Abstract
Coronary artery disease (CAD), a critical condition resulting from systemic inflammation, metabolic dysfunction, and gut microbiota dysbiosis, poses a global public health challenge. ALW-II-41-27, a specific inhibitor of the EphA2 receptor, has shown anti-inflammatory prosperities. However, the impact of ALW-II-41-27 on atherosclerosis has not been elucidated. This study aimed to examine the roles of pharmacologically inhibiting EphA2 and the underlying mechanism in ameliorating atherosclerosis. ALW-II-41-27 was administered to apoE−/− mice fed a high-fat diet via intraperitoneal injection. We first discovered that ALW-II-41-27 led to a significant reduction in atherosclerotic plaques, evidenced by reduced lipid and macrophage accumulation, alongside an increase in collagen and smooth muscle cell content. ALW-II-41-27 also significantly lowered plasma and hepatic cholesterol levels, as well as the colonic inflammation. Furthermore, gut microbiota was analyzed by metagenomics and plasma metabolites by untargeted metabolomics. ALW-II-41-27-treated mice enriched Enterococcus, Akkermansia, Eggerthella and Lactobaccilus, accompanied by enhanced secondary bile acids production. To explore the causal link between ALW-II-41-27-associated gut microbiota and atherosclerosis, fecal microbiota transplantation was employed. Mice that received ALW-II-41-27-treated mouse feces exhibited the attenuated atherosclerotic plaque. In clinical, lower plasma DCA and HDCA levels were determined in CAD patients using quantitative metabolomics and exhibited a negative correlation with higher monocytes EphA2 expression. Our findings underscore the potential of ALW-II-41-27 as a novel therapeutic agent for atherosclerosis, highlighting its capacity to modulate gut microbiota composition and bile acid metabolism, thereby offering a promising avenue for CAD.
Subject terms: Metagenomics, Applied microbiology
Introduction
Coronary artery disease (CAD), predominantly driven by atherosclerosis, continues to be a principal cause of global mortality. Atherosclerosis is a multifactorial disorder, wherein the host’s gut microbiota and its metabolites significantly contribute to its pathogenesis1. Despite interventions targeting hypertension, diabetes, hyperlipidemia, and lifestyle factors, atherosclerotic plaques are still prevalent in nearly half of the patients2, underscoring the imperative for novel therapeutic approaches to inhibit atherosclerosis.
The receptor tyrosine kinase EphA2, belonging to the Eph/ephrin family, plays a crucial role in cell migration, adhesion and differentiation, and has been identified as a potential therapeutic target in malignant tumors3. However, an accumulating body of works performed by our research team and others has delineated EphA2’s pivotal role in atherosclerosis progression. EphA2 is upregulated in the endothelial cells of human coronary atherosclerotic lesions and murine models, promoting inflammation and monocyte adhesion4. Elevated EphA2 expression is also observed in human coronary artery vascular smooth muscle cells during phenotypic transition, with its depletion attenuating plaque proliferation and matrix remodeling, inhibiting proinflammatory macrophage differentiation and reducing lipid and necrotic debris accumulation within plaques5. Furthermore, our previous findings indicate that EphA2 is overexpressed in aortic plaques and livers from atherosclerotic mice, while silence of EphA2 mitigated atherosclerotic plaque formation, decreased plasma cholesterol levels and down-regulated proinflammatory gene expression6. In particular, the LDLR-knockout murine Athsq1 locus on chromosome 4, which contains the EphA2 gene7, shares high homology with the premature myocardial infarction susceptibility locus in human chromosome 1(1q34–36)8, reinforcing EphA2’s role beyond a mere biomarker to an active player in atherosclerosis pathogenesis. EphA2’s expression and function in atherogenesis render it a promising target for atherosclerosis treatment. While EphA2-specific siRNA has demonstrated potential in vitro9, the in vivo delivery of siRNA remains a challenge10, necessitating the development of advanced EphA2-targeted therapeutic strategies. ALW-II-41-27, a type II small molecule inhibitor, has garnered attention for its efficacy in targeting the ATP-binding region and the allosteric site following the “DFG” motif of the EphA2 kinase domain. This compound demonstrates has been shown to inhibit cell survival and proliferation, suppress tumor growth and metastasis, and induce tumor regression in various malignancies such as lung11, breast12, and colorectal cancers13, due to its potent and specific inhibition of the EphA2 receptor tyrosine kinase. Moreover, ALW-II-41-27 treatment demonstrated a suppression in proinflammatory responses and a modulation in cholesterol transport14. However, the direct link between EphA2 kinase inhibition and atherosclerosis-protective effects remains to be elucidated. Moreover, the compound’s pharmacokinetic profile and oral bioavailability present challenges11 that necessitate further investigation into the mechanisms of its therapeutic effects.
