Abstract
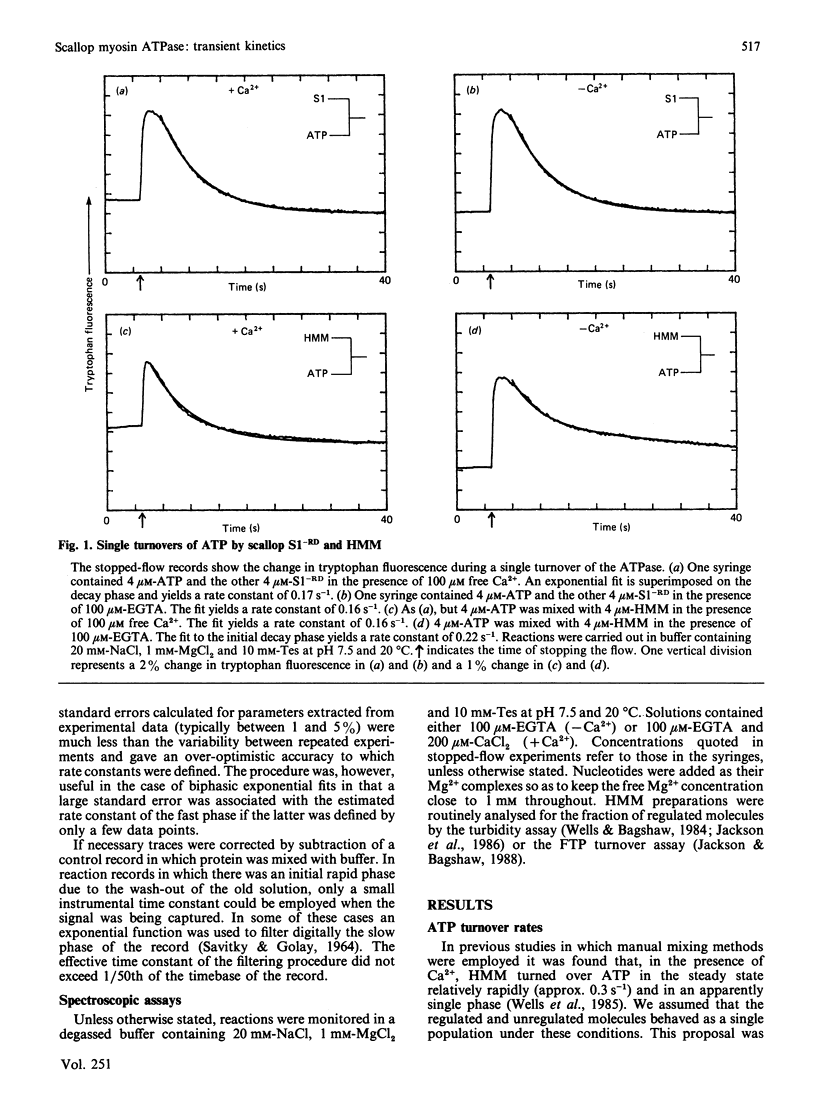
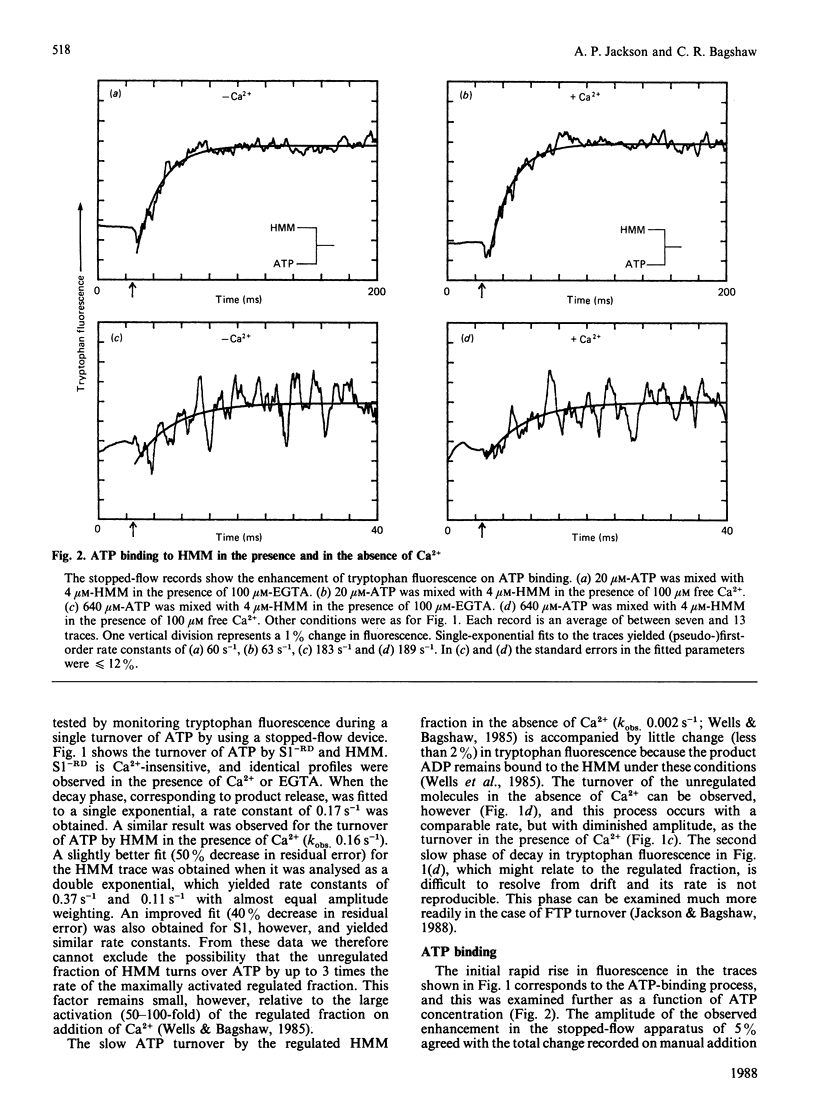
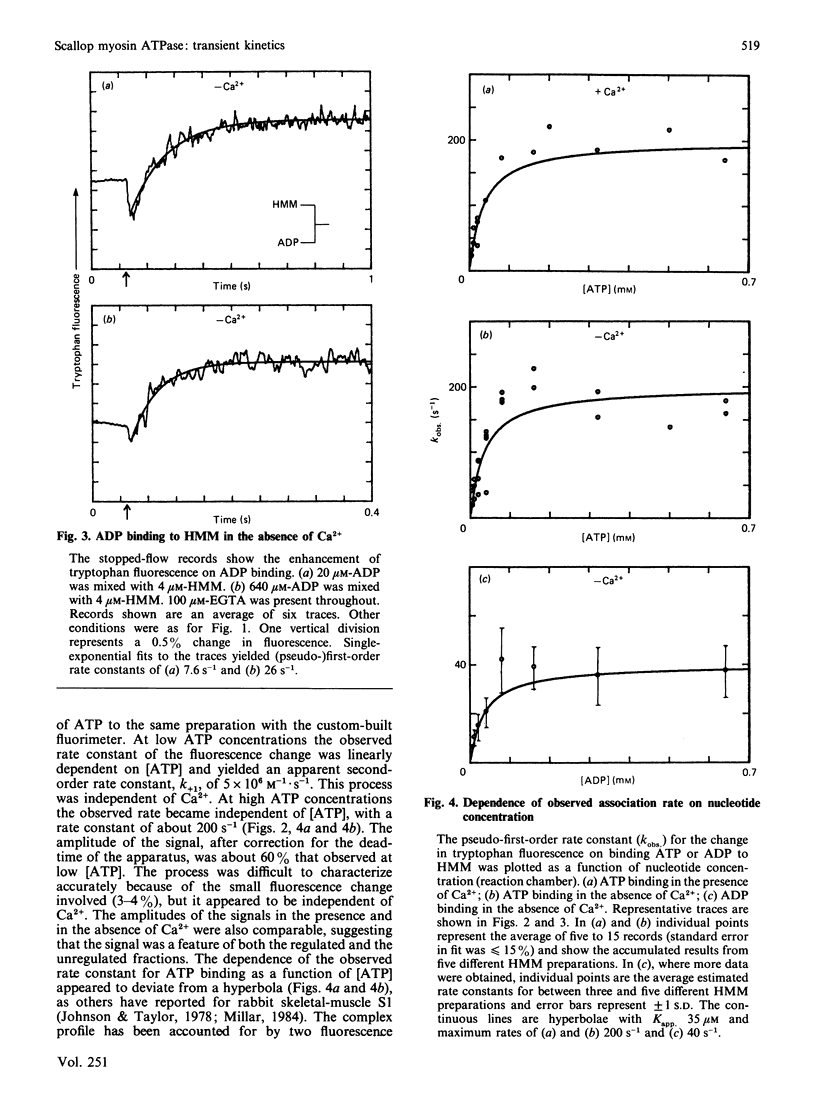
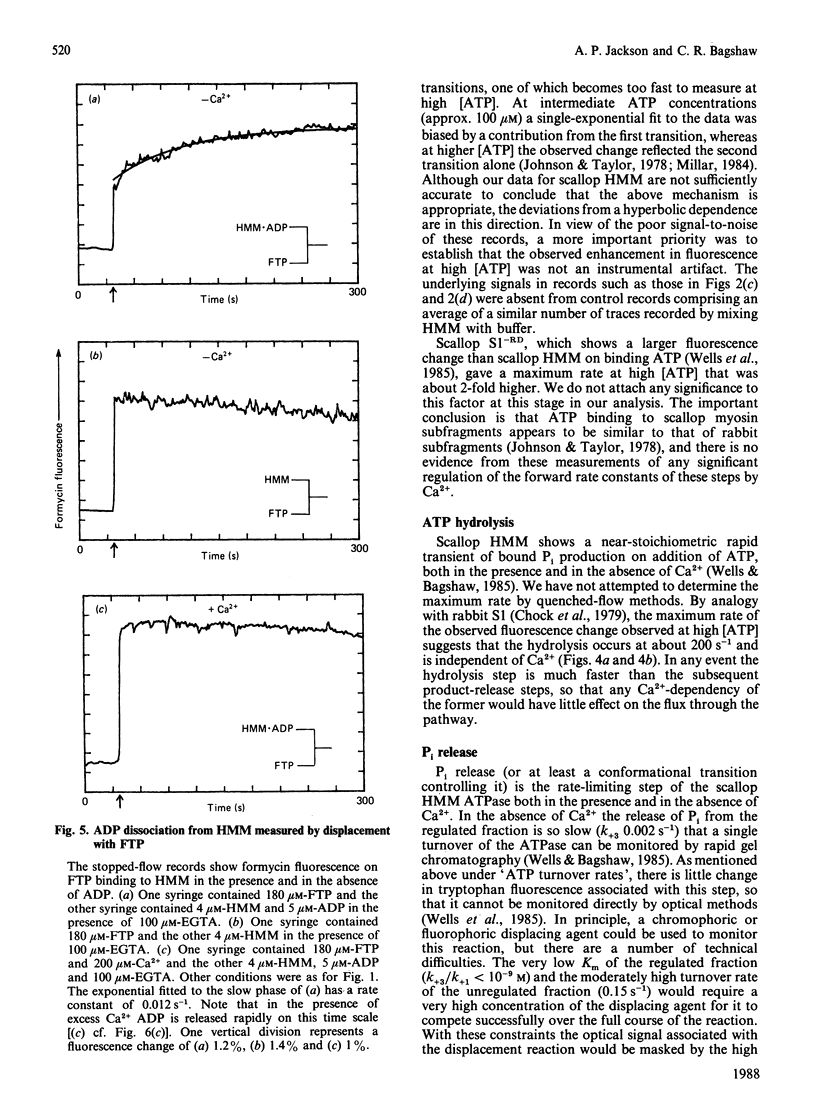
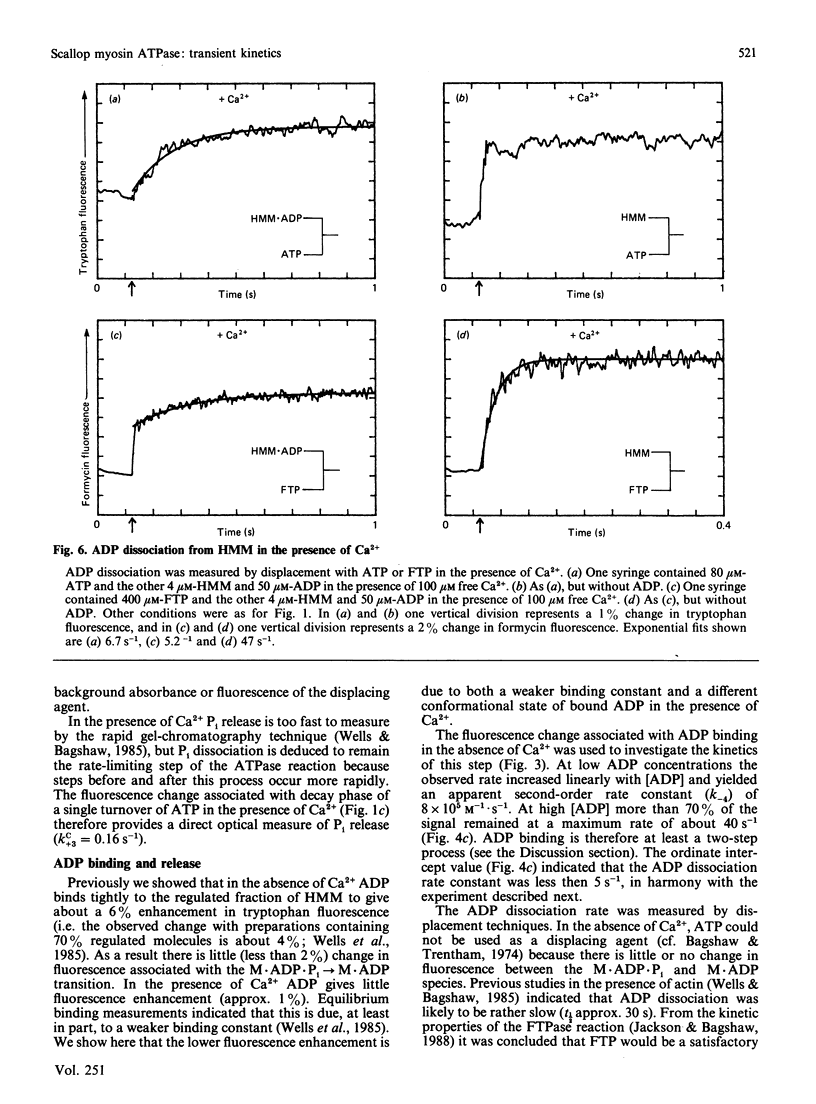
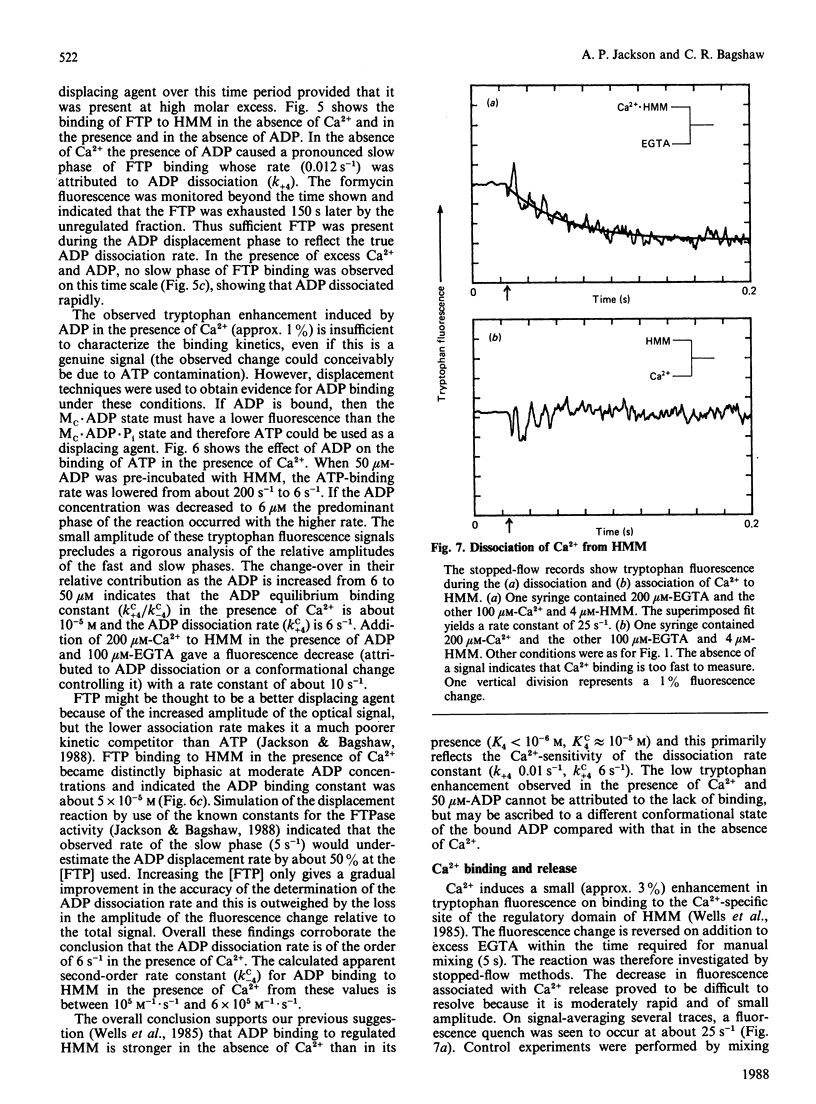
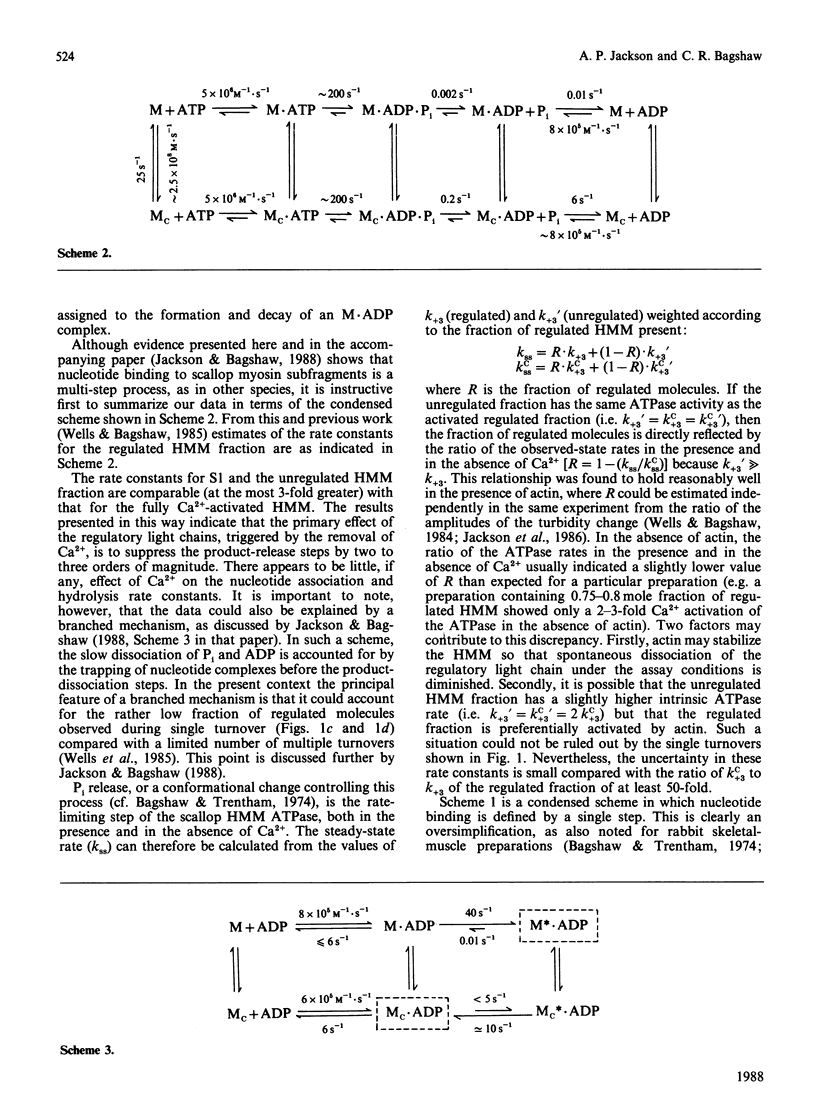
Fluorescence stopped-flow experiments were performed to elucidate the elementary steps of the ATPase mechanism of scallop heavy meromyosin in the presence and in the absence of Ca2+. ATP binding and hydrolysis, as monitored by the change in tryptophan fluorescence, appear to be Ca2+-insensitive, whereas both Pi release and ADP release are markedly suppressed in the absence of Ca2+. Rate constants for Pi release are 0.2 s-1 and 0.002 s-1 and for ADP release are 6 s-1 and 0.01 s-1 in the presence and in the absence of Ca2+ respectively. Ca2+ binding to the specific site of the regulatory domain is rapid and its release occurs at 25 s-1, consistent with the time scale of