Abstract
Introduction
The patterns of oligoprogression after first-line immune checkpoint inhibitors (ICIs) for metastatic NSCLC are yet to be well established. An increasing volume of data suggests that directed radiotherapy improves survival outcomes in patients with progression after ICIs.
Methods
A retrospective cohort study was performed on patients with metastatic NSCLC who had completed first-line programmed death-(ligand) 1 inhibitor therapy with or without chemotherapy at two high-volume cancer centers. We sought to characterize the frequency and location of oligoprogression and determine the overall survival (OS) after radiotherapy in this population.
Results
A total of 159 patients were included in the study. At first progression, 62 (39.0%) were classified as undergoing oligoprogression. Multivariate analysis confirmed the presence of brain metastases was associated with an increased likelihood of oligoprogression (OR = 2.44, p = 0.04) with most (63.2%) of these patients experiencing progression intracranially. The presence of liver metastases was associated with a decreased likelihood of oligoprogression (OR = 0.17, p < 0.01). For patients with oligoprogression, those who received radiotherapy had a longer median progression-free survival-2 (PFS2) (17 versus 11.5 mo, HR = 0.51, p = 0.02) and a longer median OS (23 versus 13 mo, HR = 0.40, p < 0.001) compared with those who did not receive radiotherapy. No difference in PFS2 or OS outcomes was observed between patients who received radiotherapy versus those who did not for systemic progression.
Conclusions
In patients with oligoprogressive metastatic NSCLC after treatment with first-line ICIs, radiotherapy significantly improves OS and PFS2 outcomes. Patients with baseline brain metastases are more likely to experience oligoprogression. Further prospective studies in directed, less heterogeneous populations of patients with metastatic NSCLC will be fundamental to optimize management.
Keywords: NSCLC, Immunotherapy, Oligoprogression, Radiotherapy
Introduction
Immune checkpoint inhibitors (ICIs) have improved survival for patients with metastatic NSCLC without oncogenic drivers. In certain patients with metastatic NSCLC, ICIs have durable efficacy, with 18.4% to 31.9% of patients alive at 5 years.1, 2, 3 However, most patients will experience progression either owing to primary or acquired resistance.
It has become evident that the pattern of progression is an important prognostic factor for patients with metastatic NSCLC. Patients who progress in limited sites, termed oligoprogression, have improved survival outcomes compared with those who progress in multiple sites; systemic progression.4 Recently, a consensus definition for oligoprogression was formulated as a subclassification of oligometastatic disease.5,6 Oligometastatic disease is a term initially defined by Hellman and Weichselbaum7 as metastases to a single or limited number of organs. The ESTRO-ASTRO consensus classification defines oligoprogression as new or enlarging oligometastases in patients undergoing active systemic therapy.6 However, the definition of “limited metastases” remains somewhat ambiguous and open to interpretation by clinicians. In cases of oligoprogression, contemporary guidelines recommend local ablative therapy with radiotherapy - stereotactic ablative radiation therapy (SABR) or conventional radiotherapy to extend the benefit of the preceding therapy.8
Some studies have shown the benefit of local therapy to extend overall survival (OS) and time to tumor progression on next-line treatment (progression-free survival-2 [PFS2]) in patients with oligoprogressive NSCLC.4,9, 10, 11 However, studies in NSCLC describing the benefits of local therapy have generally included patients with multiple tumor types,12 or those progressing after treatment with second-line ICIs4,10 or tyrosine kinase inhibitors.9,11 Therefore, the role of local therapy in oligoprogressive NSCLC without oncogenic drivers for patients treated with first-line ICIs is poorly understood owing to prior heterogeneity in study populations. In addition, there is a paucity of data regarding the management of oligoprogression after programmed death-(ligand) 1 (PD-[L]1) inhibitors and chemotherapy.
In this study, we sought to characterize the frequency and location of oligoprogression and determine the benefit of radiotherapy in patients with metastatic NSCLC treated with first-line PD-(L)1 inhibitors with or without chemotherapy.
Materials and Methods
Patients and Treatment
A retrospective cohort study was performed and data were extracted from the electronic medical records at two Australian tertiary cancer care centers from January 2017 to January 2022. Patients with locally advanced or metastatic NSCLC without a targetable oncogenic driver (EGFR, anaplastic lymphoma kinase, and ROS1 negative) who received at least one dose of first-line anti–PD-1 with or without platinum-doublet chemotherapy (either carboplatin or cisplatin and pemetrexed for nonsquamous tumors and taxane or gemcitabine for squamous tumors). Patients who had progressed on first-line therapy were included. Radiological assessments were performed locally. Approval from the Institutional Ethics Review Board (Western Sydney Local Health District Human Research Ethics Committee; 2020/ETH02064) was obtained. A waiver of consent was obtained from the Human Research Ethics Committee for this study, permitting the research to proceed without obtaining individual consent from participants.
Data Collection
Patient demographics, tumor histopathology, systemic therapy details, tumor response, sites of progression, management of progression, and survival outcomes were collected. Details regarding the management of oligoprogression were collected including systemic therapy type, radiotherapy type, site, and dose. The equivalent dose in 2 Gy fractions (EQD2) was calculated and therapy was categorized by low dose (<45 Gy EQD2) or high dose (≥45 Gy EQD2) using an α/β ratio of 10.
Management of Progression
Decisions regarding the management of oligoprogression or systemic progression were made by the treating medical oncologist and radiation oncologist with the input of a multidisciplinary team for difficult cases. In general, if the location of oligoprogression was amenable to radiotherapy and patient performance status allowed, radiotherapy to treat disease progression was administered. In patients who had radiotherapy after systemic progression, radiotherapy was often used for symptom control.
Disease Assessment
Computed tomography (CT) of the chest, abdomen, and pelvis (3-mm slices) was obtained at baseline (before starting treatment), then every 12 to 16 weeks or more frequently, according to institutional practice. Patients either underwent an additional baseline fluorodeoxyglucose-positron emission tomography scan or whole-body bone scan to assess for bone metastases, if clinically indicated, before systemic therapy. All patients had central nervous system (CNS) imaging before the commencement of systemic therapy, either with CT or magnetic resonance imaging (MRI). In patients with known brain metastases, patients were followed up with an MRI brain, or CT if the MRI was contraindicated, every 12 to 16 weeks. All patients had repeat CNS imaging at the progression of the disease, either with CT or MRI to confirm CNS involvement at progression.
