Abstract
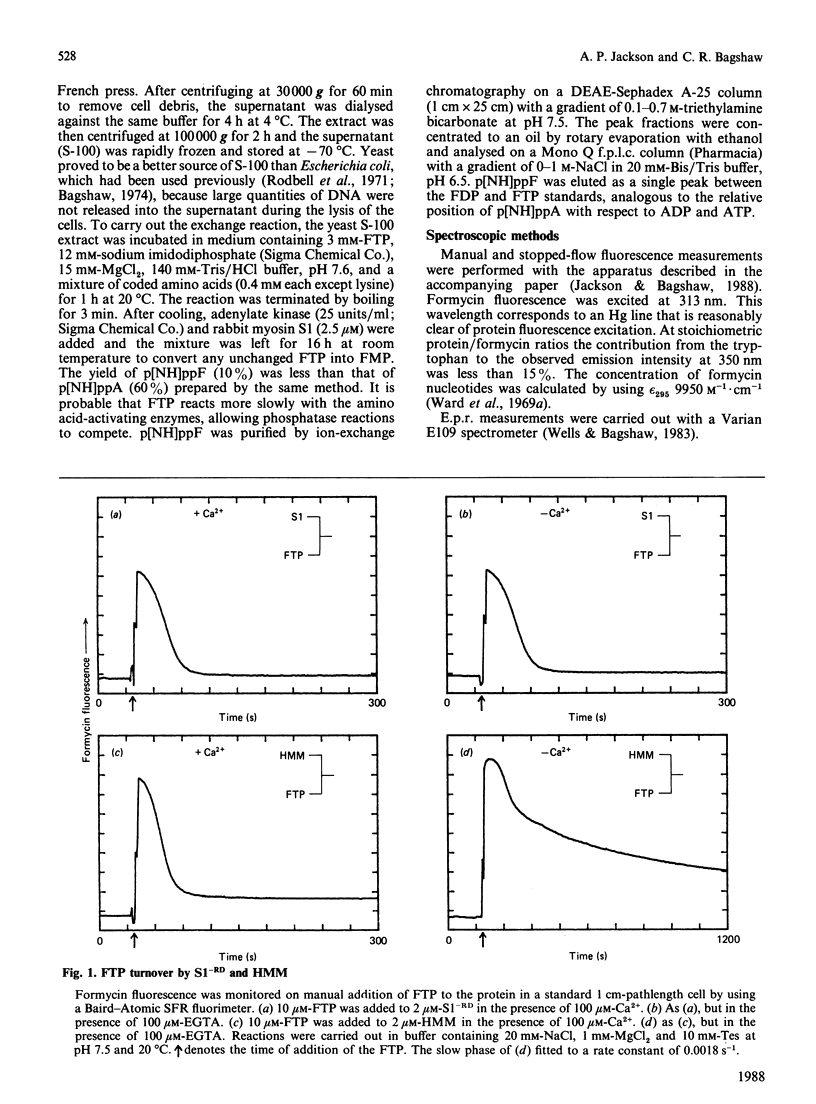
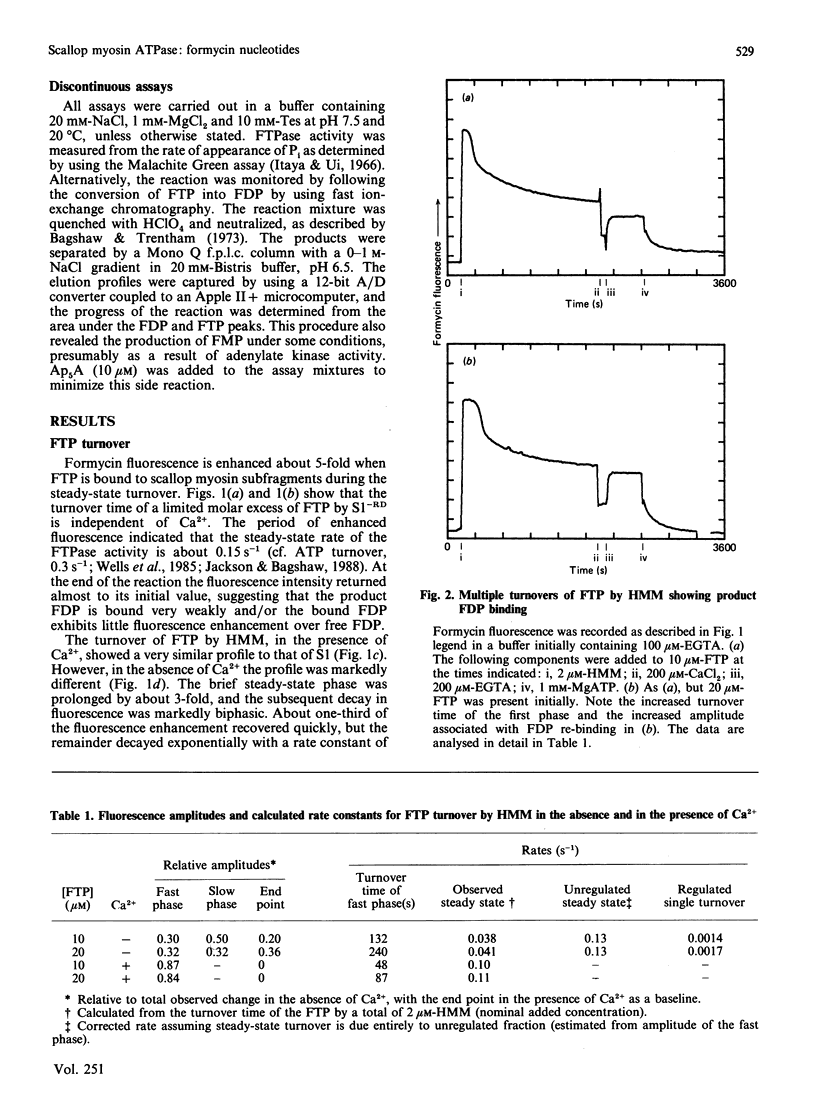
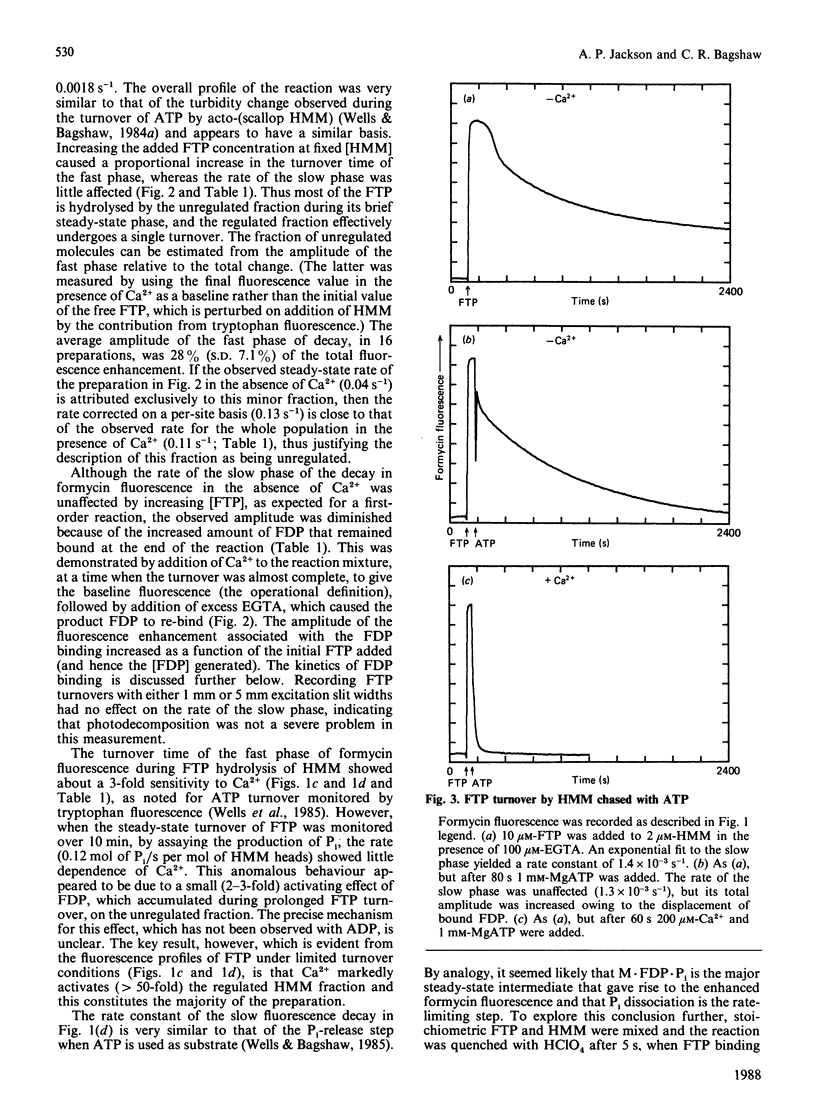
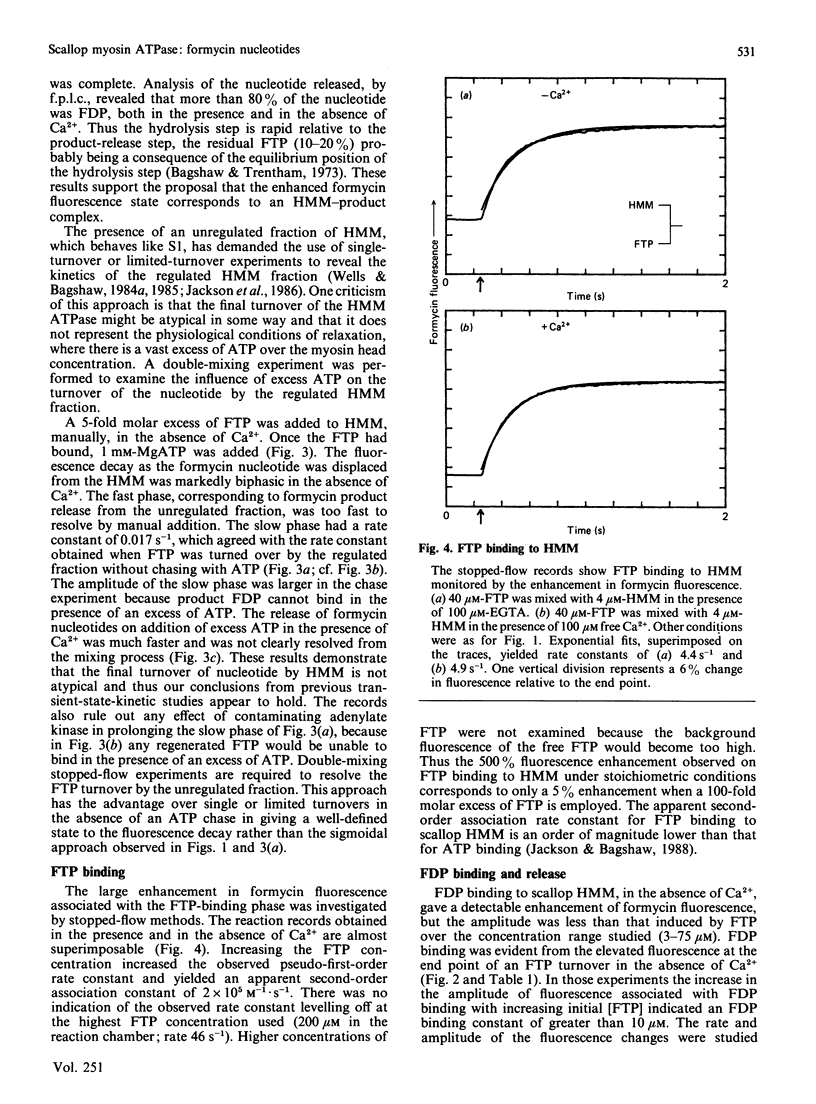
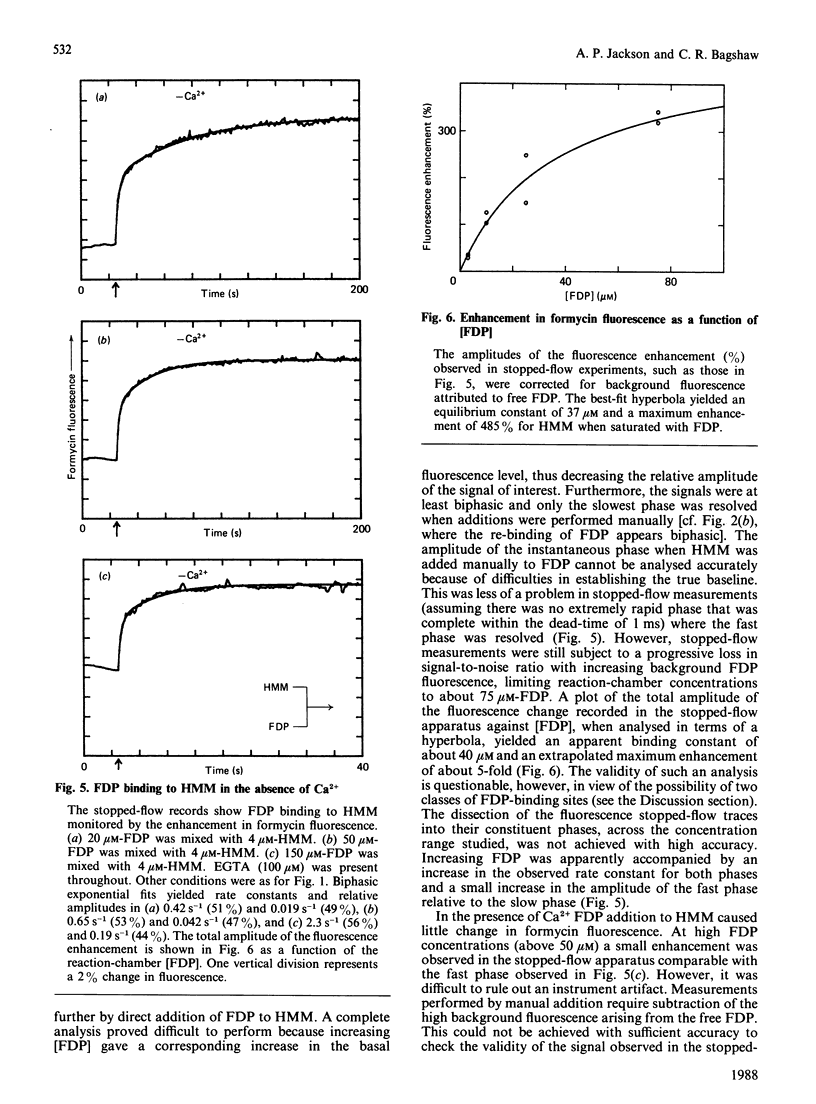
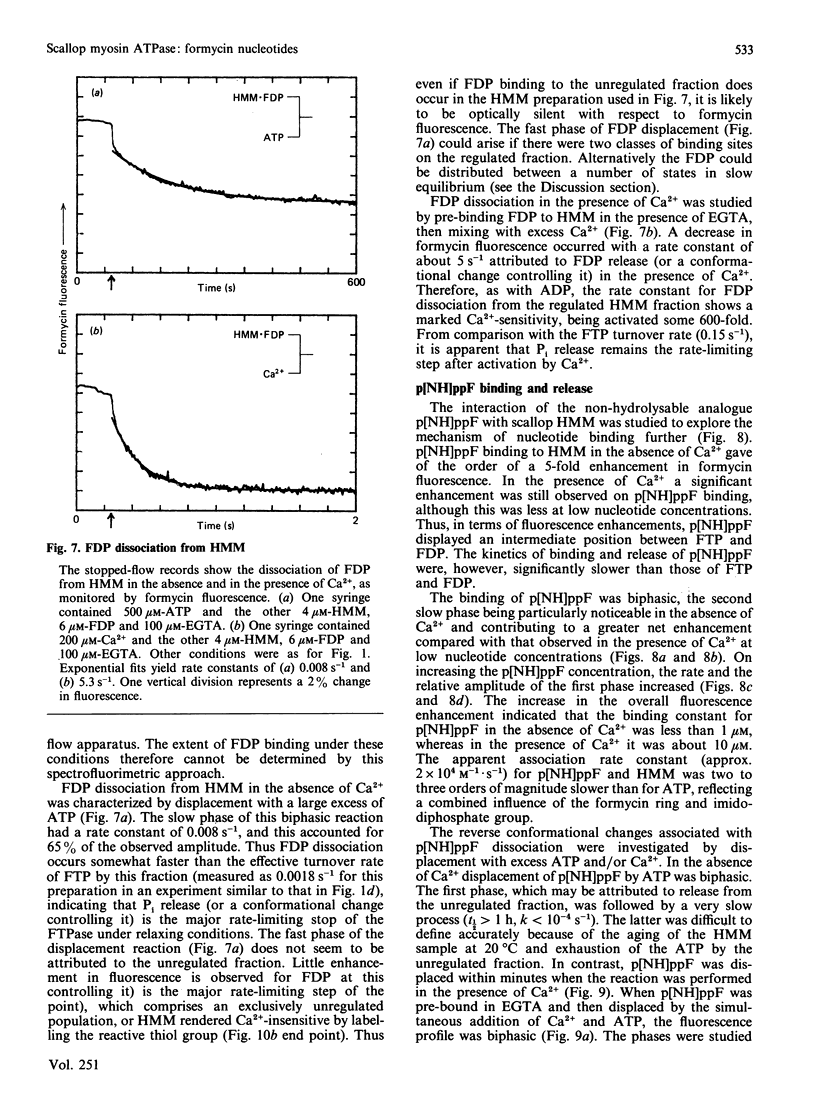
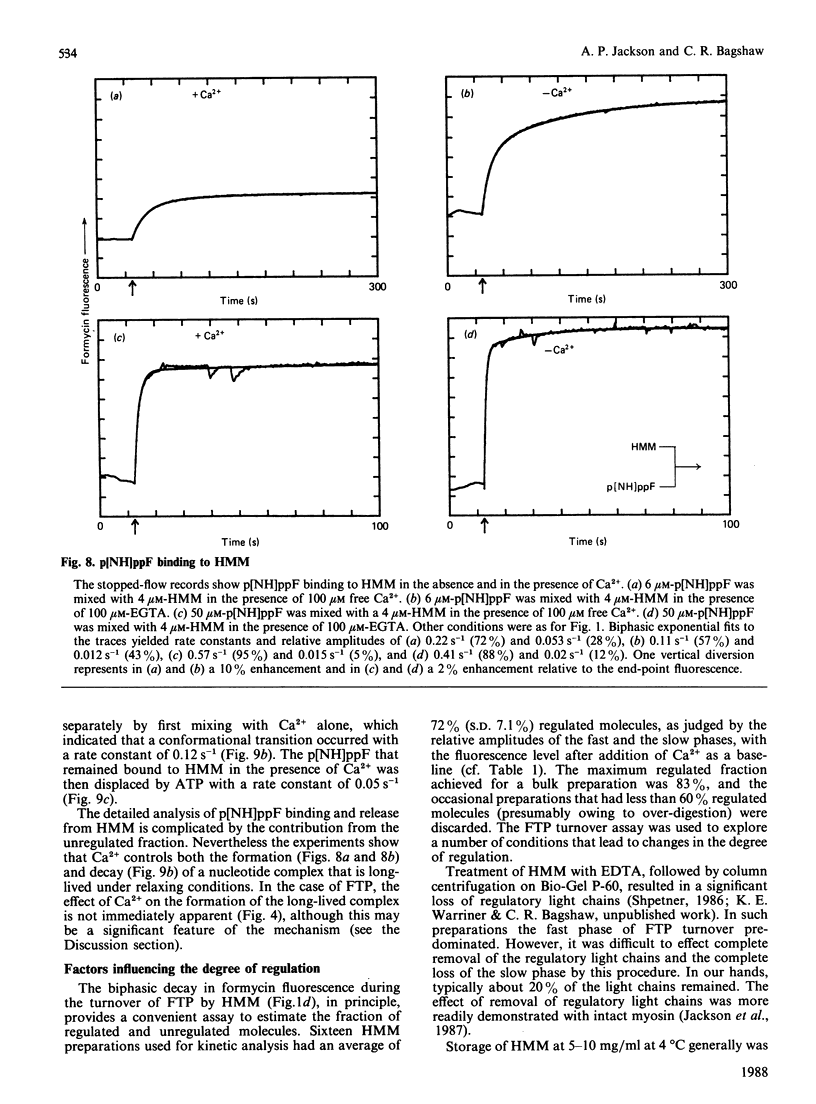
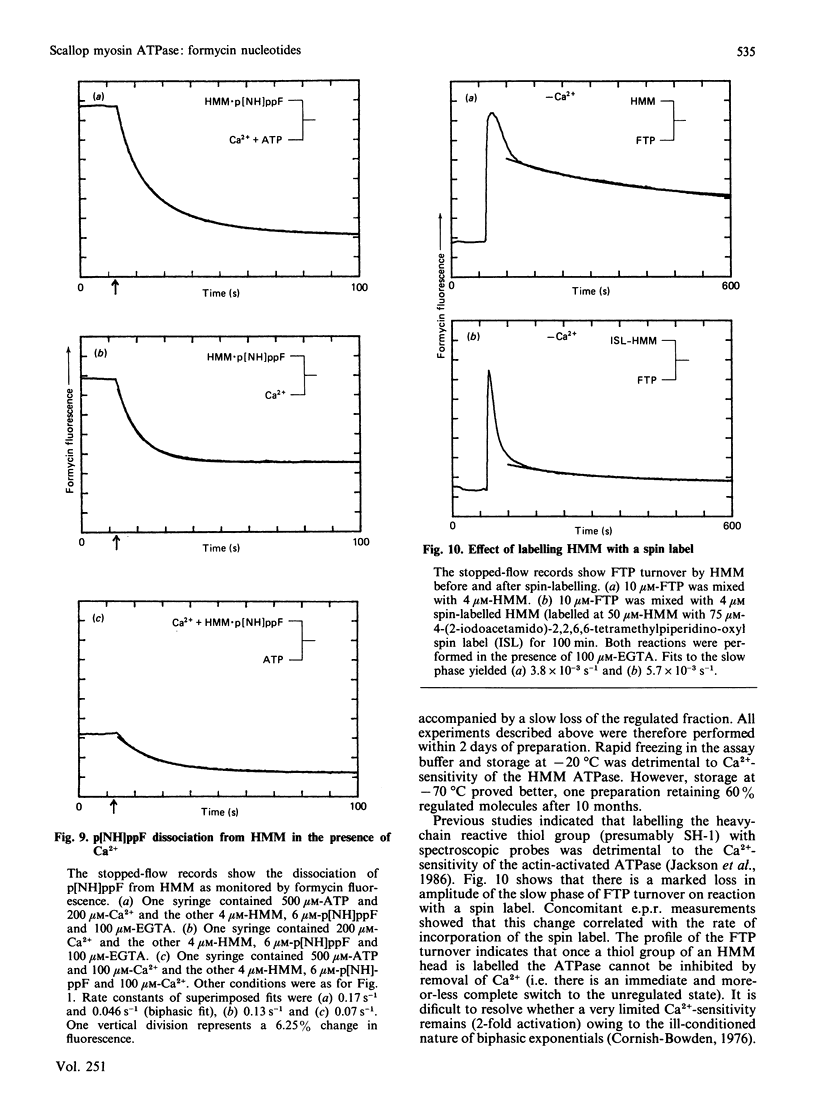
The kinetics of interaction of formycin nucleotides with scallop myosin subfragments were investigated by exploiting the fluorescence signal of the ligand. Formycin triphosphate gives a 5-fold enhancement of the emission intensity on binding to heavy meromyosin, and the profile indicates that the kinetics of binding are Ca2+-insensitive. In contrast, the subsequent product-release steps show a marked degree of regulation by Ca2+. In the absence of Ca2+ formycin triphosphate turnover by the unregulated and the regulated heavy meromyosin fractions are clearly resolved, the latter showing a fluorescence decay rate of 0.002 s-1, corresponding to the Pi-release step. In the presence of Ca2+ this step is activated 50-fold. Formycin diphosphate