a twitch of the striated adductor muscle. Nucleotide binding is a multi-step process requiring a minimum of three states. In such a model Ca2+ controls the rate of conformational changes at the active site in both the forward and the reverse direction, leading to a large dependence of the rate of nucleotide release, but a lesser effect on the overall equilibrium position. The kinetic trapping of nucleotides and Pi at the active site, in the absence of Ca2+, appears to be a fundamental step in suppressing the interaction of the myosin head with the thin filaments in relaxed molluscan muscle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Eccleston J. F., Eckstein F., Goody R. S., Gutfreund H., Trentham D. R. The magnesium ion-dependent adenosine triphosphatase of myosin. Two-step processes of adenosine triphosphate association and adenosine diphosphate dissociation. Biochem J. 1974 Aug;141(2):351–364. doi: 10.1042/bj1410351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. J., Bagshaw C. R. The kinetics of bivalent metal ion dissociation from myosin subfragments. Biochem J. 1986 Jan 1;233(1):173–177. doi: 10.1042/bj2330173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. J., Bagshaw C. R. The mechanism of regulatory light chain dissociation from scallop myosin. Biochem J. 1986 Jan 1;233(1):179–186. doi: 10.1042/bj2330179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler P. D., Sellers J. R., Szent-Györgyi A. G. Cooperativity in scallop myosin. Biochemistry. 1981 Jan 6;20(1):210–216. doi: 10.1021/bi00504a035. [DOI] [PubMed] [Google Scholar]
- Chock S. P., Chock P. B., Eisenberg E. The mechanism of the skeletal muscle myosin ATPase. II. Relationship between the fluorescence enhancement induced by ATP and the initial Pi burst. J Biol Chem. 1979 May 10;254(9):3236–3243. [PubMed] [Google Scholar]
- Chock S. P., Eisenberg E. The mechanism of the skeletal muscle myosin ATPase. I. Identity of the myosin active sites. J Biol Chem. 1979 May 10;254(9):3229–3235. [PubMed] [Google Scholar]