Initial progression of disease (progression-free survival-1 [PFS1]) after commencement of anti–PD-1 with or without chemotherapy was dichotomized as oligoprogression; defined as progression in ≤three sites, within one to two anatomical locations, or systemic progression; defined as progression in greater than three sites. Oligoprogression was further characterized as repeat (oligometastatic disease at original diagnosis) or induced (polymetastatic disease at original diagnosis) in keeping with the European Society for Radiotherapy & Oncology-European Society for Medical Oncology guidelines.5
Statistical Analysis
The outcomes assessed included: (1) OS and PFS2 after receipt of radiotherapy for the management of oligoprogression; (2) OS and PFS2 after receipt of radiotherapy in the overall cohort and the systemic progression cohort; (3) OS, PFS1, and PFS2 in the oligoprogression cohort compared with the systemic progression cohort and (4) to determine the clinical predictors of oligoprogression.
Descriptive analysis was used to assess baseline characteristics and management of progression. Continuous variables were summarized using medians and interquartile ranges (IQR) and categorical variables were summarized using proportions. A chi-square test was employed to compare categorical variables between two independent groups. Univariate and multivariate logistic regression was performed to identify factors predictive of oligoprogression. Factors for regression analyses included baseline clinical characteristics, such as age, sex, Eastern Cooperative Oncology Group performance status, tumor histopathology, site of metastases, the volume of disease, and oligometastatic versus polymetastatic disease and size of metastatic deposits. Multicollinearity was assessed using variance inflation factor, variables with a variance inflation factor of approximately 1 were considered independent of the others. Baseline characteristics with a p values ≤0.05 from the univariate logistic regression analysis were included in the multivariate model.
OS, PFS1, and PFS2 were calculated from the date of commencement of first-line treatment to the date of an event (either death [OS], first progression event [PFS1], or second progression event after first progression [PFS2]). Patients without a clinical event were censored at the last follow-up date. Kaplan-Meier Curves were formulated and survival differences between groups were compared using Cox proportional hazards tests. Median survival and associated 95% confidence interval (CI) were calculated. All statistical analyses were performed using R (version 4.2.3; R Foundation for Statistical Computing, Vienna, Austria) and some graphics were created using GraphPad Prism (version 10.0; GraphPad Software, Boston, MA) and BioRender.
Results
Patient Characteristics
Two hundred and two patients were treated with first-line ICI with or without chemotherapy across the two centers. One hundred and fifty-nine (78.7%) of these had progressed at the time of analysis and these patients were included in the final cohort for analysis (Supplementary Fig. 1). At baseline, before the commencement of first-line systemic therapy, the median age was 68 (IQR: 60–75) (Table 1). The Eastern Cooperative Oncology Group performance status was 0 to 1 in 137 patients (86.2%) and ≥2 in 22 patients (13.8%). Ninety-three (58.5%) had adenocarcinoma, 44 (27.7%) had squamous cell carcinoma, 18 (11.3%) had undifferentiated large cell carcinoma, two (1.3%) had adenosquamous carcinoma, and two (1.3%) had NSCLC, not otherwise specified. PD-L1 status was <1% in 36 (22.6%), 1–49% in 33 (20.8%), ≥50% in 76 (47.8%). In 14 patients (8.8%), PD-L1 was not available. Twenty-four patients (15.1%) were never smokers, and 135 (84.9%) had a history of smoking (either current or ex-smokers). Seventy-seven (48.4%) were treated with anti–PD-1 monotherapy and 82 (51.6%) were treated with platinum-doublet chemotherapy plus anti–PD-1 (chemoimmunotherapy). Ninety-two patients (57.9%) received upfront radiotherapy before systemic therapy (Supplementary Table 1).
Table 1.
Baseline Characteristics
| Characteristic | All N = 159, n (%) |
Oligoprogression n = 62, n (%) | Systemic Progression n = 97, n (%) |
|---|---|---|---|
| PFS1a | 4 mo (95% CI: 4–6) | 7 mo (95% CI: 5–11) | 3 mo (95% CI: 2–5) |
| Ageb | 68 (60–75) | 69 (62–74) | 67 (59–75) |
| Tumor type | |||
| Adenocarcinoma | 93 (58.5) | 34 (54.8) | 59 (60.8) |
| Squamous cell carcinoma | 44 (27.7) | 17 (27.4) | 27 (27.8) |
| Undifferentiated large cell carcinoma | 18 (11.3) | 10 (16.1) | 8 (8.2) |
| Adenosquamous carcinoma | 2 (1.3) | 0 (0) | 2 (2.1) |
| Other | 2 (1.3) | 1 (1.6) | 1 (1.0) |
| Treatment | |||
| Immunotherapy | 77 (48.4) | 27 (43.5) | 50 (51.5) |
| Chemoimmunotherapy | 82 (51.6) | 35 (56.5) | 47 (48.5) |
| Upfront RT | |||
| No upfront RT | 92 (57.9) | 31 (50.0) | 61 (62.9) |
| Upfront RT | 67 (42.1) | 31 (50.0) | 36 (37.1) |
| ECOG PS | |||
| 0 | 50 (31.4) | 20 (32.3) | 30 (30.9) |
| 1 | 87 (54.7) | 37 (59.7) | 50 (51.5) |
| 2 | 17 (10.7) | 5 (8.1) | 12 (12.4) |
| 3 | 5 (3.1) | 0 (0) | 5 (5.2) |
| Smoking status | |||
| Never-smoker | 24 (15.1) | 8 (12.9) | 16 (16.5) |
| Ex-smoker | 92 (57.9) | 37 (59.7) | 55 (56.7) |
| Current smoker | 43 (27.0) | 17 (27.4) | 26 (26.8) |
| PD-L1 status | |||
| <1% | 36 (22.6) | 16 (25.8) | 20 (20.6) |
| 1–49% | 33 (20.8) | 8 (12.9) | 25 (25.8) |
| ≥50% | 76 (47.8) | 37 (59.7) | 39 (40.2) |
| Not tested | 14 (8.8) | 1 (1.6) | 13 (13.4) |
| Oligometastatic at diagnosis | |||
| Oligometastatic | 30 (18.9) | 16 (25.8) | 14 (14.4) |
| Polymetastatic | 129 (81.1) | 46 (74.2) | 83 (85.6) |
| Volume of disease | |||
| <5 metastases | 30 (18.9) | 16 (25.8) | 14 (14.4) |
| 5–20 metastases | 96 (60.4) | 36 (58.1) | 60 (61.9) |
| >20 metastases | 33 (20.8) | 10 (16.1) | 23 (23.7) |
| Brain metastases | |||
| Not present | 122 (76.7) | 43 (69.4) | 79 (81.4) |
| Present | 37 (23.3) | 19 (30.6) | 18 (18.6) |
| Lung metastases | |||
| Not present | 82 (51.6) | 38 (61.3) | 44 (45.4) |
| Present | 77 (48.4) | 24 (38.7) | 53 (54.6) |
| Liver metastases | |||
| Not present | 133 (83.6) | 59 (95.2) | 74 (76.3) |
| Present | 26 (16.4) | 3 (4.8) | 23 (24.7) |
| Adrenal metastases | |||
| Not present | 130 (81.8) | 54 (87.1) | 76 (78.4) |
| Present | 29 (18.2) | 8 (12.9) | 21 (21.6) |
| Pleural effusion/metastases | |||
| Not present | 107 (67.3) | 45 (72.6) | 62 (63.9) |
| Present | 52 (32.7) | 17 (27.4) | 35 (36.1) |
| Lymph node metastases | |||
| Not present | 27 (17.0) | 13 (21.0) | 14 (14.4) |
| Present | 132 (83.0) | 49 (79.0) | 83 (85.6) |
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; IQR, interquartile range; PD-L1, programmed death-ligand 1; PFS1, Progression-free survival 1; RT, radiotherapy.
