Abstract
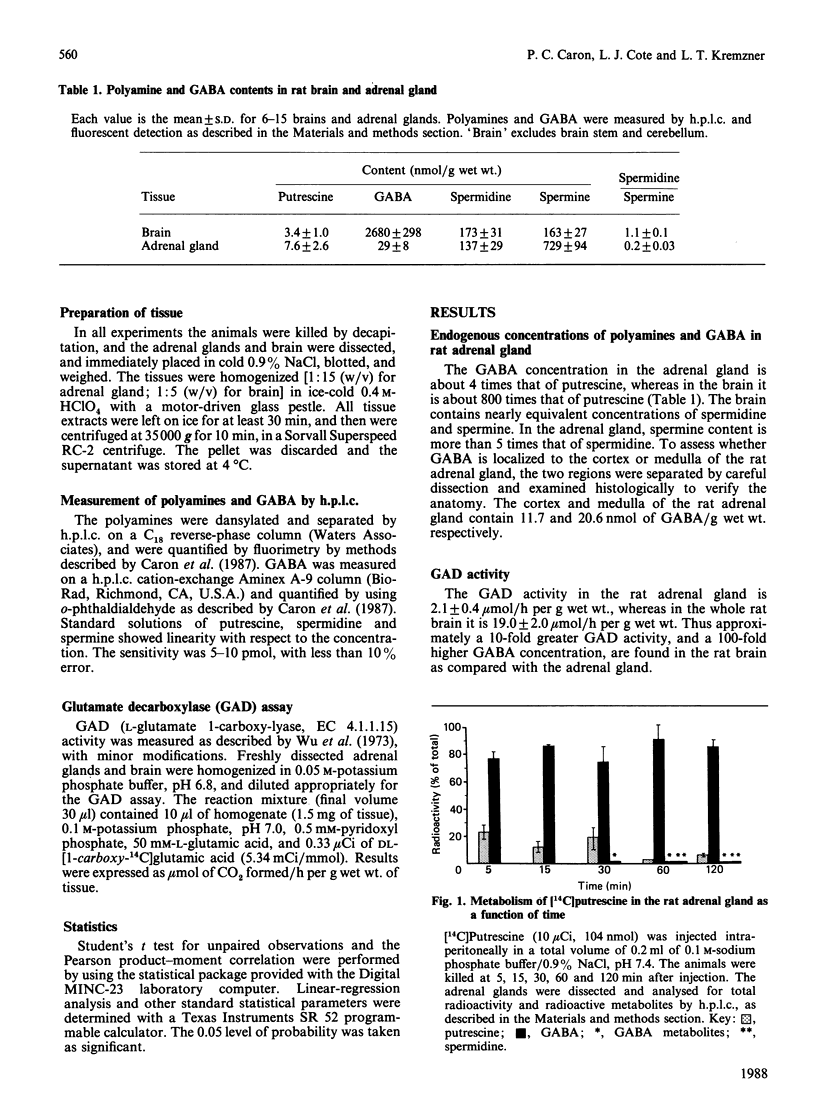
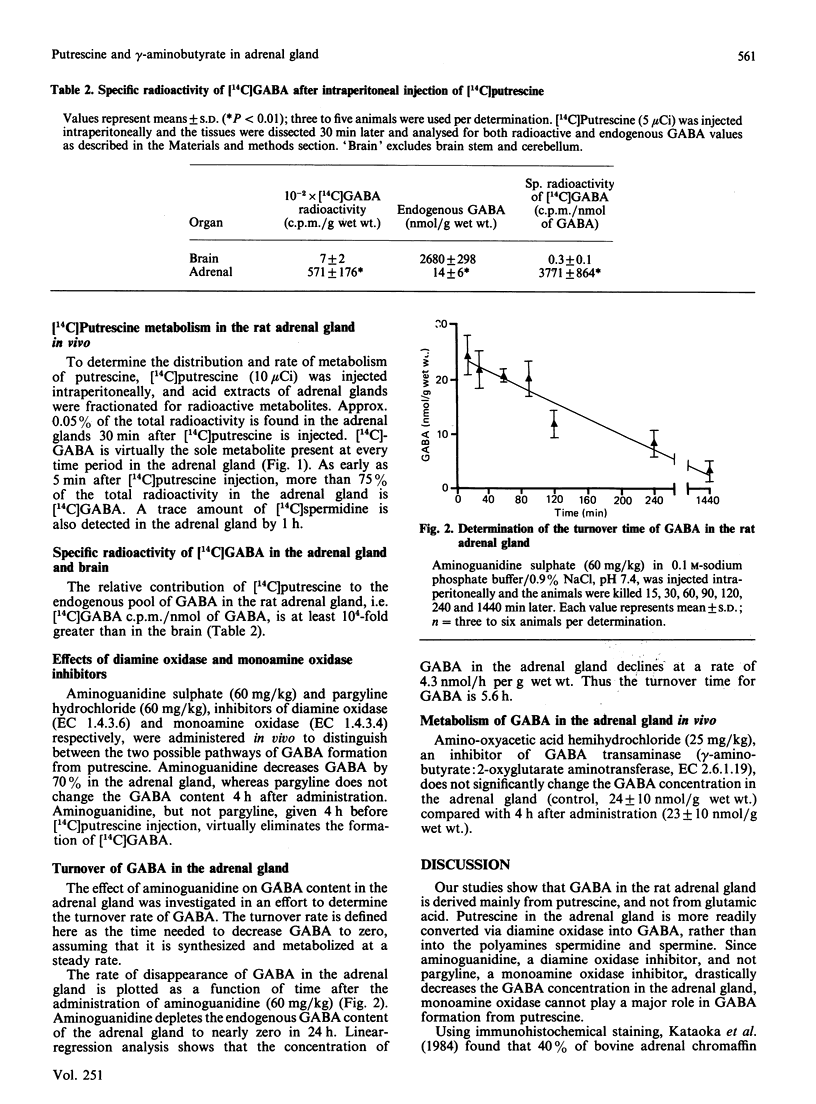
Putrescine is the major source of gamma-aminobutyric acid (GABA) in the rat adrenal gland. Diamine oxidase, and not monoamine oxidase, is essential for GABA formation from putrescine in the adrenal gland. Aminoguanidine, a diamine oxidase inhibitor, decreases the GABA concentration in the adrenal gland by more than 70% after 4 h, and almost to zero in 24 h. Studies using [14C]putrescine confirm that [14C]GABA is the major metabolite of putrescine in the adrenal gland. Inhibition of GABA transaminase by amino-oxyacetic acid does not change the GABA concentration in the adrenal gland, as compared with the brain, where the GABA concentration rises. With aminoguanidine, the turnover time of GABA originating from putrescine in the adrenal gland is 5.6 h, reflecting a slower rate of GABA metabolism compared with the brain. Since GABA in the adrenal gland is almost exclusively derived from putrescine, the role of GABA may relate to the role of putrescine as a growth factor and regulator of cell metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apud J. A., Tappaz M. L., Celotti F., Negri-Cesi P., Masotto C., Racagni G. Biochemical and immunochemical studies on the GABAergic system in the rat fallopian tube and ovary. J Neurochem. 1984 Jul;43(1):120–125. doi: 10.1111/j.1471-4159.1984.tb06686.x. [DOI] [PubMed] [Google Scholar]
- Bernasconi R., Maitre L., Martin P., Raschdorf F. The use of inhibitors of GABA-transaminase for the determination of GABA turnover in mouse brain regions: an evaluation of aminooxyacetic acid and gabaculine. J Neurochem. 1982 Jan;38(1):57–66. doi: 10.1111/j.1471-4159.1982.tb10853.x. [DOI] [PubMed] [Google Scholar]
- Bormann J., Clapham D. E. gamma-Aminobutyric acid receptor channels in adrenal chromaffin cells: a patch-clamp study. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2168–2172. doi: 10.1073/pnas.82.7.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotti F., Apud J. A., Melcangi R. C., Masotto C., Tappaz M., Racagni G. Endocrine modulation of gamma-aminobutyric acidergic innervation in the rat fallopian tube. Endocrinology. 1986 Jan;118(1):334–339. doi: 10.1210/endo-118-1-334. [DOI] [PubMed] [Google Scholar]