Currently, gut microbiota is increasingly recognized as an important metabolic and endocrine organ, which may be subject to CAD and targeted treatment because it can metabolize biologically active molecules, drugs, and their precursors, and control their bioavailability. Gut microbiota changes over time and in response to different diets and treatments. Gut microbial metabolites, such as trimethylamine N-oxide, as our previous report, accelerates the progression of atherosclerosis15, whereas short-chain fatty acids and secondary bile acids (SBAs) confer cardioprotective effects16,17. Primary bile acids (PBAs), synthesized from cholesterol in the liver, are secreted into the duodenum where they undergo gut microbiota-dependent modifications to form SBAs. Studies have observed reducing SBAs production in both inflammation-prone patients and mice, while treatment of SBAs restoration holds a potential for alleviating inflammation and atherosclerosis18–20. As a result, the exploration of therapeutic strategies targeting gut microbiome for the treatment of atherosclerosis is gaining momentum. ALW-II-41-27 has been shown to mitigate colonic oxidative stress and reduce the production of inflammatory cytokines such as TNF-α, IL-6, IL-17, and ICAM-121. Our research has confirmed that a pro-inflammatory colonic phenotype could disrupt the composition and function of gut bacteria15, yet direct data whether inactivating EphA2 alleviating atherosclerosis via manipulating the gut microbiome are lacking.
In this study, we present compelling evidence that ALW-II-41-27 halted the progression of atherosclerotic plaques in mice on a high-fat diet (HFD). Mechanistic insights revealed an increase in the abundance of SBAs-producing gut microbial strains, correlating with an elevated plasma levels of SBAs in ALW-II-41-27–treated mouse models. Furthermore, our findings highlight aberrant bile acid metabolism and EphA2 expression in CAD patients. These results underscore the therapeutic potential of ALW-II-41-27 in restoring gut microbiome and mitigating atherosclerosis.
Results
EphA2 inhibitor attenuates atherosclerosis development in HFD-fed apoE−/− mice
To delineate the protective impact of the EphA2 inhibitor ALW-II-41-27 on atherosclerosis, we subjected eight-week-old apolipoprotein E-deficient (apoE−/−) mice to a 12-week HFD followed by daily intraperitoneal injections of ALW-II-41-27 or saline for an additional 4 weeks (Fig. 1A). Mice treated with ALW-II-41-27 exhibited a significant reduction in weight gain (Fig. 1B). Enzymatic assays revealed no significant differences in ALT and AST levels between the treatment groups (Supplementary Fig. 1). Histological examination indicated that ALW-II-41-27 mitigated hepatic steatosis and lipid deposition in HFD-fed mice (Fig. 1C), primarily due to decreased hepatic cholesterol content (Fig. 1D). ALW-II-41-27 also significantly reduced plasma total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) levels compared to saline-injected controls, without affecting triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) levels (Fig. 1E). Atherosclerotic plaque formation in the aorta was assessed using Oil Red O staining. ALW-II-41-27 treatment significantly decreased atherosclerotic plaque area in the whole aorta (17.78% vs. 23.25% in saline-injected mice) (Fig. 1F) and at the aortic root (15.71% vs. 26.15% in saline-injected mice) (Fig. 1G). These results collectively indicate that ALW-II-41-27 has beneficial effects in attenuating HFD-associated atherosclerosis in apoE−/− mice.
Fig. 1. EphA2 inhibitor ALW-II-41-27 attenuates the development of atherosclerosis in HFD-fed apoE−/− mice.
A Schematic of the mouse experimental protocol. Eight-week-old male apoE−/− mice fed a 12-week HFD and then received an intraperitoneal injection of ALW-II-41-27 (30 mg/kg) once a day while the vehicle group received the same dosage of saline. Treatment with ALW-II-41-27 or saline was performed for 4 weeks. Mice at 24 weeks of age were subjected to tissue collection and subsequent evaluation. B Body weight was monitored weekly after administration. C Representative liver sections were stained by oil red O or H&E staining. D Levels of hepatic cholesterol and triglycerides were determined. E Plasma levels of lipids were determined. F Representative images of oil red O-stained en face aortas. G Cross-sections of the aortic roots were stained with oil red O for lipids. All n = 8. Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. the vehicle group. NS indicates not significant.