Median (95% CI).
Median (IQR).
At baseline, 30 (18.9%) had oligometastatic disease, 129 (81.1%) had polymetastatic disease, 37 (23.3%) had brain metastases, 26 (16.4%) had liver metastases, 29 (18.2%) had adrenal metastases and 52 (32.7%) pleural effusion or metastases.
While all patients were negative for EGFR, anaplastic lymphoma kinase, and ROS1, 105 patients (66.0%) underwent further next-generation sequencing for other mutations (Supplementary Table 2). Forty-two (26.4%) had additional oncogene mutations found including 30 (18.9%) with a KRAS mutation; of which nine had a KRAS G12C mutation.
Of the 37 patients with baseline brain metastases, 32 (76.2%) had local therapy before systemic therapy. Twelve underwent surgery followed by cavity stereotactic radiosurgery (SRS), 15 underwent SRS alone, three patients had whole brain radiotherapy, two underwent surgery alone and five had no upfront local therapy (Supplementary Table 3). Patients who did not receive upfront local therapy were asymptomatic, did not require steroids, and had lesions ≤1.5 cm.
PFS1
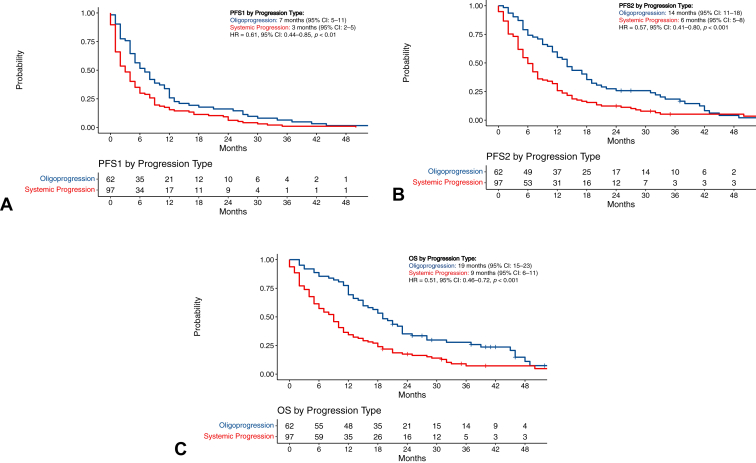
At first progression, 62 (39.0%) were classified as oligoprogression, while 97 (61.0%) experienced systemic progression. With a median follow-up of 41 months (IQR: 31–59), the median PFS1 for patients experiencing oligoprogression was 7 months (95% CI: 5–11 mo) versus 3 months (95% CI: 2–5 mo) for patients who experienced systemic progression (hazard ratio [HR] = 0.61, 95% CI: 0.44–0.85, p < 0.01; Fig. 1A).
Figure 1.
Survival outcomes by progression type. (A) PFS1 by oligoprogression versus systemic progression; (B) PFS2 by oligoprogression versus systemic progression; (C) OS by oligoprogression versus systemic progression. CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS1, progression-free survival-1; PFS2, progression-free survival-2.
Patients who were classified as oligometastatic at diagnosis had a median PFS1 of 5 months (95% CI: 3–8 mo) versus 4 months (95% CI: 4–6 mo) for patients classified as polymetastatic (HR = 0.86, 95% CI: 0.57–1.28, p = 0.5; Supplementary Fig. 2).