- Gutfreund H. Kinetic analysis of the properties and reactions of enzymes. Prog Biophys Mol Biol. 1975;29(2):161–195. doi: 10.1016/0079-6107(76)90022-5. [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Bagshaw C. R. Kinetic trapping of intermediates of the scallop heavy meromyosin adenosine triphosphatase reaction revealed by formycin nucleotides. Biochem J. 1988 Apr 15;251(2):527–540. doi: 10.1042/bj2510527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Taylor E. W. Intermediate states of subfragment 1 and actosubfragment 1 ATPase: reevaluation of the mechanism. Biochemistry. 1978 Aug 22;17(17):3432–3442. doi: 10.1021/bi00610a002. [DOI] [PubMed] [Google Scholar]
- Konno K., Arai K., Watanabe S. Fluorescence intensity and UV absorption changes accompanying dissociation and association of regulatory light chain of scallop adductor myosin. J Biochem. 1983 Oct;94(4):1061–1066. doi: 10.1093/oxfordjournals.jbchem.a134448. [DOI] [PubMed] [Google Scholar]
- Lehman W., Szent-Györgyi A. G. Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol. 1975 Jul;66(1):1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar N. C., Geeves M. A. The limiting rate of the ATP-mediated dissociation of actin from rabbit skeletal muscle myosin subfragment 1. FEBS Lett. 1983 Aug 22;160(1-2):141–148. doi: 10.1016/0014-5793(83)80954-5. [DOI] [PubMed] [Google Scholar]
- Rall J. A. Mechanics and energetics of contraction in striated muscle of the sea scallop, Placopecten magellanicus. J Physiol. 1981 Dec;321:287–295. doi: 10.1113/jphysiol.1981.sp013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Watterson J. G. Symmetry and asymmetry in the contractile protein myosin. Biochimie. 1981 Apr;63(4):291–299. doi: 10.1016/s0300-9084(81)80117-4. [DOI] [PubMed] [Google Scholar]
