Abstract
Infectious entry of enveloped viruses is thought to proceed by one of two mechanisms. pH-dependent viruses enter the cells by receptor-mediated endocytosis and are inhibited by transient treatment with agents that prevent acidification of vesicles in the endocytic pathway, while pH-independent viruses are not inhibited by such agents and are thought to enter the cell by direct fusion with the plasma membrane. Nearly all retroviruses, including amphotropic murine leukemia virus (MuLV) and human immunodeficiency virus type 1, are classified as pH independent. However, ecotropic MuLV is considered to be a pH-dependent virus. We have examined the infectious entry of ecotropic and amphotropic MuLVs and found that they were equally inhibited by NH4Cl and bafilomycin A. These agents inhibited both viruses only partially over the course of the experiments. Agents that block the acidification of endocytic vesicles also arrest vesicular trafficking. Thus, partial inhibition of the MuLVs could be the result of virus inactivation during arrest in this pathway. In support of this contention, we found that that the loss of infectivity of the MuLVs during treatment of target cells with the drugs closely corresponded to the loss of activity due to spontaneous inactivation at 37°C in the same period of time. Furthermore, the drugs had no effect on the efficiency of infection under conditions in which the duration of infection was held to a very short period to minimize the effects of spontaneous inactivation. These results indicate that the infectious processes of both ecotropic and amphotropic MuLVs were arrested rather than aborted by transient treatment of the cells with the drugs. We also found that infectious viruses were efficiently internalized during treatment. This indicated that the arrest occurred in an intracellular compartment and that the infectious process of both the amphotropic and ecotropic MuLVs very likely involved endocytosis. An important aspect of this study pertains to the interpretation of experiments in which agents that block endocytic acidification inhibit infectivity. As we have found with the MuLVs, inhibition of infectivity may be secondary to the block of endocytic acidification. While this strongly suggests the involvement of an endocytic pathway, it does not necessarily indicate a requirement for an acidic compartment during the infectious process. Likewise, a lack of inhibition during transient treatment with the drugs would not preclude an endocytic pathway for viruses that are stable during the course of the treatment.
Infectious entry of enveloped viruses into target cells proceeds by specific binding of the virus to cellular receptors, followed by fusion of the viral and cellular membranes. The viral envelope protein mediates both receptor specificity and membrane fusion (26, 51). Two distinct pathways of virus entry have been reported. Fusion of the virus with the plasma membrane at extracellular pH, termed pH independent, is exemplified by human immunodeficiency virus type 1 (HIV-1) (27, 28, 45), human T-cell leukemia virus (28), the amphotropic murine leukemia virus (MuLV) 4070A (28, 35), and the feline endogenous retrovirus RD114 (28). A second pathway, termed pH dependent, proceeds by receptor-mediated endocytosis and subsequent acidification of endocytic vesicles which is believed to trigger a conformational change in the viral envelope protein that renders it fusogenic. Examples of described pH-dependent viruses are Semliki Forest virus (18), vesicular stomatitis virus (VSV) (28, 51), ecotropic MuLV (MuLV-E) (2, 28, 35), and influenza virus (51). In the case of influenza virus, a prototypical pH-dependent virus, a spring-like conformational change in the hemagglutinin envelope protein at low pH mediates fusion (8).
The most commonly used criterion for pH-dependent entry is the inhibition of viral infection by lysosomotropic weak bases (e.g., NH4Cl, chloroquine, and amantidine) or carboxylic ionophores (e.g., monensin) (1, 2, 14, 17, 18, 29, 35). Lysosomotropic weak bases become protonated within acidic vescicles and then cannot readily diffuse back out of the vesicles. Thus, the bases raise the pH within these vesicles by functioning as a proton sink (11, 30, 36). Carboxylic ionophores facilitate the exchange of protons in acidic vesicles for potassium ions in the cytoplasm, which also results in an elevation of the pH in acidic vesicles (11, 30, 36). Recently, bafilomycin A1 (BFLA1) and concanamycin A have been used to determine the requirement of acidic endosomal compartments for viral entry (16, 40). Both agents are specific and potent inhibitors of the vacuolar H+-ATPase resulting in the inhibition of endosome and lysosome acidification (7, 12). Other, more indirect criteria to distinguish pH-dependent and pH-independent pathways of entry include the ability of a low-pH pulse to induce fusion of virions bound to cells or vesicles (6, 17, 22, 26, 34), the pH sensitivity of viral envelope protein mediated cell-cell fusion (41, 52), and the ability of mild acid treatment to inactivate extracellular viral particles (28).
To date, MuLV-E and mouse mammary tumor virus are the only mammalian retroviruses classified as pH dependent. The latter is considered pH dependent on the basis of fusion of cells induced by moderately low pH (pH 5 to 5.5) (42). In the case of MuLV-E, this classification is based on a partial inhibition of infection, where approximately 20% of the infectivity remains following treatment of target cells with NH4Cl (2, 28, 35). In contrast, other pH-dependent viruses, such as VSV, exhibit nearly complete inhibition of infection upon treatment with NH4Cl (27, 28). MuLV-E is also unique among pH-dependent viruses in that host cell entry of bound particles cannot be facilitated by a low-pH pulse and cell-free virions have not been observed to be inactivated by exposure to moderately low pH (pH 5) (28, 35). Other mammalian retroviruses, including amphotropic MuLV (MuLV-A) and HIV, are classified as pH-independent viruses and are thought to infect cells by directly fusing to the plasma membrane. However, MuLV-A infectivity appears to exhibit some sensitivity to NH4Cl (ca. 15 to 20%) (28, 35), and reports of HIV sensitivity to NH4Cl range from 0% (24) to 95% (27, 28).
In this report we have reexamined the distinction between the infectious entry pathways of MuLV-E and MuLV-A. Our data indicate that the two MuLV types employ similar receptor-mediated endocytic pathways for infection. Furthermore, inhibition by agents that prevent acidification of the endocytic pathway is the result of spontaneous inactivation of the viruses and does not necessarily indicate a requirement for an acidic compartment during the infectious process.
MATERIALS AND METHODS
Viruses, cell lines, and viral vector production.