- De Mello F. G., Bachrach U., Nirenberg M. Ornithine and glutamic acid decarboxylase activities in the developing chick retina. J Neurochem. 1976 Oct;27(4):847–851. doi: 10.1111/j.1471-4159.1976.tb05145.x. [DOI] [PubMed] [Google Scholar]
- Erdö S. L., Rosdy B., Szporny L. Higher GABA concentrations in fallopian tube than in brain of the rat. J Neurochem. 1982 Apr;38(4):1174–1176. doi: 10.1111/j.1471-4159.1982.tb05368.x. [DOI] [PubMed] [Google Scholar]
- Fogel W. A., Biegański T., Maśliński C. Gamma-aminobutyric acid (GABA) formation from putrescine in guinea-pig liver during ontogenesis. Comp Biochem Physiol C. 1982;73(2):431–434. doi: 10.1016/0306-4492(82)90148-4. [DOI] [PubMed] [Google Scholar]
- Gerber J. C., 3rd, Hare T. A. Gamma-aminobutyric acid in peripheral tissue, with emphasis on the endocrine pancreas: presence in two species and reduction by streptozotocin. Diabetes. 1979 Dec;28(12):1073–1076. doi: 10.2337/diab.28.12.1073. [DOI] [PubMed] [Google Scholar]
- Grillo M. A. Metabolism and function of polyamines. Int J Biochem. 1985;17(9):943–948. doi: 10.1016/0020-711x(85)90238-1. [DOI] [PubMed] [Google Scholar]
- Heby O. Role of polyamines in the control of cell proliferation and differentiation. Differentiation. 1981;19(1):1–20. doi: 10.1111/j.1432-0436.1981.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Kataoka Y., Gutman Y., Guidotti A., Panula P., Wroblewski J., Cosenza-Murphy D., Wu J. Y., Costa E. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremzner L. T., Hiller J. M., Simon E. J. Metabolism of polyamines in mouse neuroblastoma cells in culture: formation of GABA and putreanine. J Neurochem. 1975 Dec;25(6):889–894. doi: 10.1111/j.1471-4159.1975.tb04423.x. [DOI] [PubMed] [Google Scholar]
- Martin de Rio R., López M. S. Effects of aminooxyacetic acid on in vivo gamma-aminobutyric acid system of rat oviduct. Gen Pharmacol. 1983;14(2):281–283. doi: 10.1016/0306-3623(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Martin del Rio R., Caballero A. L. Presence of gamma-aminobutyric acid in rat ovary. J Neurochem. 1980 Jun;34(6):1584–1586. doi: 10.1111/j.1471-4159.1980.tb11247.x. [DOI] [PubMed] [Google Scholar]
- Martín del Rio R. Gamma-aminobutyric acid system in rat oviduct. J Biol Chem. 1981 Oct 10;256(19):9816–9819. [PubMed] [Google Scholar]