- Shibata-Sekiya K. Reaction intermediates of myosin ATPase from scallop adductor muscles: nonidentical two-headed structure of striated adductor muscle myosin. J Biochem. 1982 Oct;92(4):1151–1162. doi: 10.1093/oxfordjournals.jbchem.a134031. [DOI] [PubMed] [Google Scholar]
- Smith P. D., Liesegang G. W., Berger R. L., Czerlinski G., Podolsky R. J. A stopped-flow investigation of calcium ion binding by ethylene glycol bis(beta-aminoethyl ether)-N,N'-tetraacetic acid. Anal Biochem. 1984 Nov 15;143(1):188–195. doi: 10.1016/0003-2697(84)90575-x. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Transient kinetics of adenosine 5'-diphosphate and adenosine 5'-(beta, gamma-imidotriphosphate) binding to subfragment 1 and actosubfragment 1. Biochemistry. 1982 Mar 16;21(6):1284–1294. doi: 10.1021/bi00535a028. [DOI] [PubMed] [Google Scholar]
- Vibert P., Szentkiralyi E., Hardwicke P., Szent-Györgyi A. G., Cohen C. Structural models for the regulatory switch of Myosin. Biophys J. 1986 Jan;49(1):131–133. doi: 10.1016/S0006-3495(86)83622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmsley A. R., Lowe A. G. Multifit: a flexible non-linear least squares regression program in BASIC. Comput Methods Programs Biomed. 1985 Nov;21(2):113–118. doi: 10.1016/0169-2607(85)90070-7. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Calcium regulation of molluscan myosin ATPase in the absence of actin. Nature. 1985 Feb 21;313(6004):696–697. doi: 10.1038/313696a0. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Segmental flexibility and head-head interaction in scallop myosin. A study using saturation transfer electron paramagnetic resonance spectroscopy. J Mol Biol. 1983 Feb 15;164(1):137–157. doi: 10.1016/0022-2836(83)90090-6. [DOI] [PubMed] [Google Scholar]
- Wells C., Warriner K. E., Bagshaw C. R. Fluorescence studies on the nucleotide- and Ca2+-binding domains of molluscan myosin. Biochem J. 1985 Oct 1;231(1):31–38. doi: 10.1042/bj2310031. [DOI] [PMC free article] [PubMed] [Google Scholar]