Plasmid pM-MuLV-K, which contains a complete genome of the ecotropic Moloney MuLV (M-MuLV), was obtained from A. Dusty Miller (Fred Hutchinson Cancer Research Center, Seattle, Wash.). M-MuLV was harvested from NIH 3T3 cells transfected with the plasmid. A plasmid containing the complete genome of the amphotropic MuLV 4070A (4070A 11RC) was obtained from Genetic Therapy Inc., Gaithersburg, Md. MuLV 4070A was harvested from NIH 3T3 cells transfected with this plasmid. All cell lines were grown in Dulbecco's modified Eagle's medium supplemented with 5% heat-inactivated calf serum (Gibco/BRL, Grand Island, N.Y.) and 2 mM l-glutamine (Gibco/BRL). The cell line 293T/17 was obtained from the American Type Culture Collection (CRL 11268).
MuLV-A and MuLV-E vectors were generated from PA317 (31) and PE501 (32) prepackaging cells, respectively. PA317 cells were transduced with an MuLV-E-based viral particle containing the G1nβgSVNa retroviral vector (Genetic Therapy) and cultured in the presence of G418 (0.6 mg/ml; Gibco/BRL) to select for transduced cells. This vector contains the gene encoding Eicherichia coli β-galactosidase (β-Gal) with the simian virus 40 (SV40) large T-antigen nuclear localization signal (nβ-Gal) driven by the cytomegalovirus (CMV) promoter as well as the gene conferring neomycin phosphotransferase resistance (Neo) driven by the SV40 promoter. Similarly, PE501 cells were transduced with an MuLV-A-based viral particle that contains the LAPSN retroviral vector (Clontech Laboratories, Inc., Palo Alto, Calif.) and selected with G418. This vector contains the human placental alkaline phosphatase (AP) gene driven by the M-MuLV long terminal repeat as well as the Neo gene driven by the SV40 promoter. The resultant cell lines, termed PA317/nβ-gal and PE501/AP, release amphotropic particles containing G1nBgSVNa and ecotropic particles containing LAPSN, respectively. MuLV-A and MuLV-E vectors were also generated by infection of NIH-3T3 cells stably transduced with either the G1nβgSVNa or LAPSN vector. The cells were infected with ecotropic M-MuLV or amphotropic MuLV 4070A and passaged for approximately 2 weeks. The supernatants from these cells consist of virions containing the original MuLV genome as well as virions containing the vector. The cells were grown in 800-cm2 roller bottles (Corning, Inc., Corning, N.Y.), and supernatants were collected in 30 ml of medium at 12-h intervals. Supermatants were then filtered through 0.45-μm-pore-size cellulose acetate syringe filters (Millipore) and frozen immediately at −80°C. No significant differences in our results were obtained with virions generated by the prepackaging cell lines compared to those generated by infection of cells harboring the retroviral vectors. Thus, data obtained with virions generated from either source were combined in our analyses.
A transient three-plasmid expression system was used to generate MuLV-based vectors pseudotyped with the envelope (G) protein of VSV (VSV-G) (44). 293T/17 cells were transfected with plasmids pHIT60, pHIT112 (obtained from A. Kingsman, University of Oxford), and pVSV-G. Plasmid pHIT60 expresses the M-MuLV Gag and Pol proteins from the CMV promoter and possesses an SV40 origin of replication. Plasmid pHIT112 contains a retroviral vector carrying the nβ-Gal gene driven by the CMV promoter and also possesses the SV40 origin of replication. Plasmid pVSV-G contains the gene encoding VSV-G driven by the CMV promoter. Virions were harvested from 293T/17 cells 72 h after transfection with the three plasmids.
Vector assays.
Target cells for all assays were NIH 3T3 cells. Cells were seeded onto 60mm-diameter dishes (Corning) at 1.2 × 105 cells per dish 18 to 20 h before the addition of viral supernatants. Three to six replica dishes were infected for each determination in all experiments. After infections under various conditions described below, the cells were grown to confluence (approximately 5 days) and developed for detection of foci of transduced cells. The substrates 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; Denville Scientific) and nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate (NBT-BCIP; Boehringer Mannheim) were used to stain for nβ-Gal and AP foci, respectively. For X-Gal staining, cells monolayers were washed with phosphate-buffered saline (PBS) containing calcium and magnesium (PBS plus Ca2+-Mg2+; Irvine Scientific, Irvine, Calif.) and then fixed in dishes for approximately 10 min with 0.5% glutaraldehyde (Sigma). Cells were then washed with PBS without Ca2+-Mg2+ and 2 ml of X-Gal solution (5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl2, and 1 mg of X-Gal/ml in PBS without Ca2+-Mg2+) was added to each dish. Cells were incubated at 37°C for approximately 24 h for development of blue foci. AP staining, done by the method of Fields-Berry et al. (14), was the same as nβ-Gal staining up to and including the glutaraldehyde fixation. Following fixing, cells were washed with PBS plus Ca2+-Mg2+ and incubated in an oven at 60°C for 10 min in order to reduce background staining from NIH 3T3 cells. Cells were then rehydrated for 10 min at room temperature with AP buffer (100 mM Tris-HCl[pH 9.5], 100 mM NaCl, 5 mM MgCl2), incubated with 1 to 2 ml of a 1/50 dilution of NBT-BCIP stock solution (Roche Molecular Biochemicals catalog no. 1 681 451) in AP buffer, and incubated at room temperature in the dark for 2 to 24 h for the development of purple foci. For the development of foci in mixed virus assays in which staining for both nβ-Gal and AP foci was required, the cells were first treated and developed for nβ-Gal as described above. After development of the blue nβ-Gal foci, the dishes were rinsed with PBS plus Ca2+-Mg2+ and incubated in an oven at 60°C for 10 min. Thereafter, the cells were treated as described above for the development of purple AP foci. Both AP and nβ-Gal foci were counted on a Nikon Eclipse E800 microscope using a Nikon 4× objective under bright-field lighting.
Assays with lysosomotropic agents.
Cells were incubated for 30 min in 1 ml of medium containing 0.05 μM BFLA1 or 50 mM NH4Cl at 37°C in 5% CO2. The addition of the agents to the medium resulted in minimal elevation of the pH (<0.1 units). The medium on each dish was then replaced with a 1-ml aliquot of the viral vector stock mixture containing 8 μg/ml of Polybrene/ml 0.05 μM BFLA1, or 50 mM NH4Cl and incubated at 37°C in 5% CO2 for the specified duration. Following the infection period, the solution on each dish was replaced with 1 ml of medium containing 0.05 μM BFLA1 or 50 mM NH4Cl and further incubated at 37°C in 5% CO2 until the total duration of treatment with NH4Cl reached 4.5 h. The cells were then rinsed with 3 ml of fresh medium, the medium was aspirated, 5 ml of fresh medium was added to each dish, and the cells were incubated at 37°C in 5% CO2 until they were confluent. In control assays, an equivalent volume of solvent (H2O for NH4Cl or 0.1% dimethyl sulfoxide for BFLA1) was added to the dishes in place of the lysosomotropic agent.