Characteristics and Predictors of Oligoprogressive Disease
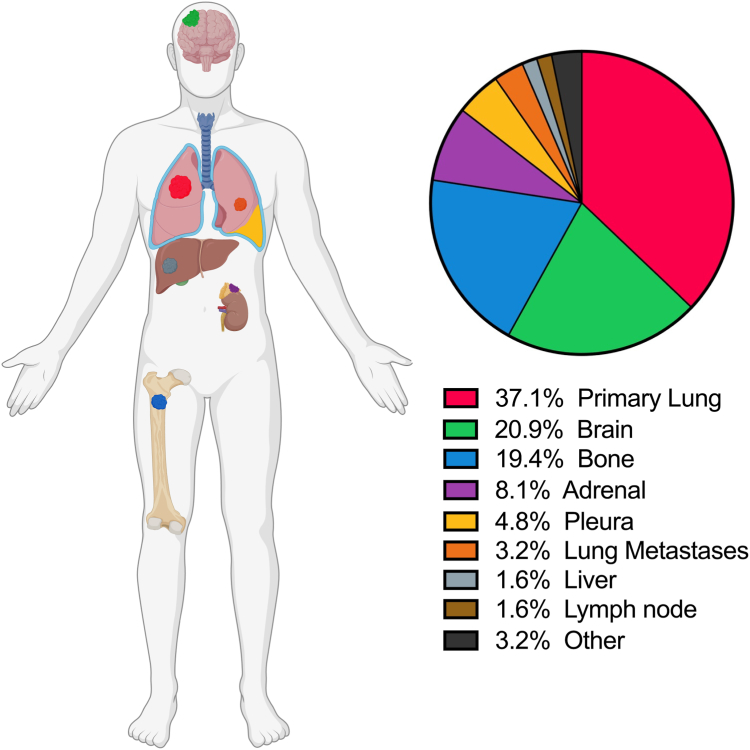
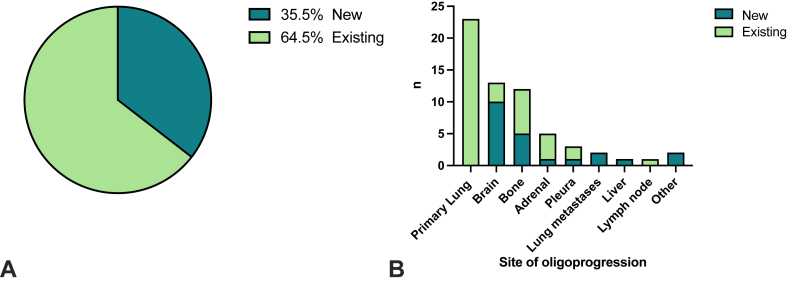
Of the 62 patients who experienced oligoprogression, 16 (25.8%) had repeat oligoprogression and 46 (74.2%) had induced oligoprogression. The most common sites of oligoprogression were the lung primary (n = 23, 37.1%), brain (n = 13, 20.9%), bone (n = 12, 19.4%), adrenal gland (n = 5, 8.1%) and other sites including lung metastases (n = 2, 3.2%), pleura (n = 3, 4.8%), liver (n = 1, 1.6%) and lymph node metastases (n = 1, 1.6%) (Fig. 2; Supplementary Table 4). Of the patients who had oligoprogression, 40 (64.5%) of these had progression in existing lesions and 22 (35.5%) had progression with the development of new lesions (Fig. 3). Patients with squamous cell carcinoma (17 of 44, 38.6%) experienced similar oligoprogression rates versus those with non-squamous disease (45 of 115, 39.1%, p = 0.9).
Figure 2.
Sites of oligoprogression.
Figure 3.
New versus existing lesions by sites of oligoprogression.
On univariate analysis, the presence of brain metastases at baseline was associated with an increased likelihood of oligoprogression at PFS1. The presence of bone and liver metastases was associated with an increased likelihood of systemic progression at PFS1. Multivariate analysis including all significant findings on univariate analysis confirmed the presence of brain metastases was associated with an increased likelihood of oligoprogression at PFS1 (OR = 2.44, 95% CI: 1.06–3.90, p = 0.04) and the presence of liver metastases was associated with increased likelihood of systemic progression at PFS1 (OR = 0.17, 95% CI: 0.04–0.54, p < 0.01) (Table 2).
Table 2.
Univariate and Multivariate Analysis Predictive of Oligoprogression at PFS1
| Characteristics | Univariatea |
Multivariatea |
|||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | VIF | |
| Age, y | 0.16 | ||||||
| <65 | 1.00 | ||||||
| ≥65 | 1.60 | 0.89–3.16 | |||||
| ECOG PS | 0.86 | ||||||
| 0-1 | 1.00 | ||||||
| ≥2 | 1.06 | 0.53–2.10 | |||||
| Sex | 0.89 | ||||||
| Female | 1.00 | ||||||
| Male | 0.95 | 0.50–1.84 | |||||
| Tumor type | 0.36 | ||||||
| Adenocarcinoma | 1.00 | ||||||
| SCC | 1.09 | 0.52–2.28 | |||||
| Large cell | 2.17 | 0.78–6.19 | |||||
| Other | 1.74 | 0.07–44.8 | |||||
| Treatment type | 0.32 | ||||||
| Immunotherapy | 1.00 | ||||||
| Chemoimmunotherapy | 1.38 | 0.73–2.63 | |||||
| Upfront radiotherapy | 0.11 | ||||||
| No | 1.00 | ||||||
| Yes | 1.69 | 0.89–3.25 | |||||
| Volume of disease | 0.16 | ||||||
| <5 metastases | 1.00 | ||||||
| 5–20 metastases | 0.53 | 0.23–1.20 | |||||
| >20 metastases | 0.38 | 0.13–1.05 | |||||
| Size | 0.08 | ||||||
| No lesions ≥5 cm | 1.00 | ||||||
| One or more lesions ≥5 cm | 1.81 | 0.93–3.53 | |||||
| Oligometastatic at diagnosis | 0.08 | ||||||
| Oligometastatic | 1.00 | ||||||
| Polymetastatic | 0.48 | 0.21–1.08 | |||||
| Adrenal metastases | 0.16 | ||||||
| Not present | 1.00 | ||||||
| Present | 0.54 | 0.21–1.26 | |||||
| Bone metastases | 0.02 | 0.08 | 1.007 | ||||
| Not present | 1.00 | ||||||
| Present | 0.46 | 0.23–0.89 | 0.53 | 0.26-1.07 | |||
| Brain metastases | 0.04 | 0.04 | 1.006 | ||||
| Not present | 1.00 | ||||||
| Present | 2.31 | 1.04–5.22 | 2.44 | 1.06-3.90 | |||
| Liver metastases | <0.001 | <0.01 | 1.008 | ||||
| Not present | 1.00 | ||||||
| Present | 0.16 | 0.04–0.50 | 0.17 | 0.04-0.54 | |||
| Lymph node metastases | 0.29 | ||||||
| Not present | 1.00 | ||||||
| Present | 0.64 | 0.27–1.48 | |||||
| Pleural metastases | 0.25 | ||||||
| Not present | 1.00 | ||||||
| Present | 0.67 | 0.33–1.33 | |||||
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; SCC, squamous cell carcinoma; VIF, variance inflation factor assessing multicollinearity.
Univariate and multivariate analysis using a logistic regression model. Numbers in bold indicate a p value ≤0.05.