release is also regulated by Ca2+, being activated from 0.008 s-1 to 5 s-1. In contrast with protein tryptophan fluorescence [Jackson & Bagshaw (1988) Biochem. J. 251, 515-526], formycin fluorescence is sensitive to conformational changes that occur subsequent to the binding step and demonstrate, directly, an effect of Ca2+ on both forward and reverse rate constants. Apart from a decrease in the apparent second-order association rate constants, formycin derivatives appear to mimic adenosine nucleotides closely in their interaction with scallop heavy meromyosin and provide a spectroscopic handle on steps that are optically silent with respect to protein fluorescence. A novel mechanism is discussed in which regulation of the formycin triphosphate activity by Ca2+ involves kinetic trapping of product complexes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashiba G., Asada T., Watanabe S. Calcium regulation in clam foot muscle. Calcium sensitivity of clam foot myosin. J Biochem. 1980 Sep;88(3):837–846. doi: 10.1093/oxfordjournals.jbchem.a133038. [DOI] [PubMed] [Google Scholar]
- Bagshaw C. R., Eccleston J. F., Eckstein F., Goody R. S., Gutfreund H., Trentham D. R. The magnesium ion-dependent adenosine triphosphatase of myosin. Two-step processes of adenosine triphosphate association and adenosine diphosphate dissociation. Biochem J. 1974 Aug;141(2):351–364. doi: 10.1042/bj1410351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Chantler P. D., Szent-Gyorgyi A. G., Eisenberg E. Regulation of molluscan actomyosin ATPase activity. J Biol Chem. 1984 Feb 25;259(4):2617–2621. [PMC free article] [PubMed] [Google Scholar]
- Collins J. H., Jakes R., Kendrick-Jones J., Leszyk J., Barouch W., Theibert J. L., Spiegel J., Szent-Györgyi A. G. Amino acid sequence of myosin essential light chain from the scallop Aquipecten irradians. Biochemistry. 1986 Nov 18;25(23):7651–7656. doi: 10.1021/bi00371a056. [DOI] [PubMed] [Google Scholar]
- Cross R. A., Cross K. E., Sobieszek A. ATP-linked monomer-polymer equilibrium of smooth muscle myosin: the free folded monomer traps ADP.Pi. EMBO J. 1986 Oct;5(10):2637–2641. doi: 