Inactivation of cell surface virions and virus entry assays.
For the entry experiments, cells were incubated with the vector mixtures for 2 h at 37°C in 5% CO2 in the presence or absence of the lysosomotropic agent. The cells were rinsed with 3 ml of ice-cold PBS plus Ca2+-Mg2+ and then incubated with 3 ml of ice-cold citric acid buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl [pH 3.0]) (48) for 30 s on ice. After aspiration of the citric acid buffer, all dishes were rinsed with 3 ml of fresh medium, the medium was aspirated, and 5 ml of fresh medium was added to each dish. Cells were incubated at 37°C in 5% CO2 until confluent, at which time dishes were stained for nβ-Gal- and AP-positive foci and scored.
Viral vector stability assay.
Mixtures of vector stock solutions were diluted in in medium containing Polybrene (8 μg/ml) and NH4Cl or BFLA1 at concentrations equal to those used in the titer assays. The vector mixtures were incubated in a 37°C water bath, and at various times medium on replicate dishes of cells was replaced with 1 ml of the viral vector mixtures. Infection of target cells was allowed to take place for 5 min at 37°C in 5% CO2. Cells were rinsed with 3 ml of fresh medium, the medium was aspirated, and 5 ml of fresh medium was added. Cells were incubated at 37°C in 5% CO2 until confluent, at which time dishes were stained for nβ-Gal- and AP-positive foci and scored.
RESULTS
Infection by MuLV-E is partially inhibited by treatment of target cells with BFLA1 or NH4Cl.
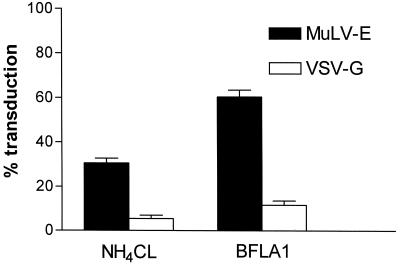
The variability of retroviral assays between different experiments is difficult to control and may be affected by differences in growth of the target cells, in cell culture media, or in the retrovirus stocks. To more carefully compare the activities of the viruses, we developed a mixed infection procedure utilizing virions that have packaged vectors containing genes encoding either β-Gal (G1nβgSVNA) or AP (LAPSN). The foci generated by the expression of these vectors are easily distinguishable and allow the assessment of infectivity of different viruses simultaneously in the same infection. In our initial experiments, we observed that transduction mediated by vectors containing VSV-G was nearly completely inhibited after treatment of cells with NH4Cl or BFLA1 (Fig. 1). In contrast, the effect of these treatments on transduction mediated by the MuLV-E SU (surface) protein was less dramatic, with a reduction in titer of 40 to 70% (Fig. 1). These results are in close agreement with previous studies that have assessed the inhibition of VSV or MuLV-E by lysosomotropic agents (2, 27, 28, 35). Experiments with BFLA1 were complicated by a toxic effect of the drug that inhibits cell growth (25, 37). NIH 3T3 cell cultures treated with BFLA1 exhibited an initial lag in growth compared to untreated cells. Moreover, infection of the cells up to 4 h after removal of the drug resulted in titers ca. 25% lower than those on untreated cells (data not shown). The data presented for BFLA1 inhibition (Fig. 1 to 3) have been normalized to reflect this observation. The inhibitory effect of BFLA1 on viral infectivity was less pronounced than that of NH4Cl in all of our experiments comparing the agents.
FIG. 1.
Partial inhibition of MuLV-E transduction by NH4Cl or BFLA1. Cells were incubated for 30 min in the presence of NH4Cl or BFLA1 and subsequently infected with a mixture of LAPSN(MuLV-E) and G1nβgSvNa(VSV-G) in medium containing either drug for a period of 2 h. The drug-virus mixture was then replaced by medium containing the drug and incubated for an additional 2 h. The medium containing the drug was then replaced by fresh medium; the culture was allowed to grow to confluence and assayed for transduction by the retroviral vectors as described in Materials and Methods. Percentages of transduction were calculated from the mean values of parallel assays performed in the absence of the drugs. Data for cells treated with NH4Cl represent the means and SEM of 12 determinations in two separate experiments; data for cells treated by BFLA1 represent the means and SEM of 10 determinations in two separate experiments.
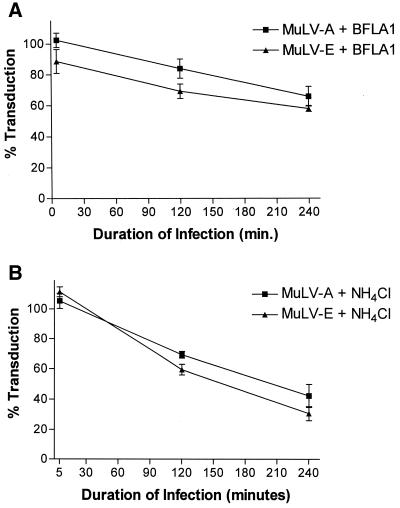
FIG. 3.
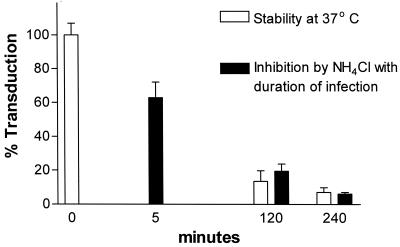
Duration of infection and inhibition of MuLV-E and MuLV-A by BFLA1 (A) or NH4Cl (B). Cells were preincubated with medium containing the drugs and infected with a mixture of LAPSN(MuLV-E) and GlnβgSvNa(MuLV-A) in the presence of the drugs after different times of preincubation. All cultures were exposed to the drugs for a period of 4.5 h. Preincubation times were 30 min, 2.5 h, and 4 h 25 min such that the duration of infection were 4 h, 2 h, and 5 min, respectively, before removal of the drug. Percentages of transduction were calculated from the mean values of parallel assays performed in the absence of the drugs. Each data point for cells treated with NH4Cl represents the mean and SEM of 13 to 22 determination in three to four separate experiments; each data point for cells treated with BFLA1 represents the mean and SEM of 11 to 22 determination in three to four separate experiments.
The infectivities of MuLV-E and MuLV-A are correspondingly reduced on cells treated with NH4Cl or BFLA1.