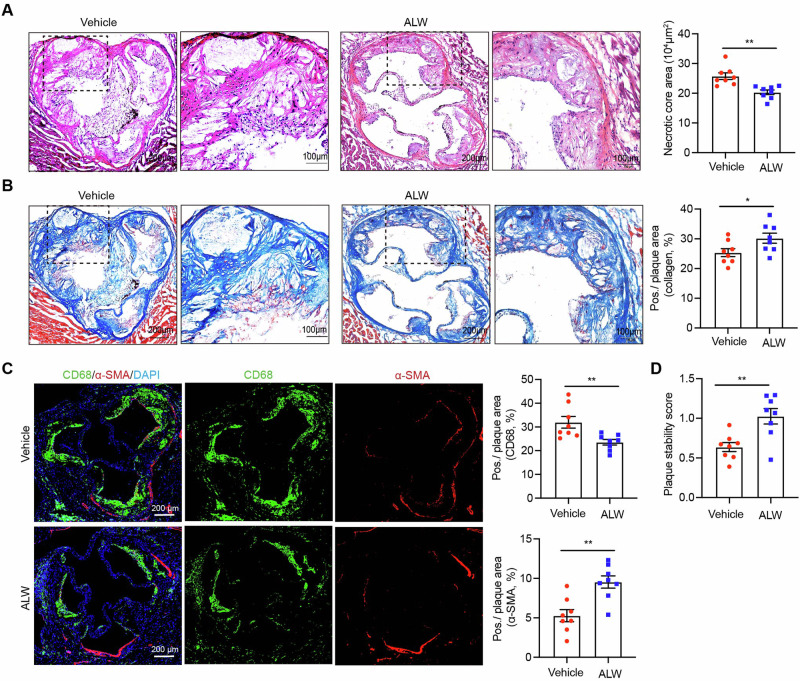
EphA2 inhibitor decreases the necrotic core area and improves plaque stability
To further investigate the effect of ALW-II-41-27 on the stabilization of plaques, the composition of the aortic root sections was detected. The necrotic core, a lipid-rich area indicative of plaque vulnerability, was significantly reduced in ALW-II-41-27–treated mice compared to the vehicle group (Fig. 2A). At same time, plaques with reduced collagen content are thought to be more vulnerable than those with a thick collagen cap22. We found that the collagen content was notably increased within the plaques from ALW-II-41-27-treated mice (Fig. 2B). In addition, the balance between the pro-inflammatory and reparative functions of macrophages and the collagen-producing abilities of smooth muscle cells within atherosclerotic plaques determines the overall stability of the plaque. To this end, immunofluorescence staining using CD68 and SMA antibodies revealed a decrease in macrophage infiltration and an increase in smooth muscle cells in the ALW-II-41-27–treated group (Fig. 2C). Furthermore, quantitative assessment of plaque stability revealed a 37.8% increase in the ALW-II-41-27–treated group relative to the vehicle group (Fig. 2D). Collectively, these findings demonstrate the potential therapeutic efficacy of ALW-II-41-27 in ameliorating atherosclerotic progression and concurrently augmenting the stability of plaques.
Fig. 2. ALW-II-41-27 enhances plaque stability in HFD-fed apoE−/− mice.
Cross-sections of the aortic roots were stained with HE for the necrotic core (A), Masson’s trichrome for collagen (B) and immunofluorescence for a-SMA and CD68 (C), and subsequent plaque stability score was determined as follows: (smooth muscle cell [SMC] area + collagen area)/(macrophage area + lipid area) (D). All n = 8. Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. the vehicle group. NS indicates not significant.
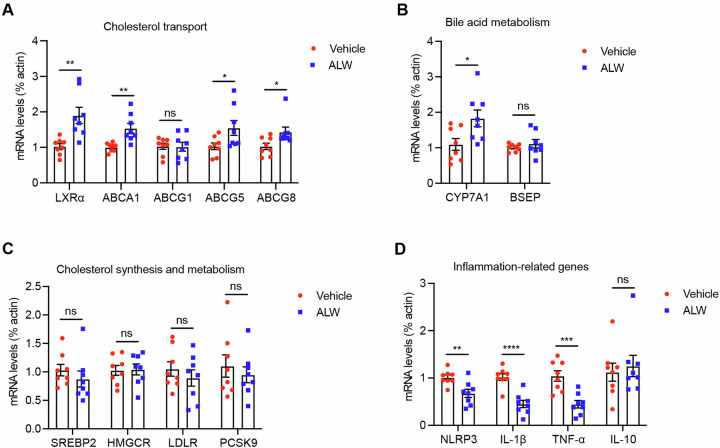
EphA2 inhibitor improves hepatic cholesterol transport and inflammatory phenotypes
Elevated hepatic expression of EphA2 has been correlated with the progression of atherogenic changes6, yet the detailed mechanisms of its role and therapeutic implications remain to be elucidated. To dissect the precise mechanism underlying ALW-II-41-27’s therapeutic efficacy, we conducted a comprehensive assessment of hepatic gene expression profiles. Specifically, the transcriptional levels of liver X receptor alpha (LXRα), ATP-binding cassette transporters A1 (ABCA1), G5 (ABCG5), and G8 (ABCG8) were markedly increased (Fig. 3A), suggesting an enhanced capacity for reverse cholesterol transport. Additionally, the expression of cytochrome P450 family 7 subfamily A member 1 (CYP7A1), a rate-limiting enzyme in bile acid synthesis, was also significantly elevated (Fig. 3B), while the expression of genes involved in cholesterol synthesis and metabolism, such as sterol regulatory element-binding protein 2 (SREBP2), 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), low-density lipoprotein receptor (LDLR), and proprotein convertase subtilisin/kexin type 9 (PCSK9), remained unchanged (Fig. 3C). Conversely, the mRNA levels of pro-inflammatory genes, including NLRP3 inflammasome component (NLRP3), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α), were substantially decreased in response to ALW-II-41-27 treatment (Fig. 3D). These data suggest that ALW-II-41-27 is capable of upregulating the expression of hepatic genes involved in cholesterol transport and bile acid synthesis, as well as suppressing pro-inflammatory genes expression.