Management of the First Progression Event
At the first progression event, 45 patients (28.3%) were treated with radiotherapy alone, 22 patients (13.8%) were treated with both radiotherapy and continuation of ICI beyond progression, nine (5.7%) continued ICI beyond progression (without receipt of radiotherapy), 56 patients (35.2%) received best supportive care and 25 patients (15.7%) had a change to systemic therapy of whom eight were enrolled on a clinical trial (Table 3). No patients had surgery at progression.
Table 3.
Management of Oligoprogression and Systemic Progression
| Management | Overall N = 159, n (%) |
Oligoprogression n = 62, n (%) | Systemic Progression n = 97, n (%) |
|---|---|---|---|
| Best supportive care | 56 (35.2) | 9 (14.5) | 47 (48.5) |
| Radiotherapy alone | 45 (28.3) | 22 (35.5) | 23 (23.7) |
| Radiotherapy and ICI continued beyond progression | 22 (13.8) | 20 (32.3) | 2 (2.1) |
| ICI continued beyond progression | 9 (5.7) | 6 (9.7) | 3 (3.1) |
| Change to systemic therapy | 25 (15.7) | 3 (4.8) | 22 (22.7) |
|
16 | 1 | 15 |
|
8 | 2 | 6 |
|
1 – atezolizumab and bevacizumab |
ICI, immune checkpoint inhibitor.
Management of the first progression event was largely dependent on whether the patient experienced oligoprogression or systemic progression. Patients who experienced oligoprogression were more likely to have radiotherapy than those who had systemic progression (67.7% versus 25.8%, p < 0.001), less likely to receive best supportive care (14.5% versus 48.5%, p < 0.001) or experience a change in systemic therapy (4.8% versus 22.7%, p < 0.001).
Forty-two patients (67.7%) received radiotherapy for the management of oligoprogression, 20 of whom also continued ICI beyond progression. The most common sites for treatment with radiotherapy included the brain (11 of 42, 26.2%), bone (11 of 42, 26.2%), and the lung primary (9 of 42, 21.4%; Supplementary Table 5). Thirty patients (30 of 42, 71.4%) received conventionally fractionated radiotherapy, nine (9 of 42, 21.4%) received intracranial SRS and three (3 of 42, 7.1%) received SABR. Eleven (11 of 42, 26.2%) received a high EQD2 dose, 28 (28 of 42, 66.7%) received low EQD2 radiotherapy doses. The exact EQD2 dose was unavailable for three patients.
Nineteen patients with baseline brain metastases had oligoprogression; 12 (63.2%) of these progression events occurred in the brain, and the other seven progression events included two in the bone, four in the lung primary, and one in lung metastasis (Supplementary Table 3). Eleven of the 12 patients (91.7%) who progressed intracranially had received prior local therapy for their CNS disease and were treated with further radiotherapy at oligoprogression. The other patient with baseline brain metastases who experienced oligoprogression intracranially had not received prior local therapy, deteriorated, and thus was treated with the best supportive care. One patient without baseline brain metastases also experienced oligoprogression in the brain.
PFS2 and OS
The median PFS2 for the entire cohort was 8 months (95% CI: 7–12 mo); the median PFS2 was 14 months (95% CI: 11–18 mo) for patients who experienced oligoprogression versus 6 months (95% CI: 5–8 mo) for patients who experienced systemic progression (HR = 0.57, 95% CI: 0.41–0.80, p < 0.001; Fig. 1B). The landmark PFS2 at 12 months was 54.8% in the oligoprogression group and 25.8% in the systemic progression group.
The median OS for the entire cohort was 12 months (95% CI: 10–15); the median OS was 19 months (95% CI: 15–23) for patients who experienced oligoprogression versus 9 months (95% CI: 6–11) for patients who experienced systemic progression (HR = 0.51, 95% CI: 0.36–0.72, p < 0.001; Fig. 1C). The landmark OS at 12 months was 69.4% in the oligoprogression group and 34.4% in the systemic progression group.
PFS2 and OS After Radiotherapy
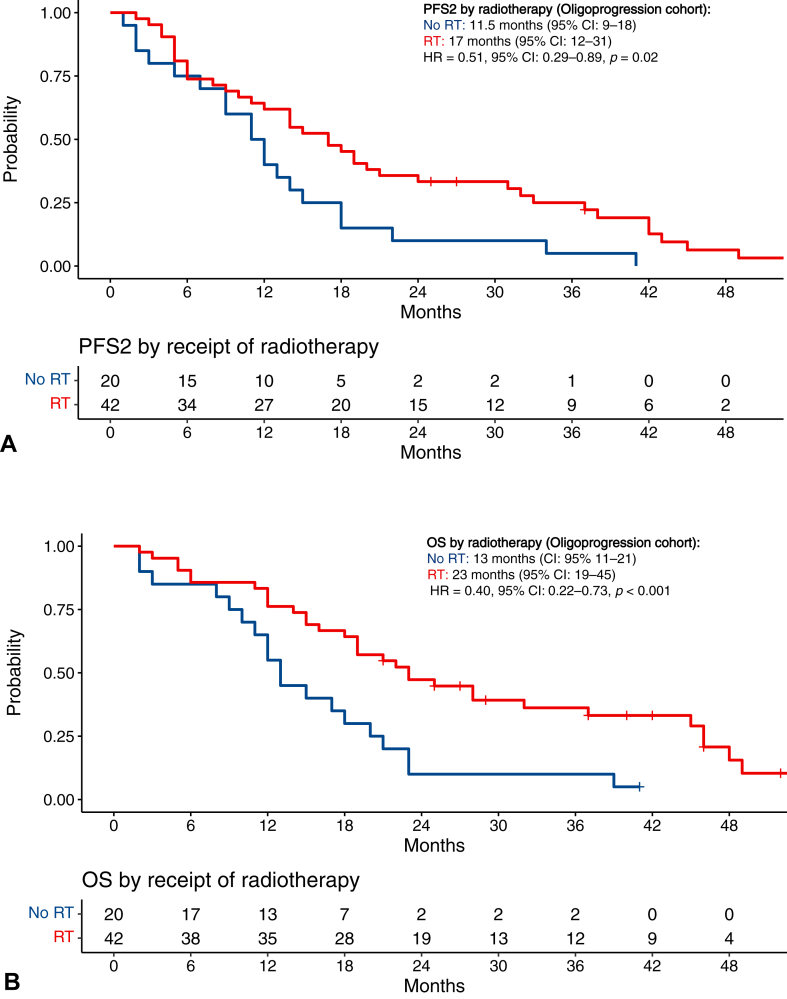
The survival outcomes for patients were compared between patients who received radiotherapy versus those who did not for the management of the progression of the disease (Supplementary Fig. 3). For patients with oligoprogression, those who received radiotherapy had a longer median PFS2 of 17 months (95% CI: 12–31) versus 11.5 months (95% CI: 9–18) (HR = 0.51, 95% CI: 0.29–0.89, p = 0.02; Fig. 4A). Those who received radiotherapy also had a longer median OS of 23 months (95% CI: 19–45) versus 13 months (95% CI: 11–21) in patients who did not receive radiotherapy (HR = 0.40, 95% CI: 0.22–0.73, p < 0.001; Fig. 4B).