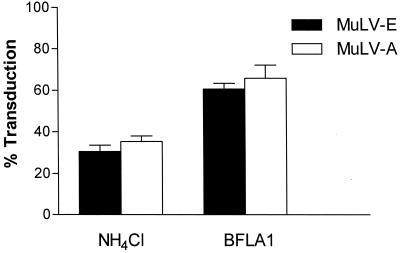
Previous studies have concluded that the mechanisms of infectious entry by MuLV-E and MuLV-A differ, with MuLV-E entering the cell through an endocytic route and MuLV-A entering by direct fusion with the plasma membrane. These conclusions were based on studies that assessed the effect of NH4Cl treatment on each virus in separate experiments. MuLV-E and MuLV-A were reported to be inhibited by 80 to 95% and by only 5 to 20%, respectively (2, 28, 35). In the present study, in which both viruses were measured simultaneously, treatment of the target cells with NH4Cl or BFLA1 resulted in substantial decreases in the infectivity of both MuLV types (Fig. 2). We found no statistically significant quantitative difference between the inhibition of ecotropic and amphotropic MuLVs by the agents. The effect of NH4Cl on the infectivity of the viruses in different experiments ranged from 55 to 85% inhibition. However, the differences between ecotropic and amphotropic MuLVs within each experiment were quite low (0.8 to 10.4%).
FIG. 2.
Inhibition of MuLV-E and MuLV-A by NH4Cl or BFLA1. Cells were treated with the drugs and infected with a mixture of LAPSN(MuLV-E) and G1nβgSvNa(MuLV-A) in the presence of the drugs as described in the legend to Fig. 1. Data for cells treated with NH4Cl represent the means and SEM of 21 determinations in four separate experiments. Percentages of transduction were calculated from the mean values of parallel assays performed in the absence of the drugs. Data for cells treated with BFLA1 represent the means and SEM of 22 determinations in four separate experiments.
Inhibition of MuLV-A and MuLV-E by lysosomotropic agents is inversely proportional to the duration of the infection and parallels the stability of the viruses.
Studies of the effects of lysosomotropic agents on viral infectivity involve infection during a transient treatment with the drug. Typically, the cells are exposed to the agent before infection with the virus, during exposure to the virus, and for a period of time after infection. In most cases, the lysosomotropic agent is present 3 to 4 h after the initiation of infection. Given the incomplete inhibition that we observed with the MuLVs, it seemed possible that inhibition of viral infectivity by lysosomotropic drugs might be a static phenomenon rather than an abortive one and that upon removal of the drug, the infectious process of any remaining viable viruses might resume. If this were the case, the loss of infectivity would reflect the stability of the virus during the time of the assay rather than a direct effect of the inhibitory drug. Moreover, inhibition observed in the presence of the drug would be expected to lessen with a shorter duration of infection. In the experiments described above, the cells were exposed to the inhibitors for 30 min before infection and for 4 h after the initiation of infection. We also tested the effects of the agents on infections of shorter duration. To control for drug effects on the cells, the times of preincubation of the cells with NH4Cl or BFLA1 were adjusted such that all of the cells were treated with the agents for a total of 4.5 h. We found that the inhibitory effect on infection of amphotropic and ecotropic MuLVs was inversely proportional to the time of infection in the presence of NH4Cl or BFLA1 (Fig. 3). Indeed, no significant inhibition by either agent was observed when the infection period was shortened to 5 min (Fig. 3).
Our results indicated that NH4Cl and BFLA1 had little inhibitory effect during a 5-min infection time, even though total exposure of the cells to the drugs was the same as for the 2- and 4-h infections. Thus, the inhibition observed during treatment with NH4Cl or BFLA1 was ultimately the result of a loss of infectivity of the virions over the time of the assay rather than an effect of the drugs on the cells.
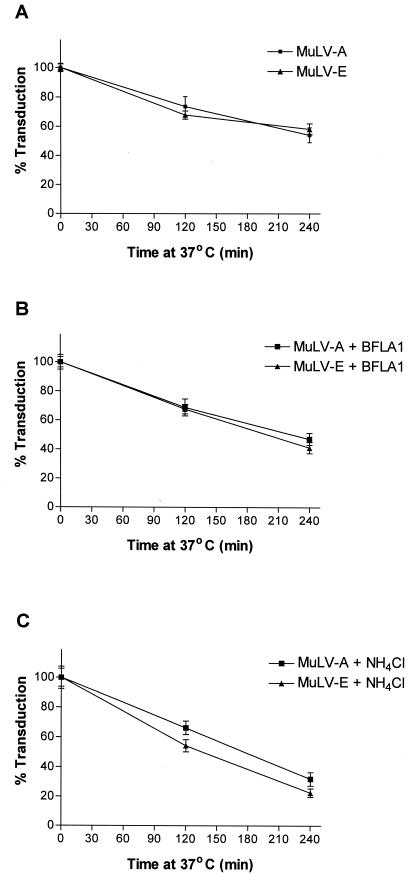
The stabilities (half-lives) of ecotropic and amphotropic MuLVs have been reported to be in the range of 2 to 8 h at 37°C (3, 4, 39), coincidentally the same range as the duration of treatment with the lysosomotropic agents in most reported experiments. We examined the spontaneous inactivation of the viruses in medium without the addition of the drugs as well as in the presence of NH4Cl or BFLA1. In medium or in medium containing BFLA1, about 45 to 60% of the infectivity remained after 4 h at 37°C (Fig. 4A and B). However, viruses incubated at 37°C in the presence of 50 mM NH4Cl retained only about 20 to 35% of their infectivity (Fig. 4C). The extents of loss of infectivity of the ecotropic and amphotropic MuLVs were similar to one another under all conditions tested. The results closely paralleled those for cells infected for various time intervals in the presence of the drugs (Fig. 3). Thus, the loss of activity due to spontaneous inactivation of ecotropic and amphotropic MuLVs may completely account for the loss of infectivity observed in the presence of lysosomotropic agents. With that consideration, the lower stability of the MuLVs in the presence of NH4Cl is quite consistent with our observation that NH4Cl inhibits infectivity to a greater extent than BFLA1 (Fig. 1 to 3).
FIG. 4.