Fig. 3. ALW-II-41-27 modulates hepatic genes expression.
The genes expression involved in cholesterol transport (A), bile acid metabolism (B), cholesterol synthesis and metabolism (C) and inflammation (D) were determined by RT-qPCR. All n = 8. Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. the vehicle group. NS indicates not significant.
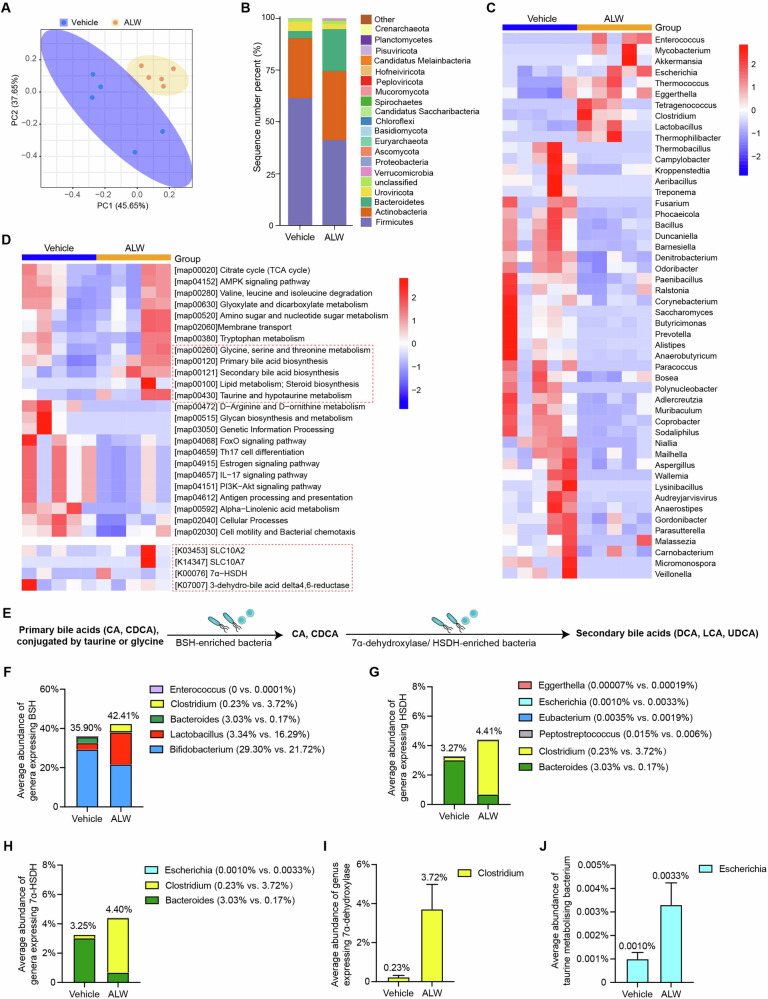
ALW-II-41-27 modulates the gut microbial composition and function
To further identify the gut microbial domains that ALW-II-41-27 modifies, fecal samples from apoE−/− mice with or without ALW-II-41-27 treatment were subjected to shotgun metagenomic sequencing. Principal Coordinate Analysis (PCoA) depicted a distinct gut microbiome structure between the treatment groups (Fig. 4A), indicative of a treatment-induced shift in microbial composition. At the phylum level, the gut microbiota of both groups was predominantly composed of Firmicutes and Bacteroidetes, which together with Actinobacteria, constituted approximately 90% of the community. Notably, ALW-II-41-27 treatment resulted in a 32.8% reduction in Firmicutes and a 4.8-fold increase in Bacteroidetes compared to the vehicle group, with percentages of 41.2% versus 61.3% and 20.3% versus 3.5%, respectively (Fig. 4B). Further analysis revealed that ALW-II-41-27 administration was associated with increased diversity and abundance of specific genera, including Enterococcus, Mycobacterium, Akkermansia, Escherichia, Thermococcus, Eggerthella, Clostridium and Lactobacillus in their gut microbiota, accompanied by a marked decrease in the abundance of Thermobacillus, Campylobacter, Aeribacillus, Fusarium, Ralstonia, Corynebacterium, Paracoccus, Prevotella, Polynucleobacter, Wallemia, Micromonospora and Veillonella in treated mice compared to saline-injected controls (Fig. 4C). Taxonomic comparison identified 569 common species, with 196 and 251 endemic species in ALW-II-41-27–treated and control mice, respectively (Supplementary Fig. 2A). Linear Discriminant Analysis Effect Size (LEfSe) analysis highlighted several species with significantly different abundances, including an increase in Akkermansia muciniphila, Lactobacillus paragasseri, Lactobacillus jensenii, and Lactobacillus intestinalis, which serve as the factors contributing to bile acid synthesis, inflammation and atherosclerosis23–25 (Supplementary Fig. 2B). Consistently, species belonging to Akkermansia and Lactobacillus demonstrated an inverse correlation with plaque areas and other established risk factors for instability plaques, such as necrotic core area and CD68-positive area (Supplementary Fig. 3). Given that Helicobacter possesses pro-inflammatory and lipid-affecting properties26, particularly H. pylori, which has been proven to induce the downregulation of EphA2 receptor27, we further analyzed the abundance of Helicobacter species. However, no significant difference was observed in Helicobacter, and gastric H. pylori was undetectable in both groups (Supplementary Fig. 4).