Figure 4.
PFS2 and OS after radiotherapy in the oligoprogression cohort. (A) PFS2 after radiotherapy for oligoprogression; (B) OS after radiotherapy for oligoprogression. CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS2, progression-free survival-2; RT, radiotherapy.
In the 42 patients treated with radiotherapy for oligoprogression, the best response of the treated region was assessed in 37 patients. Five patients were unable to be assessed for response owing to rapid deterioration and death. Of the treated lesions, 10 (10 of 37, 27.0%) had partial response, 14 (14 of 37, 37.8%) had stable disease and 13 (13 of 37, 35.1%) experienced progressive disease (Supplementary Fig. 4A). The best response of the untreated disease regions was also assessed, three (3 of 37, 8.1%) with partial response, 15 (15 of 37, 40.5%) with stable disease, and 19 (19 of 37, 51.4%) with progressive disease (Supplementary Fig. 4B). Four (9.5%) remained stable without progression, 11 (26.2%) progressed systemically, six (14.3%) progressed in the radiotherapy-treated lesion or field, 19 (45.2%) progressed in a lesion outside of the radiotherapy field and two (4.8%) died before evaluation of progression location.
For patients with systemic progression, when comparing patients who received radiotherapy versus those who did not, there was no difference in median PFS2 or OS outcomes (Supplementary Fig. 5A and B).
Discussion
In this large multicenter study assessing progression-dependent survival outcomes of patients with metastatic NSCLC on first-line immunotherapy, we confirm the following findings: (1) oligoprogression correlates with improved survival over systemic progression; (2) in patients who have oligoprogressive disease there is a survival benefit from radiotherapy and (3) patients who have brain metastases at baseline are more likely to experience oligoprogression.
In our study, 39.0% of patients experienced oligoprogression. Patients with oligoprogression after first-line therapy had improved median PFS2 and OS compared with those who had systemic progression. The rate of oligoprogression in our cohort is similar to a recent study by Friedes et al.13 who report oligoprogression in 39.9% of patients. Higher rates of oligoprogression, between 10–20%, were reported in a prior study of NSCLC without oncogenic drivers.4 This may be attributed to our population being limited to patients who progressed on first-line therapy, supporting the observation that oligoprogression is more common after first-line rather than later-line therapy.4 However, this remains lower than the reported rates in studies of oncogene-addicted NSCLC which are reported as high as 73%.14,15 The improved survival outcomes in the oligoprogression population compared with systemic progression, support the findings of prior studies within heterogenous NSCLC cohorts.4,9,11,13
Patients who had oligoprogression also had a longer PFS1 compared with patients who experienced systemic progression. This suggests that patients who have a prolonged benefit from ICIs are more likely to experience oligoprogression. This has been revealed in the prior retrospective study by Rheinheimer et al.4 and indicates there may be a biological basis for the development of oligoprogression that also underpins the mechanism behind immunotherapy resistance.
Patients with brain metastases were more likely to experience oligoprogression (OR = 2.44, p = 0.04). Studies of oncogene-addicted NSCLC have also revealed an increased proportion of oligoprogression in the brain versus extracranial sites.14, 15, 16 Brain metastases have a unique tumor immune microenvironment which can lead to local adaptive resistance and increased probability of limited intracranial progression.17, 18, 19 In contrast, patients with liver metastases were more likely to experience systemic progression (OR = 0.17, p < 0.01). Liver metastases harbor immunotherapy resistance in both lung cancer and melanoma studies.20,21 Studies have demonstrated the unique tumor microenvironment of liver metastases that explains local immune resistance.19,22 Immunosuppressive cytokine profiles have also been observed in patients with melanoma liver metastases.23 This may explain the predisposition of patients with liver metastases to develop progression systemically.
Patients with oligoprogression who received radiotherapy had a longer median OS and PFS2 compared with those who did not receive radiotherapy. This benefit was not seen in patients with systemic progression. In our cohort, 42 patients (67.7%) received radiotherapy for the management of oligoprogression, with 22 (35.5%) receiving radiotherapy alone and 20 (32.3%) continuing ICI post-radiotherapy. Radiotherapy can reinvigorate local immunostimulatory effects or cause direct cell death in immunotherapy-resistant clones. In the 22 patients who received radiotherapy alone, the impact of prior ICI exposure may be enduring, modifying the immune environment to potentially boost subsequent therapies like radiotherapy, and may explain why this population had improved survival outcomes compared with those who did not receive radiotherapy.
Most patients in our oligoprogression cohort received conventionally fractionated radiotherapy, rather than SABR or SRS, and were treated with lower EQD2 doses. It should be noted that most prospective studies assessing radiotherapy in oligoprogression or oligometastatic disease primarily explore the efficacy of SABR. Given the retrospective nature of our cohort, the precise rationale for treatment decisions was not always clear. However, radiotherapy doses and schedules were influenced by several factors, including patient performance status, symptoms, lesion size, location, and prior radiotherapy at the same site. This suggests that specific dosing protocols may not be critically important given the observed survival benefits in patients treated with variable radiotherapy doses for oligoprogression. However, prospective dose-finding studies in this population are warranted.
