Abstract
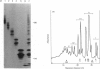
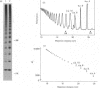
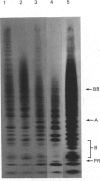
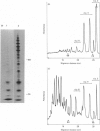
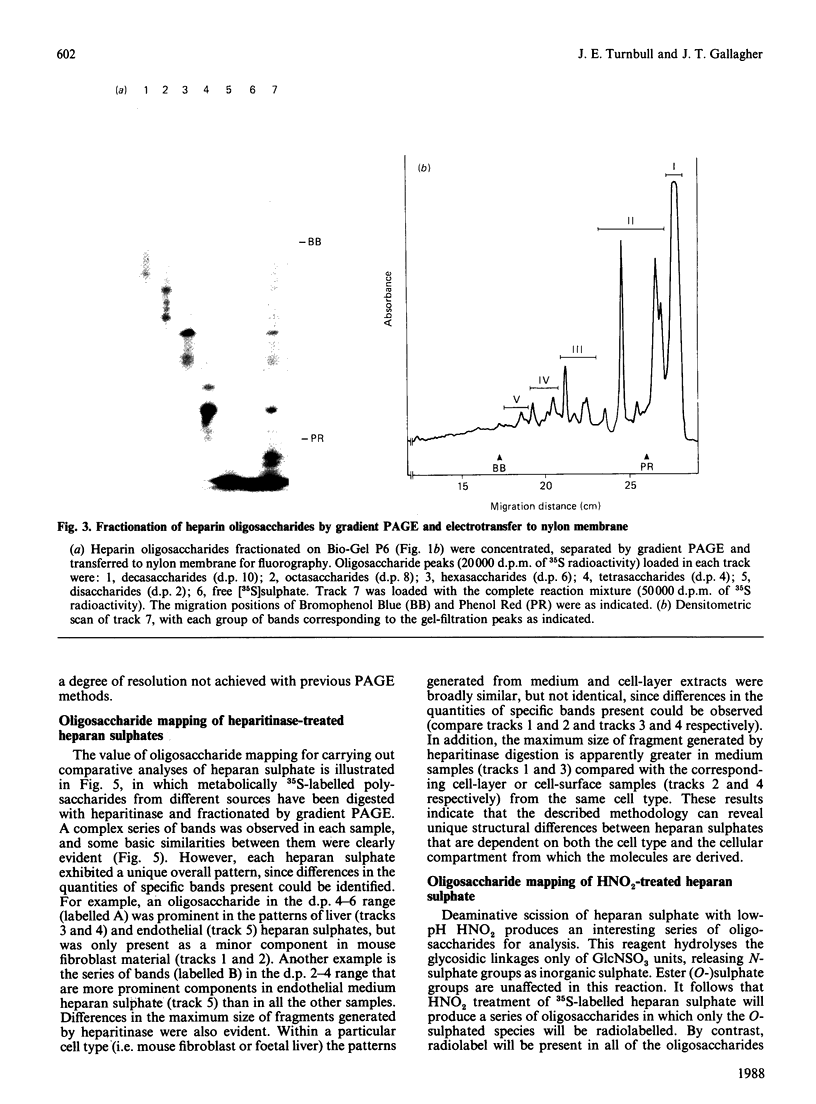
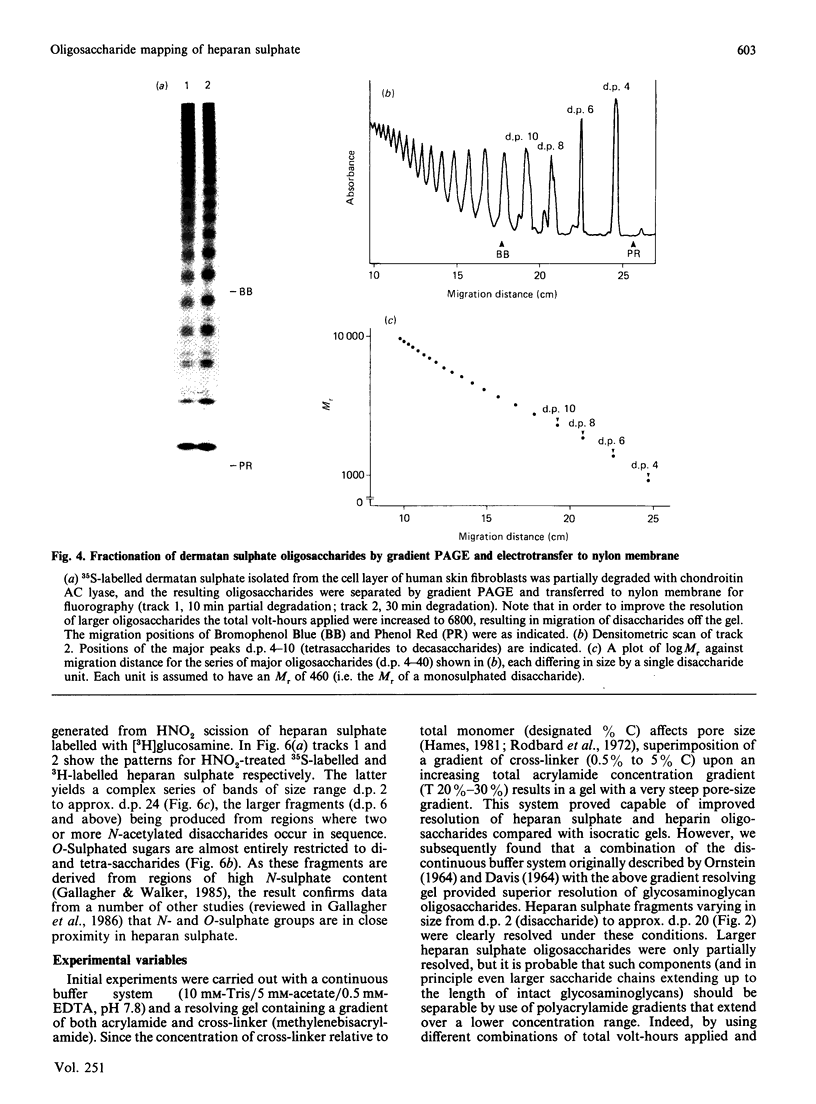
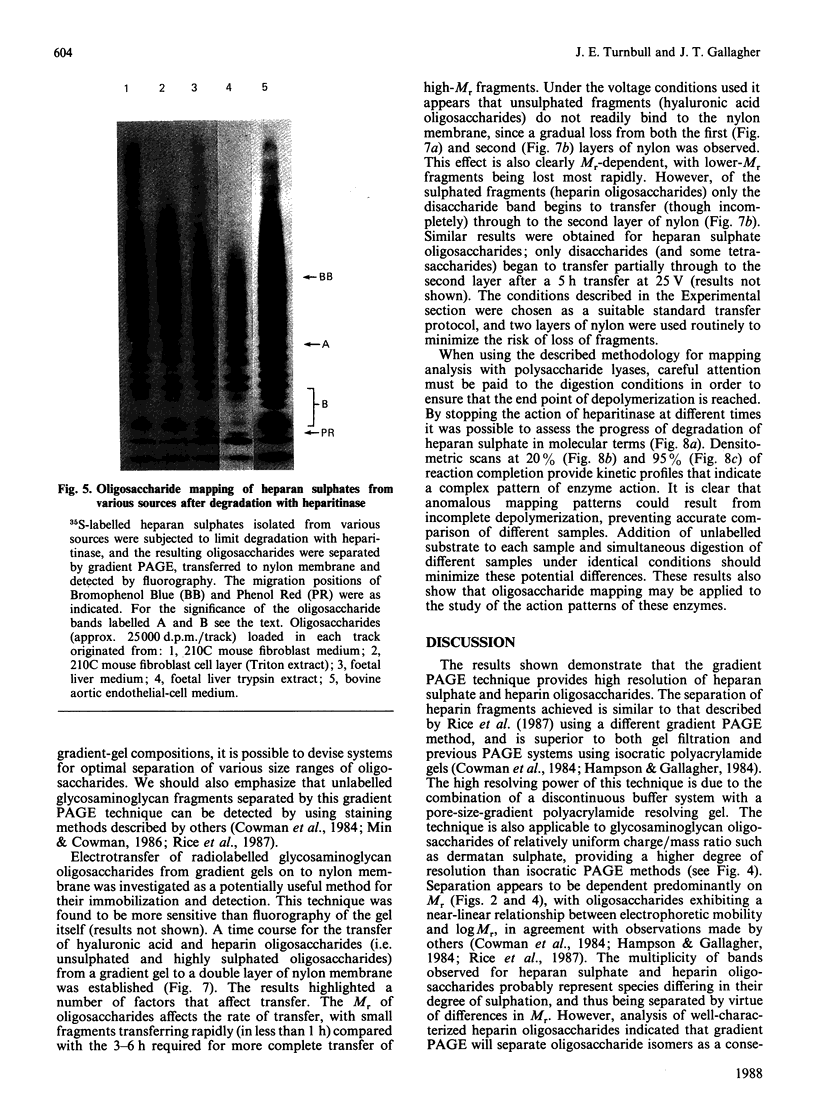
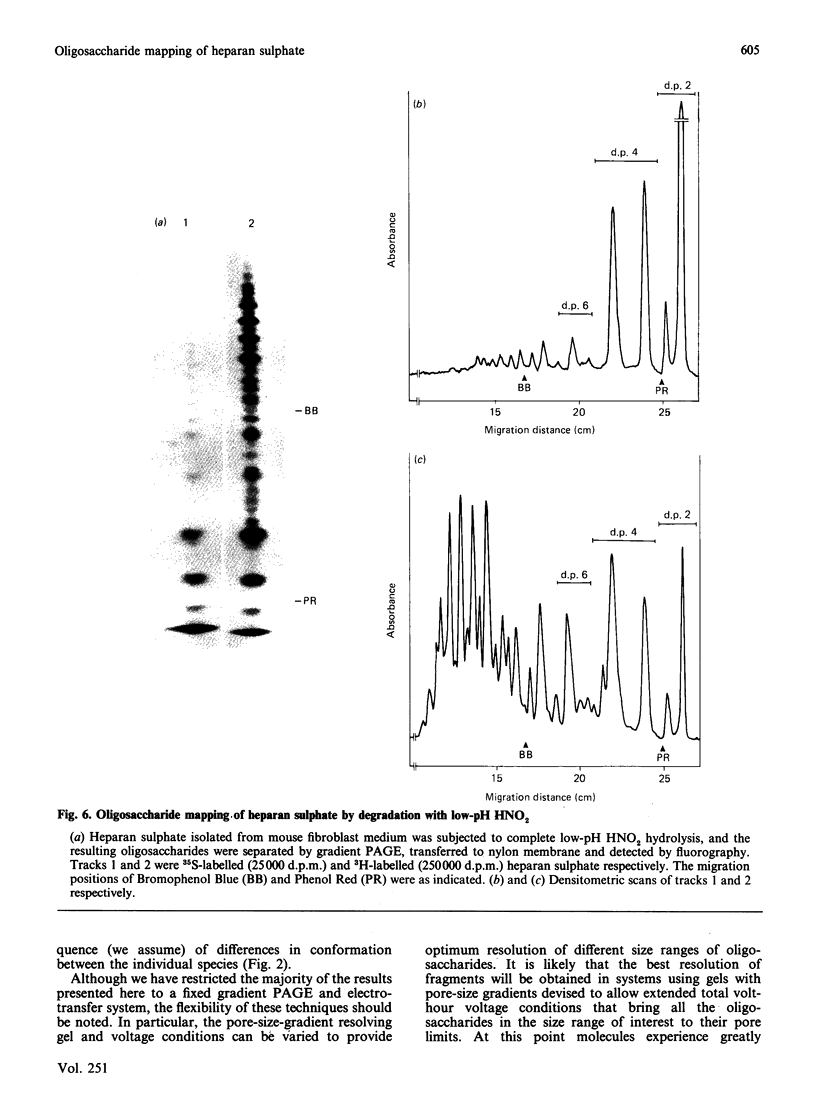
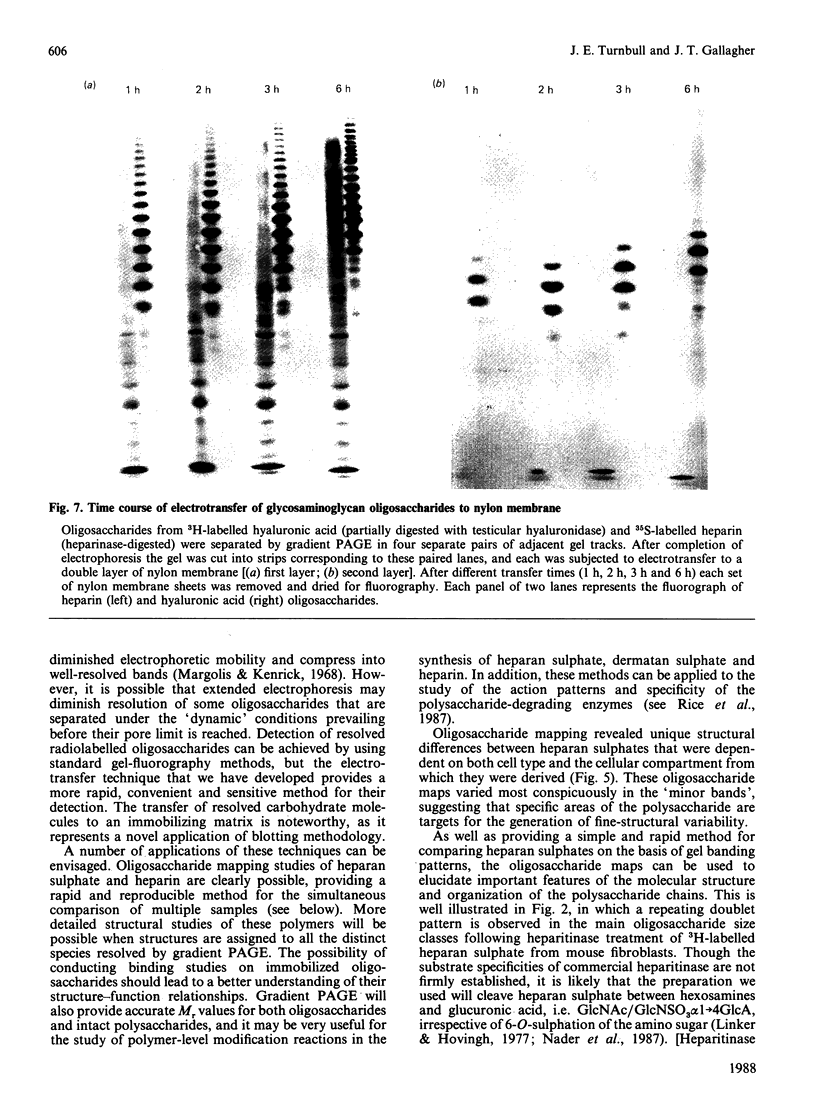
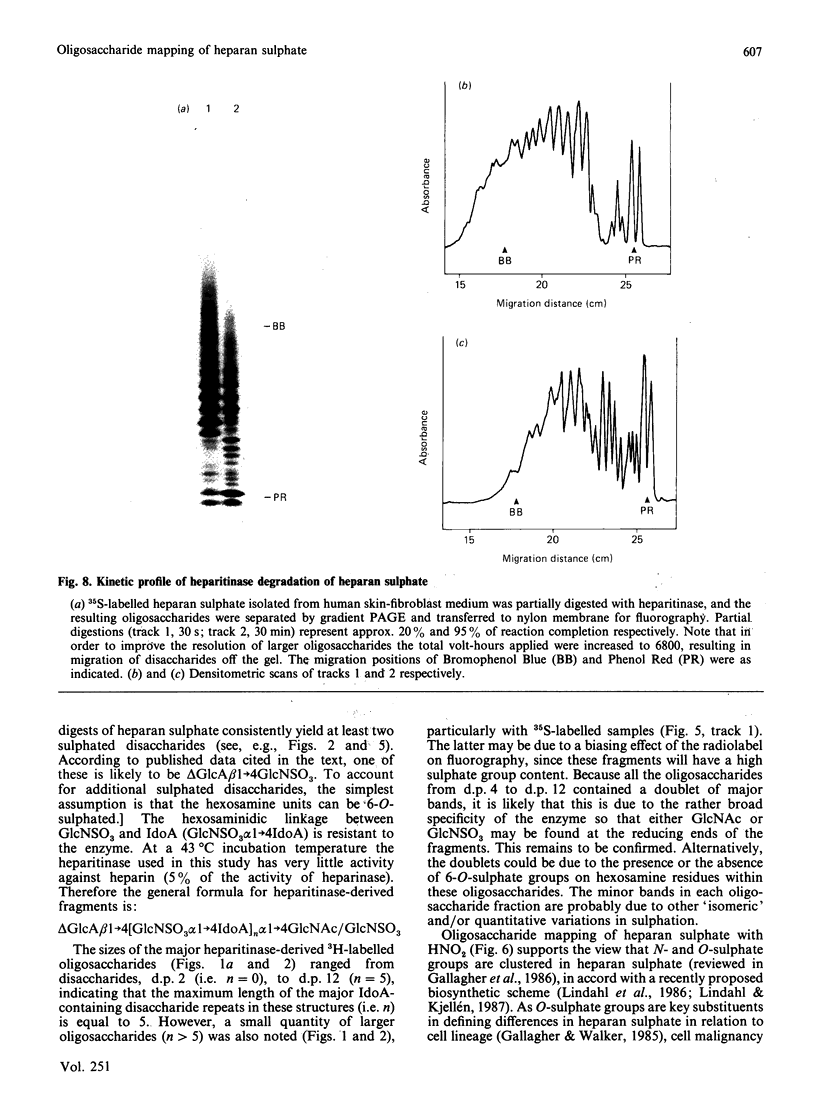
A new method that we have called 'oligosaccharide mapping' is described for the analysis of radiolabelled heparan sulphate and other glycosaminoglycans. The method involves specific enzymic or chemical scission of polysaccharide chains followed by high-resolution separation of the degradation products by polyacrylamide-gradient-gel electrophoresis. The separated oligosaccharides are immobilized on charged nylon membranes by electrotransfer and detected by fluorography. A complex pattern of discrete bands is