Fig. 4. ALW-II-41-27 alters the gut microbiome and increases secondary bile acids-producing bacteria abundance.
The fecal samples were collected from 24-week-old male atherosclerotic apoE−/− mice subjected to ALW-II-41-27 or saline administration and sequenced using metagenomics (n = 5 mice/group). A Three-dimensional PCoA showed the significant differences in the gut microbiota composition. Each data point represents one sample. B Barplot of both groups showed the different gut microbial composition at the phylum level. C Heat map of relative abundance in metagenomics sequencing analysis at the differentially genus levels. D Relative abundance changes of microbial metabolic pathways or genes involved bile acid metabolism summarized by KEGG orthology (KO entries) annotations. Red dashed box represents the pathways or genes involved in bile acid synthesis and metabolism. E Schematic representation of the bacterial enzymes and their substrates (bile acids). All PBAs are deconjugated by BSH before further modification. Deconjugated CA and CDCA are converted to DCA or LCA by 7-alpha-dehydroxylase, respectively; CDCA is converted to UDCA by HSDH/7α-HSDH. Average abundance of bacterial genera expressing specific enzymes: (F) BSH involved in primary bile acids deconjugation, (G) HSDH involved in SBAs synthesis, (H) 7α-HSDH, (I) 7α-dehydroxylase (Clostridium) and (J) taurine metabolizing bacterium (Escherchia) in the gut of mice injected with ALW-II-41-27 or saline.
Functional predictions based on KEGG and GO pathway enrichment indicated that ALW-II-41-27 treatment led to a diminished potential for estrogen, IL-17, and PI3K-Akt signaling pathways, alongside an upregulation of genes involved in lipid metabolism, including SBA biosynthesis, taurine or glycine metabolism, diacylglycerol kinase activity, glycerophospholipid metabolic process and 3-hydroxybutyryl-CoA dehydrogenase; however, no significant alterations were observed in sodium-bile acid cotransporter family members (SLC10A2 and SLC10A7) or bile acid delta 4,6-reductase (Fig. 4D and Supplementary Fig. 5).
We then examined the bacterial taxa that perform the crucial steps of SBAs biosynthesis. PBAs arriving at the intestines undergo conjugation modification with taurine or glycine, followed by deconjugation through gut bacterial enzyme bile salt hydrolase (BSH), and subsequently, the 7α-hydroxy group was removed by hydroxysteroid dehydrogenase (HSDH) and 7α-HSDH, leading to the production of SBAs28 (Fig. 4E). We observed that 42.41% of the gut bacteria in ALW-II-41-27-injected mice belong to the genera Bifidobacterium, Lactobacillus, Bacteroides, Clostridium and Enterococcus, which express BSH29, and a slight elevation in the total abundance of these genera was observed (35.90% in the vehicle group, Fig. 4F). Similar trends were observed in gut bacteria abundance known to express HSDH or 7α-HSDH30 (Fig. 4G, H). Moreover, gut microbiome in mice received ALW-II-41-27 exhibited a markedly elevated abundance in bacteria that expressed 7α-dehydroxylase and metabolize taurine, which was mainly explained by increased abundance in Clostridium and Escherichia (Fig. 4I, J). These findings imply that ALW-II-41-27 modulates gut microbiota composition and enriches the gut microbiome with bacteria capable of enhancing BAs production.