Spontaneous inactivation of MuLV-E and MuLV-A in the presence of NH4Cl or BFLA1. A mixture of LAPSN(MuLV-E) and G1nβgSvNa(MuLV-A) was incubated in a water bath at 37°C in medium or in medium containing 0.05 μM BFLA1 or 50 mM NH4Cl. Aliquots of the virus mixture were removed at 0, 2, and 4 h and assayed for transduction activity as described in Materials and Methods. Mean transduction titers obtained after 0 h of incubation were considered to be 100%, and percent transduction after 2 and 4 h was calculated relative to the mean titers obtained at 0 h. Data for virus incubated in medium (A), medium with BFLA1 (B), and medium with NH4Cl (C) represent the means and SEM of 15 determinations at each time in four separate experiments.
It was of interest to determine if the inhibition by lysosomotropic agents on infectivity mediated by VSV-G could be accounted for by inactivation during the course of treatment. We found that inhibition of the vector mediated by VSV-G was also inversely related to the time of infection (Fig. 5). During a 4-h infection in the presence of NH4Cl, transduction by the vector was nearly completely inhibited. However, inhibition was only 80% during a 2-h infection and less than 40% during a 5-min infection. Experiments examining the spontaneous inactivation of the vector in NH4Cl indicated that 80 to 85% of its activity was lost after incubation for 2 h at 37°C and nearly all activity was gone after 4 h (Fig. 5). Similar results were obtained in the presence of BFLA1 (data not shown). Spontaneous inactivation of the VSV-G vector was also determined in medium without the addition of drugs. In each case, the assays were done as mixed infections with an MuLV-E vector as an internal control. After 2 h at 37°C, the average transduction activity remaining for the MuLV-E vector in these experiments was 80.8% ± 2.4% (standard error of the mean [SEM]) while the average transduction activity remaining for the VSV-G vector was 18.2% ± 2.1% (SEM). These results indicated that transduction mediated by VSV-G was much more labile than that mediated by the MuLV SU proteins. Thus, much of the loss of infectivity of this vector in the presence of lysosomotropic agents could be attributed to spontaneous inactivation, similar to the case for MuLVs, even though entry mediated by VSV-G likely requires an acidic compartment for entry (51, 52).
FIG. 5.
Correlation of spontaneous inactivation with the inhibition of VSV-G by NH4Cl. To determine the effect of the duration of infection on inhibition by NH4Cl, cells were preincubated in medium containing NH4Cl and infected with G1nβgSvNa(VSV-G) in the presence of NH4Cl after different times of preincubation as described for Fig. 3. The data represent the mean and SEM of 9 to 15 determinations at each time in two to four separate experiments. For thermal stability, G1nβgSvNa(VSV-G) was incubated in a water bath at 37°C in the presence of NH4Cl. Aliquots of the virus mixture were removed at 0, 2, and 4 h and assayed for transduction activity as described in Materials and Methods. Mean transduction titers obtained after 0-h incubation were considered to be 100%, and percent transduction after 2 and 4 h was calculated relative to the mean titers obtained at 0 h. Each value represents the mean and SEM of six determinations at each time in two separate experiments.
Infectious ecotropic and amphotropic MuLVs are internalized during treatment with NH4Cl.
The results presented above suggested that lysosomotropic agents inhibited the progression of the infectious process only during the course of treatment and that upon removal of the drug, the infection proceeded for viruses that had not been inactivated through spontaneous or other degradative processes. Thus, the inhibition of infectivity of both amphotropic and ecotropic MuLVs may have been the result of degradation during arrest in an endosomal pathway. Alternatively, it was conceivable that prevention of lysosomal acidification may inhibit fusion of the viruses with the plasma membrane, although it has been reported that several lysosomotropic agents do not significantly inhibit virus binding or internalization (2, 17). If fusion with the plasma membrane were inhibited, loss of viral infectivity would be the result of degradation of viruses bound to the cell surface. To distinguish between these alternatives, we examined the internalization of ecotropic and amphotropic MuLV infectivity in presence or absence of NH4Cl.
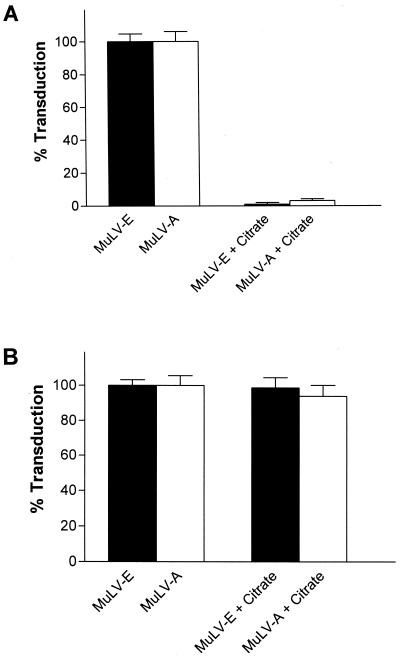
Assays to examine virus entry in the presence of the lysosomotropic agents required the specific inactivation of cell surface virions. Treatment of cells for a very short time with citrate-buffered saline at pH 3.0 has been reported to inactivate herpesviruses (48) as well as MuLV-E (21). Prior to performing the entry experiments, we tested the ability of citrate buffer to inactivate ecotropic and amphotropic virions at the cell surface. In agreement with the previous reports, we found that treatment of cells with ice-cold citrate-buffered saline at pH 3.0 for 30 s abolished 97 to 100% of the activity of ecotropic and amphotropic virions that had been previously bound to the cell surface at 4°C for 2 h (Fig. 6A). Furthermore, when the vectors were added after treatment of the cells with citrate, transduction efficiency was not significantly different from that for cells treated with PBS (Fig. 6B). Thus, citrate treatment did not have a deleterious effect on the cultures that would diminish their ability to be infected.
FIG. 6.
Inactivation of cell surface-bound MuLV-E and MuLV-A by treatment with citrate buffer (pH 3.0). (A) Cells were incubated for 2 h at 4°C with a mixture of LAPSN(MuLV-E) and G1nβgSvNa(MuLV-A); the cells washed and then mock treated or treated with citrate buffer (pH 3) as described in Materials and Methods. Each value represents the mean and SEM of 12 determinations in two separate experiments. (B) Cells were mock treated or treated with citrate buffer (pH 3) as described in the text. Immediately after treatment the cells were infected for 2 h at 37°C with a mixture of LAPSN(MuLV-E) and G1nβgSvNa(MuLV-A). Each value represents the mean and SEM of eight determinations in two separate experiments.