10.1002/j.1460-2075.1986.tb04545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston J. F., Trentham D. R. The interaction of chromophoric nucleotides with subfragment 1 of myosin. Biochem J. 1977 Apr 1;163(1):15–29. doi: 10.1042/bj1630015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody R. S., Hofmann W. Stereochemical aspects of the interaction of myosin and actomyosin with nucleotides. J Muscle Res Cell Motil. 1980 Mar;1(1):101–115. doi: 10.1007/BF00711928. [DOI] [PubMed] [Google Scholar]
- Hardwicke P. M., Wallimann T., Szent-Györgyi A. G. Light-chain movement and regulation in scallop myosin. Nature. 1983 Feb 10;301(5900):478–482. doi: 10.1038/301478a0. [DOI] [PubMed] [Google Scholar]
- Itaya K., Ui M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966 Sep;14(3):361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- Jackson A. P., Bagshaw C. R. Transient-kinetic studies of the adenosine triphosphatase activity of scallop heavy meromyosin. Biochem J. 1988 Apr 15;251(2):515–526. doi: 10.1042/bj2510515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick-Jones J., Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory light chains in myosins. J Mol Biol. 1976 Jul 15;104(4):747–775. doi: 10.1016/0022-2836(76)90180-7. [DOI] [PubMed] [Google Scholar]