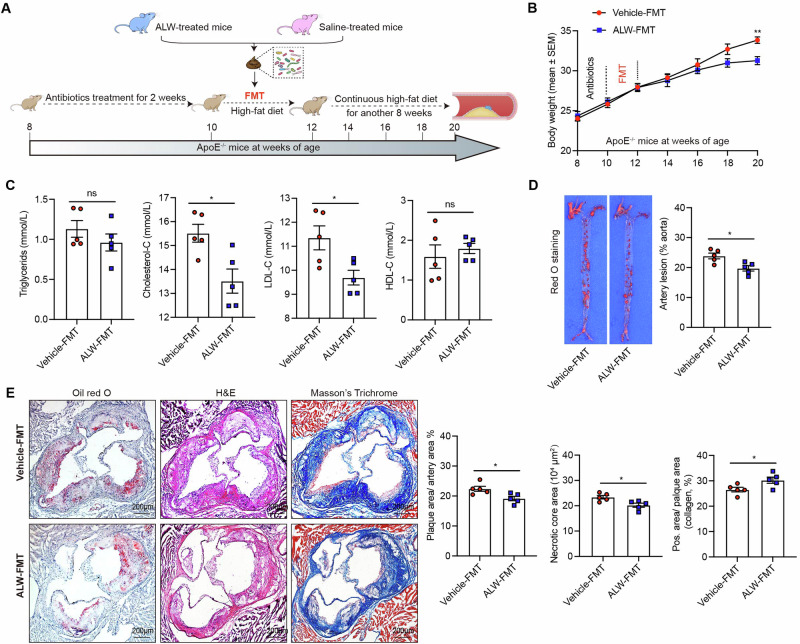
Fecal microbiota transplantation (FMT) from-ALW-II-41-27-treated donors ameliorates atherosclerosis in HFD-fed apoE−/− recipients
To elucidate whether the ameliorative effects of ALW-II-41-27 on atherosclerosis are mediated by gut microbiota, fecal microbiota from ALW-II-41-27-treated donor mice (ALW-FMT) or saline-treated mice (vehicle-FMT) were isolated and transplanted into recipient mice maintained on a HFD (Fig. 5A). Recipient mice that underwent ALW-FMT demonstrated a significant reduction in body weight at week 20 (Fig. 5B). Plasma lipid profiles were also favorably affected, with lowered levels of TC and LDL-C observed in the ALW-FMT group compared to the vehicle-FMT group (Fig. 5C). Furthermore, histological assessment of atherosclerotic plaques revealed a decreased lesion area in the ALW-FMT recipients, suggesting a reduction in atherosclerotic burden (Fig. 5D). Additionally, a significant reduction in the necrotic core areas and elevation in collagen content were observed in the ALW-FMT group (Fig. 5E). These results collectively indicate that the beneficial effects of ALW-II-41-27 on atherosclerosis are modulated by gut microbiota.
Fig. 5. Fecal transplant from ALW-treated mice attenuates atherosclerosis in HFD-fed apoE−/− recipient mice.
A FMT experimental design. 8-week-old male apoE−/− mice were treated with antibiotics for 2 weeks and gavaged with the fecal suspension of either vehicle or ALW donor mice for another 2 weeks. Both vehicle-FMT (n = 5) and ALW-FMT (n = 5) were supplemented with HFD for another 8 weeks. Mice at 20 weeks of age were subjected to tissue collection and subsequent atherosclerotic plaques assessment. B Body weight was monitored biweekly after antibiotics treatment. C Plasma levels of lipids were determined. D Representative images of en face Oil Red O staining of aorta and quantification of lesion area. E Representative Oil Red O staining for lipids, H&E staining for the necrotic core and Masson’s trichrome for collagen of aortic roots (scale bar=200 μm). Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01 vs. the vehicle-FMT group. NS indicates not significant.
EphA2 inhibitor-mediated alterations in colonic inflammation are associated with gut microbial status
Subsequently, we assessed the mechanism by which EphA2 inhibitor to orchestrate the gut microbiota from the perspective of colonic inflammatory phenotypes. Nuclear factor-κB (NF-κB) and inducible nitric oxide synthase (iNOS) are established as key regulators of intestinal inflammation15. Immunofluorescence analysis of colonic sections revealed that ALW-II-41-27 administration significantly reduced the expression of phosphorylated NF-κB p65 and iNOS in F4/80-positive macrophages (Fig. 6A, B), indicative of attenuated inflammatory signaling. To identify whether alterations in ALW-II-41-27 influence inflammatory signals, and consequently, the expression of inflammatory genes, we analyzed the expression levels of transcriptional levels of both pro-inflammatory and anti-inflammatory makers in colonic tissues. We found that ALW-II-41-27 treatment resulted in downregulation of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, while concurrently upregulating anti-inflammatory markers IL-10, TGF-β, and Arg-1 (Fig. 6C).
Fig. 6. The attenuation of pro-inflammatory colonic phenotype by ALW-II-41-27 is connected to alterations in the gut microbiota.
Double-immunofluorescence staining for F4/80 and phosphate-NF-κB p65 (A) and iNOS (B) in colonic sections were performed. C Relative mRNA levels of proinflammatory markers and anti-inflammatory cytokines (to β-actin) in the colon tissues were assessed by RT-qPCR (n = 8). Data are shown as the mean ± SEM. D Pearson correlations between variable bacteria and established inflammatory markers were analyzed. The color scale is indicative of the strength of correlation, ranging from −0.5 (strong negative correlation) to 0.5 (strong positive correlation). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Additionally, to elucidate the link between ALW-II-41-27-induced alterations in colonic inflammation and gut microbiota composition, we conducted a correlation analysis (Fig. 6D). Gut bacteria capable of BSH and 7α-dehydrogenase, including Eggerthella, Escherichia and Clostridium, exhibited positive correlations with the expression of anti-inflammatory genes Arg-1, IL-10, and TGF-β, and negative correlations with the expression of pro-inflammatory genes IL-6 and TNF-α, implying a potential contributory role of colonic inflammatory phenotypes in gut microbiota homeostasis.