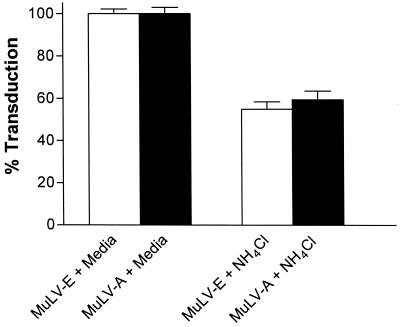
To investigate the effect of NH4Cl on virus entry, cells were infected for 2 h at 37°C in medium containing NH4Cl and immediately treated with cold citrate-buffered saline at pH 3.0 to inactivate virus on the cell surface. Viruses that were internalized during the 2-h period would be resistant to citrate treatment. From experiments described above (Fig. 3B), we expected a 30 to 40% decline in activity during infection of cells for 2 h in the presence of NH4Cl as a result of degradation during the arrest of the infectious process. However, if the infectious process were halted at the cell surface, treatment with citrate should have abolished nearly all activity. Compared to untreated cultures, we observed only about a 40% decrease in activity of both MuLV-E and MuLV-A during the 2 h infection (Fig. 7), a decrease attributable to the virus degradation expected during the infection period. Thus, the arrest of infectivity by NH4Cl was not due to inhibition of virus entry but rather occurred in an intracellular compartment. Importantly, we observed that infectivity of both MuLV-E and MuLV-A was internalized in the presence of NH4Cl, again indicating that infectious entry of both virus types was by a similar mechanism.
FIG. 7.
Effect of NH4Cl on entry of MuLV-E and MuLV-A. Cells were incubated for 30 min at 37°C in medium or medium containing NH4Cl and then infected with a mixture of LAPSN(MuLV-E) and G1nβgSvNa(MuLV-A) for 2 h in the presence or absence of the base. The cells were then treated with citrate buffer (pH 3) as described in the text. Each value represents the mean and SEM of 10 determinations in three separate experiments.
DISCUSSION
Previous studies have reported a quantitative difference between the effects of lysosomotropic agents on MuLV-E and MuLV-A infectivity. Based on these differences, it was concluded that MuLV-E is pH dependent and enters the cell through endocytosis, while MuLV-A is pH independent and enters the cell by direct fusion with the plasma membrane (2, 28, 35). In contrast to previous reports, we did not observe a significant difference between the effects of the lysosomotropic agents on the infectivity of MuLV-A and MuLV-E in these studies. The agents equally inhibited both viruses. This is attributed, we believe, to the more stringent control of variables in our assays, in which both viruses were assayed simultaneously from a mixed virus stock in the same infection. In this regard, we found a greater variability between different experiments with the same virus than between ecotropic and amphotropic MuLVs within each experiment.
In agreement with previous reports, we observed only a partial inhibition of the MuLVs compared to inhibition of the prototypic pH-dependent VSV. Although this might reflect alternative routes of entry for MuLVs other than endocytosis, it seemed plausible that partial inhibition might be the result of an arrest in the progression of an endocytic pathway of infection. Since the cells are typically treated only transiently with the lysosomotropic agents, the inhibition might simply reflect a loss in infectivity of the viruses while halted in the infectious process. Upon removal of the inhibitor, the infection would proceed for any remaining viable viruses. Two lines of published investigations are consistent with this interpretation. First, the half-life of murine retroviruses has been reported to be in the range of the duration of exposure to the drugs in most experiments involving lysosomotropic agents (3, 4, 39). Second, in addition to blocking acidification of late endosomes and lysosomes, it is well documented that lysosomotropic drugs arrest the transport of fluid-phase markers, ligands, receptors, and viruses through the endocytic pathway (9, 19, 49, 50). Moreover, the drugs are reported not to significantly affect the initial internalization from the plasma membrane. Our experiments demonstrated that the inhibitory effects of the lysosomotropic agents on MuLV-E and MuLV-A were inversely proportional to the duration of infection and paralleled the spontaneous inactivation of the viruses. These experiments provide compelling evidence that the inhibitory effect of the agents is static, arresting rather than aborting the infectious process. Furthermore, in agreement with earlier studies on endocytic internalization cited above, we demonstrated that the disappearance of the viruses from the cell surface was not inhibited during treatment with the NH4Cl. These results indicate that the infectious process was arrested in an intracellular compartment and that infection by both MuLV-A and MuLV-E very likely proceeds through endocytosis.
Our results suggest that virus internalized in the presence of NH4Cl or BFLA1 is arrested in an endocytic compartment. Recently, Mothes et al. (33) reported that MuLV-E infection of the avian DF-1 cell line expressing the ecotropic MCAT-1 receptor was not inhibited by BFLA1. This result was based on assays detecting viral DNA synthesis at various times shortly after infection. In contrast, infection by avian leukosis virus and pseudotypes of MuLV-E encapsulated in the avian leukosis virus envelope were blocked by BFLA1. This result is somewhat surprising considering studies indicating that other agents that block endosomal acidification inhibit MuLV-E infectivity in murine cells (2, 28, 35). It is possible that this system is not entirely analogous to the natural host systems studied by others and in the present work. For example, interactions of the murine ecotropic receptor with components of the avian cell that influence virion entry could differ from interactions with components of murine cells. In this regard, it has been reported that MuLV-E enters several murine cell lines by endocytosis but enters the highly transformed XC rat cell line by fusion with the plasma membrane (28). Infection of both 3T3 and XC cells was inhibited by disruption of actin filaments; however, disruption of microtubules inhibited MuLV-E infection of 3T3 cells but not of the XC cells (21). These results suggest that interactions of the virus-receptor complex with cytoskeletal components play a crucial role in virus entry and may influence the route of infection. Alternatively, MuLV-E may enter both murine and avian cells by the same mechanism. In that were the case, the results of Mothes et al. (33) suggest that a halt in the infectious process observed with BFLA1 would occur after reverse transcription. Synthesis of complete transcripts of MuLV-E appears to be limited to reverse transcription complexes in the cytoplasm that emerge subsequent to fusion of the viral and cellular membranes (13). However, coupling of the fusion process with the synthesis of complete DNA transcripts is not well understood. Thus, it is possible that DNA synthesis proceeded in MuLV-E envelope-containing virions arrested within an endosomal compartment. Last, inhibition by BFLA1 may differ mechanistically from inhibition by NH4Cl. Several effects of BFLA1 on cells and cellular processes that have not been found in cells treated with NH4Cl have been described and may be independent of endosomal acidification (10, 20, 38, 43, 46). It cannot be excluded that BFLA1 inhibits infectivity at a stage subsequent to fusion and DNA synthesis.