- Munson K. B., Smerdon M. J., Yount R. G. Cross-linking of myosin subfragment 1 and heavy meromyosin by use of vanadate and a bis(adenosine 5'-triphosphate) analogue. Biochemistry. 1986 Nov 18;25(23):7640–7650. doi: 10.1021/bi00371a055. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Sekine T., Grammer J., Yount R. G. The essential light chains constitute part of the active site of smooth muscle myosin. Nature. 1986 Nov 6;324(6092):78–80. doi: 10.1038/324078a0. [DOI] [PubMed] [Google Scholar]
- Prince H. P., Trayer H. R., Henry G. D., Trayer I. P., Dalgarno D. C., Levine B. A., Cary P. D., Turner C. Proton nuclear-magnetic-resonance spectroscopy of myosin subfragment 1 isoenzymes. Eur J Biochem. 1981 Dec;121(1):213–219. doi: 10.1111/j.1432-1033.1981.tb06451.x. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rossomando E. F., Jahngen J., Eccleston J. F. A fluorometric-high-performance liquid chromatographic assay procedure for several enzymatic activities using formycin analogs of adenosine 5'-mono, 5'-tri, and cyclic 3',5'-monophosphate as substrates. Anal Biochem. 1981 Sep 1;116(1):80–88. doi: 10.1016/0003-2697(81)90325-0. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Watterson J. G. Symmetry and asymmetry in the contractile protein myosin. Biochimie. 1981 Apr;63(4):291–299. doi: 10.1016/s0300-9084(81)80117-4. [DOI] [PubMed] [Google Scholar]
- Shoham M., Steitz T. A. Crystallographic studies and model building of ATP at the active site of hexokinase. J Mol Biol. 1980 Jun 15;140(1):1–14. doi: 10.1016/0022-2836(80)90353-8. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Trybus K. M., Taylor E. W. Transient kinetics of adenosine 5'-diphosphate and adenosine 5'-(beta, gamma-imidotriphosphate) binding to subfragment 1 and actosubfragment 1. Biochemistry. 1982 Mar 16;21(6):1284–1294. doi: 10.1021/bi00535a028. [DOI] [PubMed] [Google Scholar]