ALW-II-41-27 induces the plasma metabolic alterations in HFD-fed mice
Untargeted metabolomics approach has been demonstrated to be useful for studying the impact of drugs on gut microbiota. In this context, we analyzed plasma metabolites after 4 weeks of administering ALW-II-41-27 via intraperitoneal injection to mice fed with HFD. PCoA revealed a remarkable difference in the metabolic profiles of plasma samples (Fig. 7A). Compared to the control group, the ALW-II-41-27-treated group exhibited a 5.90% reduction in plasma lipid content, accompanied by a 1.59-fold increase in the enrichment of organic acids (Supplementary Fig. 6A). Overall, a focused analysis of the top 30 metabolites disclosed significant reductions in lysophosphatidylcholines (LPC18:0, LPC18:1, LPC18:2, LPC16:1) and lysophosphatidylethanolamine (LPE18:2, LPE20:4, LPE22:6) levels in the ALW-II-41-27–injected group, suggesting alterations in lipid metabolism (Supplementary Fig. 6B). Functionally, the plasma samples from ALW-II-41-27–injected mice showed enhanced enrichment of tryptophan- and bile acid-related pathways (Fig. 7B). The corresponding analyses showed that ALW-treated mice displayed a higher abundance of indole-3-acetic acid (Supplementary Fig. 7), and notably, taurocholic acid (TCA) and lithocholic acid (LCA) emerged as key metabolic markers differentiating the ALW-II-41-27 and vehicle treatments (Fig. 7C).
Fig. 7. ALW-II-41-27 alters plasma metabolism and secondary bile acid-related metabolic pathways in HFD-fed apoE−/− mice.
The plasma samples were collected from 24-week-old male atherosclerotic apoE−/− mice subjected to ALW-II-41-27 or saline administration and sequenced using untargeted metabolomics (n = 5 mice/group). A Three-dimensional PCoA showed the significant differences in metabolites composition and structure in the plasma samples. B The metabolic pathways enrichment in the differential metabolites between ALW-II-41-27 and saline-treated groups. We found taurine and hypotaurine metabolism and secondary bile acid biosynthesis were enriched in plasma samples. C Distribution of the discriminative markers in plasma metabolites for ALW-II-41-27-injected mice classification was shown using random forest methods. Left plot listed the most important 15 metabolites. The bigger of value of x-axis, the more important of metabolites. Right plot represents the heatmap of corresponding metabolites abundance. D Plotted in the bar graph are total bile acids (mean ± SEM) in the plasma of mice injected with ALW-II-41-27 or saline. E Ratio of SBAs to PBAs in the plasma of mice injected with ALW-II-41-27 or saline. F Pie graphs are the mean per cent of the bile acids. G The relative abundance of SBAs was analyzed. H The relative abundance of PBAs was analyzed. I, Correlations between the relative abundance of bile acids and gut microbiota were analyzed. J Correlations between the relative abundance of bile acids and established cholesterol and bile acids-related genes were analyzed. The color scale is indicative of the strength of correlation, ranging from −0.5 (strong negative correlation) to 0.5 (strong positive correlation). K Correlations between LCA and DCA and aortic lesion area were analyzed. Pearson correlation coefficients (r) and p values are presented in the graph. Data are shown as the mean ± SEM. *p < 0.05, **p < 0.01. NS indicates not significant.
Altered bile acid metabolic pathways prompted us to analyze their distinct components and abundances. Non-significant difference was found in total bile acids intensity (Fig. 7D). However, the plasma bile acid composition varied between groups, with ALW-II-41-27–injected mice exhibiting a higher percentage of SBAs (Fig. 7E, F). In the current study, SBAs including LCA and deoxycholic acid (DCA) were upregulated in mice received ALW-II-41-27 injection (Fig. 7G), while PBAs, including chenodeoxycholic acid (CDCA), TCA and glycochenodeoxycholic acid (GCDCA), exhibited conflicting results (Fig. 7H). Gut microbiota converts PBAs to SBAs, and additionally, a potential association between the gut microbiota composition and BAs modification was assessed. We found that altered proportion of gut microbiota could contribute to the production of SBAs. Fox example, the plasma levels of LCA and DCA were positively correlated with the abundances of genera Lactobacillus, Enterococcus, Eggerthella and Clostridium (Fig. 7I).
Furthermore, correlation analysis was performed to determine the potential association of BAs with mice phenomes. We observed that LCA and DCA which were enriched in plasma samples of ALW-II-41-27-treated mice were positively correlated with hepatic cholesterol transporters ABCA1 and LXRα (Fig. 7J). Similarly, parallel analysis showed that the relative abundance of LCA and DCA exhibited statistically negative correlations with aortic plaque areas (Fig. 7K). These findings showed that the therapeutic efficacy of ALW-II-41-27 against atherosclerosis is, at least in part, associated with its manipulation of gut microbiome and bile acid metabolism.