It is notable that the rate of infectivity loss that we observed after virus entry was similar to the loss exhibited by virus held at 37°C. This result may reflect common mechanisms of virus inactivation in both environments. Several different processes may contribute to the loss of infectivity of virions. Viral envelope functions required for infection include receptor binding and fusion with cellular membranes. Disruption of these functions must occur prior to fusion to effect infectivity. In this regard, at short incubations times, the loss of HIV-1 infectivity has been correlated with spontaneous shedding of the SU envelope proteins from virions (23, 29). Inactivation of virus stocks due to shedding of the envelope protein would result in the failure of the virions to sufficiently bind receptors and enter the cell. Inactivation of internalized virions by this process would likely be reflected in the dissociation of virions from the endosomal membrane, precluding subsequent fusion and entry into the cytoplasm. Our results with the VSV-G vector are likely the result of envelope protein shedding or inactivation. The VSV-G vector differs from the MuLV SU vectors only in the identity of the envelope protein, yet the infectivity was much more labile at 37°C. This might reflect a stronger association of the native MuLV SU proteins with the MuLV core compared to the heterologous VSV-G. The diminished effect of NH4Cl at shorter times of infection with the VSV-G vector suggests that the rapid loss of infectivity also occurred while arrested in an endosomal pathway, perhaps by dissociation of the virion from the endosomal membrane. It is less clear if the loss of infectivity of the MuLV SU vectors reflected disruption of viral envelope-associated functions or disruption of viral core functions. In contrast to disruption of envelope functions, deterioration of viral core functions, such as a loss of polymerase or integrase activity, could occur at any time during the infectious process.
An important aspect of this study pertains to what is actually being measured in infectivity experiments using lysomotropic agents. These agents have been routinely used to determine whether viruses enter target cells through an endocytic pathway or directly through the plasma membrane (2, 24, 27, 28, 35). An inhibitory effect of the agents on viral titer has been inferred to indicate that such viruses enter by endocytosis and require an acidic compartment during viral entry. Our results indicate that inhibition of the MuLVs by lysosomotropic agents reflects the stability of viral particles during the course of the experiments and does not address the necessity for an acidic environment. In this regard, it has recently been suggested that inhibition of human rhinovirus serotype 2 by BFLA1 may also be partially the result of trapping in early endosomes rather than a direct result of a block in endosomal acidification (5). This may also be the case for other enveloped viruses that are currently considered to be pH dependent. In this regard, much of the inhibition of VSV-G vector infectivity observed after treatment by these drugs could be accounted for by spontaneous inactivation irrespective of a requirement for an acidic compartment during viral entry. Although the degree of inhibition of the VSV-G vector by NH4Cl is in close agreement with published data on the inhibition of the native VSV (28, 51), it is not known if the spontaneous inactivation of the vector comprised of heterologous components used in the present study parallels that of the native VSV.
The lack of inhibition of viral infectivity by lysosomotropic agents has been interpreted as evidence for infectious entry by direct fusion with the plasma membrane. Our results predict that viruses whose infectious cycle involves endocytosis, but exhibit a relatively high degree of stability, would be minimally inhibited by lysosomotropic agents. A case in point may be the infectious entry of HIV-1. Most studies that have examined the effects of lysosomotropic agents on HIV-1 infection have reported minimal inhibition (27, 28, 45). In comparison to MuLVs, HIV-1 appears relatively stable at 37°C with reports of half-lives ranging from 24 to 30 h (23, 47). If the infectious entry of HIV-1 were by an endosomal route, this would not be reflected in standard assays with lysosomotropic agents, considering that its half-life is 6- to 10-fold longer than the duration of the treatment with the agents.
ACKNOWLEDGMENTS
We thank F. Malik and M. Taylor for technical assistance. We are grateful to J. Portis, K. Peterson, and S. Priola for helpful discussions.
L. Katen, M. Januszeski, and W. F. Anderson were supported by USC/GTI/Novartis.
REFERENCES
- 1.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen K B, Nexo B A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983;125:85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- 3.Andreadis S, Palsson B O. Coupled effects of polybrene and calf serum on the efficiency of retroviral transduction and the stability of retroviral vectors. Hum Gene Ther. 1997;8:285–291. doi: 10.1089/hum.1997.8.3-285. [DOI] [PubMed] [Google Scholar]
- 4.Andreadis S T, Brott D, Fuller A O, Palsson B O. Moloney murine leukemia virus-derived retroviral vectors decay intracellularly with a half-life in the range of 5.5 to 7.5 hours. J Virol. 1997;71:7541–7548. doi: 10.1128/jvi.71.10.7541-7548.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayner N, Schober D, Prchla E, Murphy R F, Blaas D, Fuchs R. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implication for viral uncoating and infection. J Virol. 1998;72:9645–9655. doi: 10.1128/jvi.72.12.9645-9655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenthal R, Schoch C, Puri A, Clague M J. A dissection of steps leading to viral envelope protein-mediated membrane fusion. Ann N Y Acad Sci. 1991;635:285–296. doi: 10.1111/j.1749-6632.1991.tb36499.x. [DOI] [PubMed] [Google Scholar]
- 7.Bowman E J, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carr C M, Kim P S. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- 9.Clague M J, Urbe S, Aniento F, Gruenberg J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- 10.D'Arrigo A, Bucci C, Toh B H, Stenmark H. Microtubules are involved in bafilomycin A1-induced tubulation and Rab5-dependent vacuolation of early endosomes. Eur J Cell Biol. 1997;72:95–103. [PubMed] [Google Scholar]
- 11.De Duve C, De Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F. Lysosomotropic agents. Biochem Pharmacol. 1974;23:2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 12.Drose S, Bindseil K U, Bowman E J, Siebers A, Zeeck A, Altendorf K. Inhibitory effect of modified bafilomycins and concanamycins on P- and V-type adenosine triphosphatases. Biochemistry. 1993;32:3902–3906. doi: 10.1021/bi00066a008. [DOI] [PubMed] [Google Scholar]
