccd_719_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novel applications of statins for bone regeneration. Shah SR, Werlang CA, Kasper FK, Mikos AG. Natl Sci Rev. 2015;2:85–99. doi: 10.1093/nsr/nwu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Statin use ameliorates survival in oral squamous cell carcinoma-data from a population-based cohort study applying propensity score matching. Spoerl S, Gerken M, Fischer R, et al. Biomedicines. 2023;11:369. doi: 10.3390/biomedicines11020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The effect of simvastatin on remodelling of the alveolar bone following tooth extraction. Wu Z, Liu C, Zang G, Sun H. Int J Oral Maxillofac Surg. 2008;37:170–176. doi: 10.1016/j.ijom.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 42.Statin medication improves five-year survival rates in patients with head and neck cancer: a retrospective case-control study of about 100,000 patients. Wüster J, Heiland M, Nahles S, Preissner R, Preissner S. Cancers (Basel) 2023;15:3093. doi: 10.3390/cancers15123093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Local delivery of simvastatin maintains tooth anchorage during mechanical tooth moving via anti-inflammation property and AMPK/MAPK/NF-kB inhibition. Xu L, Sun X, Zhu G, Mao J, Baban B, Qin X. J Cell Mol Med. 2021;25:333–344. doi: 10.1111/jcmm.16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Effect of simvastatin on bone regeneration: a histologic and histomorphometric analysis. Yaghobee S, Panjnoush M, Chokami Rafiei S, Amini Shakib P, Mahmoodi S, Rasouli-Ghahroudi AA, Poursafar F. J Oral Maxillofac Surg. 2020;78:927–934. doi: 10.1016/j.joms.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 45.New insights into the pharmacodynamic and pharmacokinetic properties of statins. Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. Pharmacol Ther. 1999;84:413–428. doi: 10.1016/s0163-7258(99)00045-5. [DOI] [PubMed] [Google Scholar]
- 46.Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. Song BL, DeBose-Boyd RA. J Biol Chem. 2006;281:25054–25061. doi: 10.1074/jbc.M605575200. [DOI] [PubMed] [Google Scholar]
- 47.Correction. J Am Coll Cardiol. 2019;73:3234–3237. doi: 10.1016/j.jacc.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Bone formation with the combination of simvastatin and calcium sulfate in critical-sized rat calvarial defect. Nyan M, Sato D, Oda M, Machida T, Kobayashi H, Nakamura T, Kasugai S. J Pharmacol Sci. 2007;104:384–386. doi: 10.1254/jphs.sc0070184. [DOI] [PubMed] [Google Scholar]
- 49.Chronic intravenous aminobisphosphonate therapy increases high-density lipoprotein cholesterol and decreases low-density lipoprotein cholesterol. Adami S, Braga V, Guidi G, Gatti D, Gerardi D, Fracassi E. J Bone Miner Res. 2000;15:599–604. doi: 10.1359/jbmr.2000.15.3.599. [DOI] [PubMed] [Google Scholar]
- 50.Efficacy of simvastatin in bone regeneration after surgical removal of mandibular third molars: a clinical pilot study. Chauhan AS, Maria A, Managutti A. J Maxillofac Oral Surg. 2015;14:578–585. doi: 10.1007/s12663-014-0697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Statin adherence in patients enrolled in the disease management program for coronary artery disease - comparison between patients' and general practitioners' self-reports and patient records. Salam B, Schrimpf A, Münster S, Bleckwenn M. Res Health Serv Reg. 2023;2:13. doi: 10.1007/s43999-023-00029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]