- Murashima Y. L., Kato T. Distribution of gamma-aminobutyric acid and glutamate decarboxylase in the layers of rat oviduct. J Neurochem. 1986 Jan;46(1):166–172. doi: 10.1111/j.1471-4159.1986.tb12940.x. [DOI] [PubMed] [Google Scholar]
- Perry T. L., Hansen S. Sustained drug-induced elevation of brain GABA in the rat. J Neurochem. 1973 Nov;21(5):1167–1175. doi: 10.1111/j.1471-4159.1973.tb07572.x. [DOI] [PubMed] [Google Scholar]
- Sangiah S., Borowitz J. L., Yim G. K. Actions of GABA, picrotoxin and bicuculline on adrenal medulla. Eur J Pharmacol. 1974 Jun;27(1):130–135. doi: 10.1016/0014-2999(74)90209-x. [DOI] [PubMed] [Google Scholar]
- Seiler N. On the role of GABA in vertebrate polyamine metabolism. Physiol Chem Phys. 1980;12(5):411–429. [PubMed] [Google Scholar]
- Sobue K., Nakjima T. Changes in concentrations of polyamines and gamma-aminobutyric acid and their formation in chick embryo brain during development. J Neurochem. 1978 Jan;30(1):277–279. doi: 10.1111/j.1471-4159.1978.tb07065.x. [DOI] [PubMed] [Google Scholar]
- Sourkes T. L., Missala K. Putrescine metabolism and the study of diamine oxidase activity in vivo. Agents Actions. 1981 Apr;11(1-2):20–27. doi: 10.1007/BF01991449. [DOI] [PubMed] [Google Scholar]
- Tanaka C. gamma-Aminobutyric acid in peripheral tissues. Life Sci. 1985 Dec 16;37(24):2221–2235. doi: 10.1016/0024-3205(85)90013-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi H., Okada Y., Seguchi H., Shimada C., Seki M., Tsutou A., Baba S. High concentration of gamma-aminobutyric acid in pancreatic beta cells. Diabetes. 1979 Jul;28(7):629–633. doi: 10.2337/diab.28.7.629. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y., Elde R. P., Morgan L. M., Kimmel J. R. Immunohistochemical studies of the GABA system in the pancreas. Neuroendocrinology. 1983;36(3):197–204. doi: 10.1159/000123456. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Matsuda T., Roberts E. Purification and characterization of glutamate decarboxylase from mouse brain. J Biol Chem. 1973 May 10;248(9):3029–3034. [PubMed] [Google Scholar]
- Yamanaka K., Yamada S., Okada T., Hayashi E. Effect of picrotoxin on adrenal catecholamine secretion. Jpn J Pharmacol. 1983 Oct;33(5):1049–1055. doi: 10.1254/jjp.33.1049. [DOI] [PubMed] [Google Scholar]