- 13.Fassati A, Goff S P. Characterization of intracellular reverse transcription complexes of Moloney murine leukemia virus. J Virol. 1999;73:8919–8925. doi: 10.1128/jvi.73.11.8919-8925.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields-Berry S C, Halliday A L, Cepko C L. A recombinant retrovirus encoding alkaline phosphatase confirms clonal boundary assignment in lineage analysis of murine retina. Proc Natl Acad Sci USA. 1992;89:693–697. doi: 10.1073/pnas.89.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuhara N, Yoshie O, Kitaoka S, Konno T, Ishida N. Evidence for endocytosis-independent infection by human rotavirus. Arch Virol. 1987;97:93–99. doi: 10.1007/BF01310737. [DOI] [PubMed] [Google Scholar]
- 16.Guinea R, Carrasco L. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J Virol. 1995;69:2306–2312. doi: 10.1128/jvi.69.4.2306-2312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helenius A, Kartenbeck J, Simons K, Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980;84:404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helenius A, Marsh M, White J. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J Gen Virol. 1982;58:47–61. doi: 10.1099/0022-1317-58-1-47. [DOI] [PubMed] [Google Scholar]
- 19.Johnson L S, Dunn K W, Pytowski B, McGraw T E. Endosome acidification and receptor trafficking: bafilomycin A1 slows receptor externalization by a mechanism involving the receptor's internalization motif. Mol Biol Cell. 1993;4:1251–1266. doi: 10.1091/mbc.4.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinoshita K, Waritani T, Noto M, Takizawa K, Minemoto Y, Nishikawa A, Ohkuma S, Nishikawa Y. Bafilomycin A1 induces apoptosis in PC12 cells independently of intracellular pH. FEBS Lett. 1996;398:61–66. doi: 10.1016/s0014-5793(96)01182-9. [DOI] [PubMed] [Google Scholar]
- 21.Kizhatil K, Albritton L M. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J Virol. 1997;71:7145–7156. doi: 10.1128/jvi.71.10.7145-7156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kooi C, Cervin M, Anderson R. Differentiation of acid-pH-dependent and -nondependent entry pathways for mouse hepatitis virus. Virology. 1991;180:108–119. doi: 10.1016/0042-6822(91)90014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layne S P, Merges M J, Dembo M, Spouge J L, Conley S R, Moore J P, Raina J L, Renz H, Gelderblom H R, Nara P L. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology. 1992;189:695–714. doi: 10.1016/0042-6822(92)90593-e. [DOI] [PubMed] [Google Scholar]
- 24.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 25.Manabe T, Yoshimori T, Henomatsu N, Tashiro Y. Inhibitors of vacuolar-type H+-ATPase suppresses proliferation of cultured cells. J Cell Physiol. 1993;157:445–452. doi: 10.1002/jcp.1041570303. [DOI] [PubMed] [Google Scholar]
- 26.Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClure M O, Marsh M, Weiss R A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988;7:513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClure M O, Sommerfelt M A, Marsh M, Weiss R A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990;71:767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- 29.McKeating J A, McKnight A, Moore J P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991;65:852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 31.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–986. [PMC free article] [PubMed] [Google Scholar]
- 33.Mothes W, Boerger A L, Narayan S, Cunningham J M, Young J A T. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell. 2000;103:679–689. doi: 10.1016/s0092-8674(00)00170-7. [DOI] [PubMed] [Google Scholar]
- 34.Nussbaum O, Loyter A. Quantitative determination of virus-membrane fusion events. Fusion of influenza virions with plasma membranes and membranes of endocytic vesicles in living cultured cells. FEBS Lett. 1987;221:61–67. doi: 10.1016/0014-5793(87)80352-6. [DOI] [PubMed] [Google Scholar]
- 35.Nussbaum O, Roop A, Anderson W F. Sequences determining the pH dependence of viral entry are distinct from the host range-determining region of the murine ecotropic and amphotropic retrovirus envelope proteins. J Virol. 1993;67:7402–7405. doi: 10.1128/jvi.67.12.7402-7405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkuma S, Shimizu S, Noto M, Sai Y, Kinoshita K, Tamura H. Inhibition of cell growth by bafilomycin A1, a selective inhibitor of vacuolar H+-ATPase. In Vitro Cell Dev Biol Anim. 1993;29A:862–866. doi: 10.1007/BF02631364. [DOI] [PubMed] [Google Scholar]
- 38.Palokangas H, Ying M, Vaananen K, Saraste J. Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitory bafilomycin A1. Mol Biol Cell. 1998;12:3561–3578. doi: 10.1091/mbc.9.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paul R W, Morris D, Hess B W, Dunn J, Overell R W. Increased viral titer through concentration of viral harvests from retroviral packaging lines. Hum Gene Ther. 1993;4:609–615. doi: 10.1089/hum.1993.4.5-609. [DOI] [PubMed] [Google Scholar]
- 40.Perez L, Carrasco L. Entry of poliovirus into cells does not require a low-pH step. J Virol. 1993;67:4543–4548. doi: 10.1128/jvi.67.8.4543-4548.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ragheb J A, Anderson W F. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redmond S, Peters G, Dickson C. Mouse mammary tumor virus can mediate cell fusion at reduced pH. Virology. 1984;133:393–402. doi: 10.1016/0042-6822(84)90405-7. [DOI] [PubMed] [Google Scholar]
- 43.Saurin A J, Hamlett J, Clague M J, Pennington S R. Inhibition of mitogen-induced DNA synthesis by bafilomycin A1 in Swiss 3T3 fibroblasts. Biochem J. 1996;313:65–70. doi: 10.1042/bj3130065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein B S, Gowda S D, Lifson J D, Penhallow R C, Bensch K G, Engleman E G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 46.Thomsen P, Rudenko O, Berezin V, Norrild B. The HPV-16 E5 oncogene and bafilomycin A1 influence cell motility. Biochim Biophy Acta. 1999;1452:285–295. doi: 10.1016/s0167-4889(99)00132-9. [DOI] [PubMed] [Google Scholar]
- 47.Tjotta E, Hungnes O, Grinde B. Survival of HIV-1 activity after disinfection, temperature and pH changes, or drying. J Med Virol. 1991;35:223–227. doi: 10.1002/jmv.1890350402. [DOI] [PubMed] [Google Scholar]
- 48.Tugizov S, Navarro D, Paz P, Wang Y, Qadri I, Pereira L. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology. 1994;201:263–276. doi: 10.1006/viro.1994.1291. [DOI] [PubMed] [Google Scholar]
- 49.van Deurs B, Holm P K, Sandvig K. Inhibition of the vacuolar H+-ATPase with bafilomycin reduces delivery of internalized molecules from mature multivesicular endosomes to lysosomes in HEp-2 cells. Eur J Cell Biol. 1996;69:343–350. [PubMed] [Google Scholar]
- 50.van Weert A W, Dunn K W, Gueze H J, Maxfield F R, Stoorvogel W. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol. 1995;130:821–834. doi: 10.1083/jcb.130.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White J, Kielian M, Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983;16:151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- 52.White J, Matlin K, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]