observed covering an oligosaccharide size range from degree of polymerization (d.p.) 2 (disaccharide) to approximately d.p. 40. Separation is due principally to differences in Mr, though the method also seems to detect variations in conformation of oligosaccharide isomers. Resolution of oligosaccharides is superior to that obtained with isocratic polyacrylamide-gel-electrophoresis systems or gel chromatography, and reveals structural details that are not accessible by other methods. For example, in this paper we demonstrate a distinctive repeating doublet pattern of iduronate-rich oligosaccharides in heparitinase digests of mouse fibroblast heparan sulphate. This pattern may be a general feature of mammalian heparan sulphates. Oligosaccharide mapping should be a valuable method for the analysis of fine structure and sequence of heparan sulphate and other complex polysaccharides, and for making rapid assessments of the molecular distinctions between heparan sulphates from different sources.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cowman M. K., Slahetka M. F., Hittner D. M., Kim J., Forino M., Gadelrab G. Polyacrylamide-gel electrophoresis and Alcian Blue staining of sulphated glycosaminoglycan oligosaccharides. Biochem J. 1984 Aug 1;221(3):707–716. doi: 10.1042/bj2210707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- David G., Van Den Berghe H. Transformed mouse mammary epithelial cells synthesize undersulfated basement membrane proteoglycan. J Biol Chem. 1983 Jun 25;258(12):7338–7344. [PubMed] [Google Scholar]
- Fedarko N. S., Conrad H. E. A unique heparan sulfate in the nuclei of hepatocytes: structural changes with the growth state of the cells. J Cell Biol. 1986 Feb;102(2):587–599. doi: 10.1083/jcb.102.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson L. A., Carlstedt I., Cöster L., Malmström A. The functions of the heparan sulphate proteoglycans. Ciba Found Symp. 1986;124:125–142. doi: 10.1002/9780470513385.ch8. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Havsmark B., Chiarugi V. P. Co-polymeric glycosaminoglycans in transformed cells. Transformation-dependent changes in the co-polymeric structure of heparan sulphate. Biochem J. 1982 Jan 1;201(1):233–240. doi: 10.1042/bj2010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Hampson I. N. Proteoglycans in cellular differentiation and neoplasia. Biochem Soc Trans. 1984 Jun;12(3):541–543. doi: 10.1042/bst0120541. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J. T., Walker A. Molecular distinctions between heparan sulphate and heparin. Analysis of sulphation patterns indicates that heparan sulphate and heparin are separate families of N-sulphated polysaccharides. Biochem J. 1985 Sep 15;230(3):665–674. doi: 10.1042/bj2300665. [DOI] [PMC free article] [PubMed] [Google Scholar]
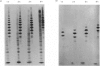
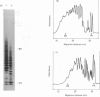
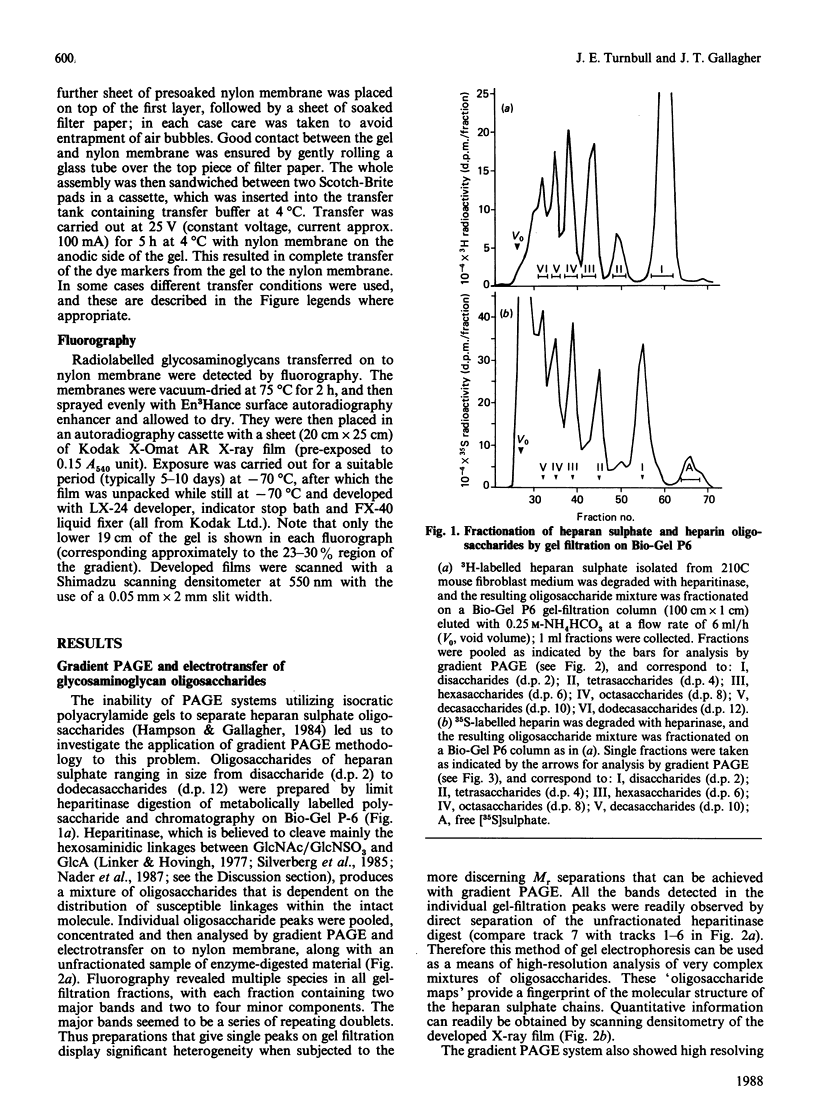
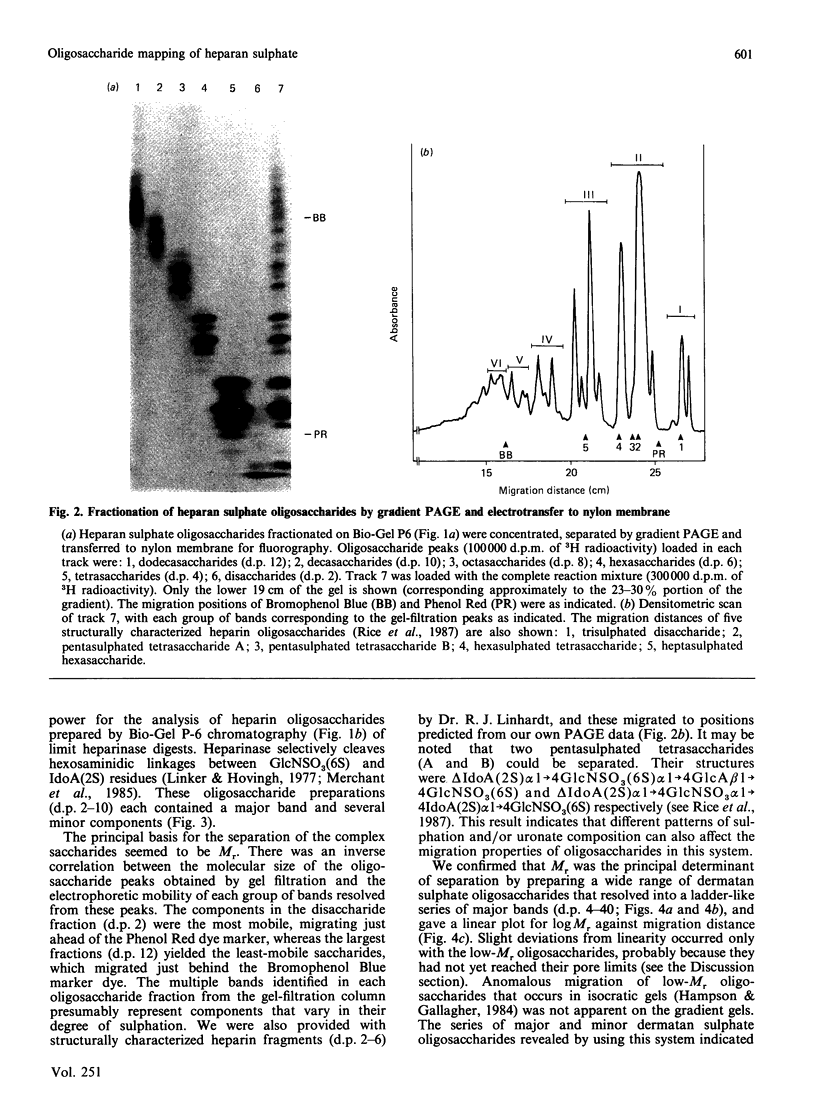
- Hampson I. N., Gallagher J. T. Separation of radiolabelled glycosaminoglycan oligosaccharides by polyacrylamide-gel electrophoresis. Biochem J. 1984 Aug 1;221(3):697–705. doi: 10.1042/bj2210697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Keller K. L., Keller J. M., Moy J. N. Heparan sulfates from Swiss mouse 3T3 and SV3T3 cells: O-sulfate difference. Biochemistry. 1980 May 27;19(11):2529–2536. doi: 10.1021/bi00552a035. [DOI] [PubMed] [Google Scholar]
- Knudson W., Gundlach M. W., Schmid T. M., Conrad H. E. Selective hydrolysis of chondroitin sulfates by hyaluronidase. Biochemistry. 1984 Jan 17;23(2):368–375. doi: 10.1021/bi00297a028. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The uses of degradative enzymes as tools for identification and structural analysis of glycosaminoglycans. Fed Proc. 1977 Jan;36(1):43–46. [PubMed] [Google Scholar]