However, there are limitations to the effect of radiotherapy on other untreated lesions. In the oligoprogression group, minimal abscopal effect was observed, with only three patients (8.1%) demonstrating a partial response in disease regions not treated with radiotherapy. While preclinical murine and cell models have revealed the induction of abscopal responses when combining radiotherapy and immunotherapy,24 the results in clinical studies have been variable. The CHEERS study evaluated the value of immunostimulatory radiotherapy by combining the use of low-dose SABR (3 × 8 Gy) and ICIs for the management of solid organ tumors and failed to reveal an improvement in PFS and OS.25 In contrast, in the SABR-COMET trial, the use of SABR to all metastatic sites in combination with standard-of-care systemic therapy, with the aim of cytoreduction, revealed an improvement in OS for patients with oligometastatic disease.26,27 Recently, a pooled analysis of two phase 2 trials has revealed hypofractionated radiotherapy can reinvigorate immunotherapy responses in immunotherapy-resistant NSCLC, leading to ongoing disease control.28
While other retrospective trials have shown that management of oligoprogression in metastatic NSCLC with radiotherapy improves survival outcomes,11,13,29 the results have been variable in prospective clinical trials. The combination of SABR and ICI therapy for oligoprogression in NSCLC and melanoma patients revealed high rates of local and systemic response in a prospective observational study.30 The Phase II STOP trial assessing SABR for oligoprogression in multiple cancer types after systemic therapy did not reveal improvement in PFS or OS compared with standard of care.31 In contrast, the CURB trial revealed an improvement in median PFS after SABR for oligoprogression compared with standard of care (10.0 versus 2.2 mo, p = 0.002) for the 59 patients with NSCLC included, with no benefit observed for the breast cancer cohort.9 The OS data for the CURB trial remains immature. The differential benefit between tumor types suggest mechanistically that, the primary site contributes to the development of and treatment response after oligoprogression. Notably, this study included patients with NSCLC with actionable driver mutations and patients treated with any line of systemic therapy.9 Radiotherapy for patients with metastatic NSCLC and oligoprogression after first-line ICIs or chemoimmunotherapy is yet to be distinctly examined within trial populations.
It is important to acknowledge that the definition and classification of oligometastatic and oligoprogressive disease has only recently been established by ASTRO and ESTRO,5,6 with oligoprogression being an umbrella term under oligometastatic disease. It is not yet clear if each state has a differing pathobiology and thus whether management should be the same. Oligoprogressive disease under this umbrella definition refers to the progression of few sites after exposure to systemic therapy, the evolution of which is thought to be complex and dynamic, influenced by alterations of the tumor microenvironment from prior therapies. For patients with de-novo oligometastatic disease, ablation with high-dose therapy may help with long-term disease control.26,27 However, the oligoprogression paradigm needs to be separately addressed with the aim of treatment of resistant tumor clones, or stimulation of antitumor immunity. Therefore, in this context using immunostimulatory doses of radiotherapy may offer the opportunity to salvage control over metastatic disease and prolong the therapeutic benefits from first-line ICI therapy.
There are several limitations of our study. Firstly, the retrospective nature of the study is associated with an inherent cohort selection bias. Because of the retrospective nature of the study, the aim of radiotherapy was difficult to ascertain and decision-making paradigms were determined by the treating clinicians. Secondly, the small sample size and inclusion of patients from two centers may have limited some detection of clinically meaningful outcomes, particularly in determining the influence of the site of radiotherapy and dosing on the outcomes. Specifically, we observed that 47.8% of patients in our cohort had a PD-L1 ≥50%, which is generally higher than the reported rates which are closer to 30%.32 Thirdly, functional fluorodeoxyglucose-positron emission tomography imaging and brain MRI were not utilized in all cases for initial staging or to confirm progression. Consequently, there is a risk that small metastases at some sites may not have been detected, although this is believed to have had a minimal impact on the results within this population.
Furthermore, the classification of oligoprogression has varied in studies both retrospective and prospective, and thus comparisons between studies are difficult to perform. The definition used in our report encompasses the new consensus definitions of oligoprogression.5,6 Further study into understanding the natural history and best practice in the management of oligometastatic disease is currently being assessed in the OligoCare cohort of the ESTRO E2—RADIatE study (NCT03818503).
In conclusion, this study provides further insights into the oligoprogression paradigm in metastatic NSCLC after first-line therapy with ICIs with or without chemotherapy. Our study provides clinically meaningful data to support the survival benefits of radiotherapy in this group for the management of oligoprogression. A deeper understanding of the mechanisms behind oligoprogression after immunotherapy in NSCLC is required to understand the biological basis for oligoprogression. Further prospective studies in directed, less heterogeneous populations of patients with metastatic NSCLC treated with first-line ICIs will be fundamental to further optimize management.
CRediT Authorship Contribution Statement
Lauren Brown: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing- Original draft preparation.
Julie Ahn: Methodology, Investigation, Data Curation, Writing- Original draft preparation.
Bo Gao: Writing- reviewing and editing.
Harriet Gee: Writing- reviewing and editing.
Adnan Nagrial: Writing- reviewing and editing.
Ines Pires da Silva: Conceptualization, Methodology, Supervision, Writing- reviewing and editing.
Eric Hau: Conceptualization, Methodology, Supervision, Writing- reviewing and editing.
Disclosure
Dr. Gee received honoraria from AstraZeneca. Dr. Nagrial is an advisory board member for Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, AstraZeneca, Pfizer Merck Serono. Dr. Silva is a consultant advisor for Merck Sharp & Dohme and received honoraria from Roche, Novartis, and Bristol-Myers Squibb. Dr. Eric Hau received honoraria and research funding from AstraZeneca, and honoraria from Novartis. The remaining authors declare no conflict of interest.
Footnotes
Cite this article as: Brown LJ, Ahn J, Gao B, et al. Radiotherapy improves survival in NSCLC after oligoprogression on immunotherapy: a cohort study. JTO Clin Res Rep. 2024;5:100695.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at [https://doi.org/10.1016/j.jtocrr.2024.100695].