- Vibert P., Craig R. Structural changes that occur in scallop myosin filaments upon activation. J Cell Biol. 1985 Sep;101(3):830–837. doi: 10.1083/jcb.101.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P., Szentkiralyi E., Hardwicke P., Szent-Györgyi A. G., Cohen C. Structural models for the regulatory switch of Myosin. Biophys J. 1986 Jan;49(1):131–133. doi: 10.1016/S0006-3495(86)83622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. C., Cerami A., Reich E., Acs G., Altwerger L. Biochemical studies of the nucleoside analogue, formycin. J Biol Chem. 1969 Jun 25;244(12):3243–3250. [PubMed] [Google Scholar]
- Ward D. C., Reich E. Conformational properties of polyformycin: a polyribonucleotide with individual residues in the syn conformation. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1494–1501. doi: 10.1073/pnas.61.4.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. C., Reich E., Stryer L. Fluorescence studies of nucleotides and polynucleotides. I. Formycin, 2-aminopurine riboside, 2,6-diaminopurine riboside, and their derivatives. J Biol Chem. 1969 Mar 10;244(5):1228–1237. [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Calcium regulation of molluscan myosin ATPase in the absence of actin. Nature. 1985 Feb 21;313(6004):696–697. doi: 10.1038/313696a0. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. Segmental flexibility and head-head interaction in scallop myosin. A study using saturation transfer electron paramagnetic resonance spectroscopy. J Mol Biol. 1983 Feb 15;164(1):137–157. doi: 10.1016/0022-2836(83)90090-6. [DOI] [PubMed] [Google Scholar]
- Wells C., Bagshaw C. R. The characterization of vanadate-trapped nucleotide complexes with spin-labelled myosins. J Muscle Res Cell Motil. 1984 Feb;5(1):97–112. doi: 10.1007/BF00713154. [DOI] [PubMed] [Google Scholar]
- Wells C., Warriner K. E., Bagshaw C. R. Fluorescence studies on the nucleotide- and Ca2+-binding domains of molluscan myosin. Biochem J. 1985 Oct 1;231(1):31–38. doi: 10.1042/bj2310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Zimmerman R. W., Trentham D. R. The ATPase kinetics of insect fibrillar flight muscle myosin subfragment-1. J Muscle Res Cell Motil. 1986 Apr;7(2):179–192. doi: 10.1007/BF01753419. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]