- Marcum J. A., Rosenberg R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem Biophys Res Commun. 1985 Jan 16;126(1):365–372. doi: 10.1016/0006-291x(85)90615-1. [DOI] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Merchant Z. M., Kim Y. S., Rice K. G., Linhardt R. J. Structure of heparin-derived tetrasaccharides. Biochem J. 1985 Jul 15;229(2):369–377. doi: 10.1042/bj2290369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H., Cowman M. K. Combined alcian blue and silver staining of glycosaminoglycans in polyacrylamide gels: application to electrophoretic analysis of molecular weight distribution. Anal Biochem. 1986 Jun;155(2):275–285. doi: 10.1016/0003-2697(86)90437-9. [DOI] [PubMed] [Google Scholar]
- Nader H. B., Dietrich C. P., Buonassisi V., Colburn P. Heparin sequences in the heparan sulfate chains of an endothelial cell proteoglycan. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3565–3569. doi: 10.1073/pnas.84.11.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Pejler G., Bäckström G., Lindahl U., Paulsson M., Dziadek M., Fujiwara S., Timpl R. Structure and affinity for antithrombin of heparan sulfate chains derived from basement membrane proteoglycans. J Biol Chem. 1987 Apr 15;262(11):5036–5043. [PubMed] [Google Scholar]
- Rice K. G., Rottink M. K., Linhardt R. J. Fractionation of heparin-derived oligosaccharides by gradient polyacrylamide-gel electrophoresis. Biochem J. 1987 Jun 15;244(3):515–522. doi: 10.1042/bj2440515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Winterbourne D. J., Mora P. T. Cells selected for high tumorigenicity or transformed by simian virus 40 synthesize heparan sulfate with reduced degree of sulfation. J Biol Chem. 1981 May 10;256(9):4310–4320. [PubMed] [Google Scholar]