Supplementary Data
References
- 1.Reck M., Rodríguez-Abreu D., Robinson A.G., et al. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50% J Clin Oncol. 2021;39:2339–2349. doi: 10.1200/JCO.21.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novello S., Kowalski D.M., Luft A., et al. Pembrolizumab plus chemotherapy in squamous non–small-cell lung cancer: 5-year update of the Phase III KEYNOTE-407 study. J Clin Oncol. 2023;41:1999–2006. doi: 10.1200/JCO.22.01990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garassino M.C., Gadgeel S., Speranza G., et al. Pembrolizumab plus pemetrexed and platinum in nonsquamous non–small-cell lung cancer: 5-year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41:1992–1998. doi: 10.1200/JCO.22.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rheinheimer S., Heussel C.P., Mayer P., et al. Oligoprogressive non-small-cell lung cancer under treatment with PD-(L)1 Inhibitors. Cancers (Basel) 2020;12:1046. doi: 10.3390/cancers12041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guckenberger M., Lievens Y., Bouma A.B., et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 6.Lievens Y., Guckenberger M., Gomez D., et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network, Inc NCCN clinical practice guidelines in oncology (NCCN Guidelines®) for non-small cell lung cancer Name V.5.2023. https://www.nccn.org/ [DOI] [PubMed]
- 9.Tsai C.J., Yang J.T., Shaverdian N., et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block [CURB] oligoprogression): an open-label, randomised, controlled, phase 2 study. Lancet. 2024;403:171–182. doi: 10.1016/S0140-6736(23)01857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenfeld A.J., Rizvi H.A., Memon D., et al. Systemic and oligo-acquired resistance to PD-(L)1 blockade in lung cancer. Clin Cancer Res. 2022;28:3797–3803. doi: 10.1158/1078-0432.CCR-22-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebadi M., Ladbury C., Liu J., et al. Stereotactic body radiation therapy for oligoprogressive and oligorecurrent non–small-cell lung cancer. Clin Lung Cancer. 2023;24:651–659. doi: 10.1016/j.cllc.2023.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Mahmood U., Huynh M.A., Killoran J.H., et al. Retrospective review of outcomes after radiation therapy for oligoprogressive disease on immune checkpoint blockade. Int J Radiat Oncol Biol Phys. 2022;114:666–675. doi: 10.1016/j.ijrobp.2022.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Friedes C., Yegya-Raman N., Zhang S., et al. Patterns of failure in metastatic NSCLC treated with first line pembrolizumab and use of local therapy in patients with oligoprogression. Clin Lung Cancer. 2024;25:50–60.e6. doi: 10.1016/j.cllc.2023.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Weickhardt A.J., Scheier B., Burke J.M., et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmid S., Klingbiel D., Aeppli S., et al. Patterns of progression on osimertinib in EGFR T790M positive NSCLC: a Swiss cohort study. Lung Cancer. 2019;130:149–155. doi: 10.1016/j.lungcan.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 16.Yu H.A., Sima C.S., Huang J., et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–351. doi: 10.1097/JTO.0b013e31827e1f83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudmeier L.J., Hoang K.B., Nduom E.K., et al. Distinct phenotypic states and spatial distribution of CD8(+) T cell clonotypes in human brain metastases. Cell Reprod Med. 2022;3 doi: 10.1016/j.xcrm.2022.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Q., Abdo R., Iosef C., et al. The spatial transcriptomic landscape of non-small cell lung cancer brain metastasis. Nat Commun. 2022;13:5983. doi: 10.1038/s41467-022-33365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway J.W., Rawson R.V., Lo S., et al. Unveiling the tumor immune microenvironment of organ-specific melanoma metastatic sites. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown L.J., da Silva I.P., Moujaber T., et al. Five-year survival and clinical correlates among patients with advanced non-small cell lung cancer, melanoma and renal cell carcinoma treated with immune check-point inhibitors in Australian tertiary oncology centres. Cancer Med. 2023;12:6788–6801. doi: 10.1002/cam4.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pires da Silva I., Lo S., Quek C., et al. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti–PD-1 therapy. Cancer. 2020;126:86–97. doi: 10.1002/cncr.32522. [DOI] [PubMed] [Google Scholar]
- 22.Tumeh P.C., Hellmann M.D., Hamid O., et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017;5:417–424. doi: 10.1158/2326-6066.CIR-16-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva I., Tasker A., Quek C., et al. Liver metastases (mets) induce systemic immunosuppression and immunotherapy resistance in metastatic melanoma. Cancer Res. 2019;79(suppl 13) 975–975. [Google Scholar]
- 24.Vanpouille-Box C., Alard A., Aryankalayil M.J., et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8 doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaas M., Sundahl N., Kruse V., et al. Checkpoint inhibitors in combination with stereotactic body radiotherapy in patients with advanced solid tumors: the CHEERS phase 2 randomized clinical trial. JAMA Oncol. 2023;9:1205–1213. doi: 10.1001/jamaoncol.2023.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palma D.A., Olson R., Harrow S., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 27.Palma D.A., Olson R., Harrow S., et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET Phase II randomized trial. J Clin Oncol. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popp I., Vaes R.D.W., Wieten L., et al. Radiotherapy to reinvigorate immunotherapy activity after acquired resistance in metastatic non-small-cell lung cancer: a pooled analysis of two institutions prospective phase II single arm trials. Radiother Oncol. 2024;190 doi: 10.1016/j.radonc.2023.110048. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z., Wei L., Li J., et al. Combing stereotactic body radiotherapy with checkpoint inhibitors after oligoprogression in advanced non-small cell lung cancer. Transl Lung Cancer Res. 2021;10:4368–4379. doi: 10.21037/tlcr-21-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chicas-Sett R., Zafra J., Rodriguez-Abreu D., et al. Combination of SABR with anti-PD-1 in oligoprogressive non-small cell lung cancer and melanoma: results of a prospective multicenter observational study. Int J Radiat Oncol Biol Phys. 2022;114:655–665. doi: 10.1016/j.ijrobp.2022.05.013. [DOI] [PubMed] [Google Scholar]
- 31.Schellenberg D., Gabos Z., Duimering A., et al. Stereotactic ablative radiotherapy for oligo-progressive cancers: results of the randomized phase II STOP trial. Int J Radiat Oncol Biol Phys. 2023;117:S58. doi: 10.1016/j.ijrobp.2024.08.031. [DOI] [PubMed] [Google Scholar]
- 32.Aggarwal C., Abreu D.R., Felip E., et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024. Ann Oncol. 2016;27:vi363. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.