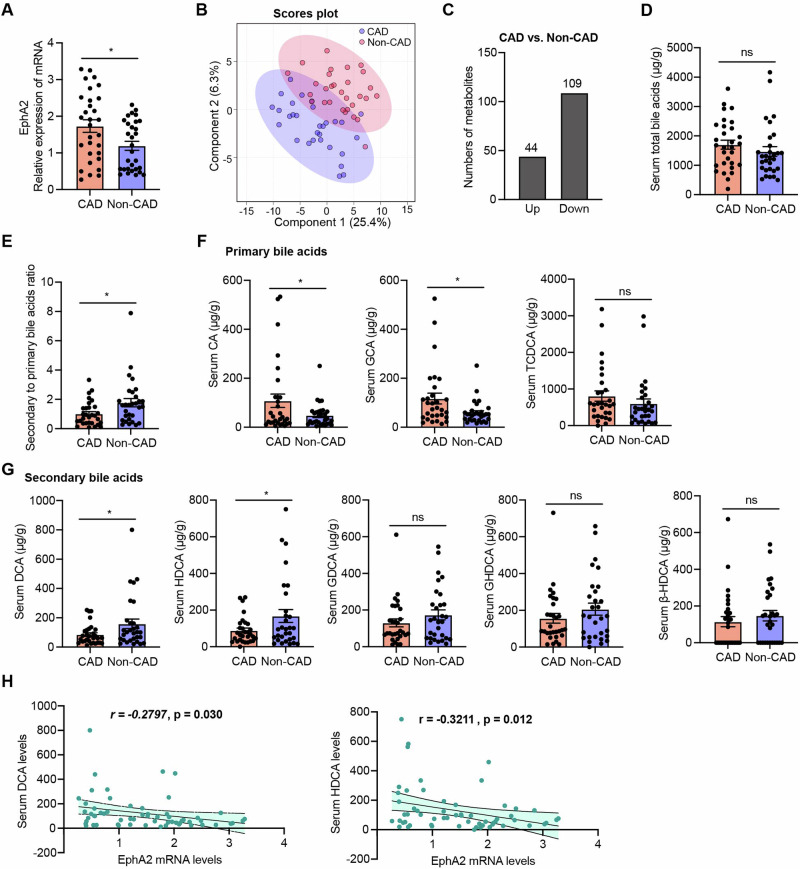
The relevance of reduced DCA and HDCA levels in patients with CAD to EphA2
To investigate the changes of circulating metabolites in clinical specimens, peripheral blood samples from 30 non-CAD patients and 30 CAD patients with a target LDL-C level of <1.8 mmol/L were collected. Baseline characteristics, including a significant prevalence of males and smokers in the CAD group, are detailed in Supplementary Table 1. The expression of EphA2 in peripheral blood mononuclear cells (PBMCs) isolated from CAD patients was significantly higher than that in PBMCs from non-CAD controls (Fig. 8A). Furthermore, quantitative analysis of plasma metabolites was conducted by stable-isotope-dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) method. PCoA depicted a comparable degree of variability among clinical samples from both groups, with no significant outliers (Supplementary Fig. 8A). Partial Least Squares Discriminant Analysis (PLS-DA) revealed a remarkable difference in the plasma metabolic profiles (Fig. 8B), highlighting the metabolic distinctions between non-CAD and CAD patients. A total of 44 metabolites were identified as differentially upregulated, while 109 metabolites were downregulated in CAD patients (Fig. 8C). A bar graph of the 20 most abundant metabolites indicated reduced plasma levels of 11Z-Eicosenoic acid and 7Z,10Z,13Z,16Z-Docosatetraenoic acid in CAD patients, alongside increased levels of hypoxanthine and inosine relative to non-CAD patients (Supplementary Fig. 8B).
Fig. 8. Correlation of EphA2 and bile acids in CAD and Non-CAD patients.
The plasma samples collected from non-CAD (n = 30) and CAD (n = 30) patients were sequenced using quantitative LC-MS/MS method. A EphA2 expression in PBMCs isolated from non-CAD and CAD patients was assessed by RT–qPCR (% β-actin). B Three-dimensional PCoA showed the significant differences in metabolites composition and structure in the plasma samples. C Barplot showed the numbers of up- and down-regulated metabolites in plasma from CAD patients compared to that from non-CAD controls. D Plotted in the bar graph are total bile acids (mean ± SEM) in the plasma of patients. E Ratio of SBAs to PBAs in the plasma of patients with or without CAD. F The relative abundance of PBAs was analyzed. G The relative abundance of SBAs was analyzed. H Correlation between EphA2 expression and DCA and HDCA was analyzed. Pearson correlation coefficients (r) and p values are presented in the graph. Data are shown as the mean ± SEM. *p < 0.05 vs. non-CAD controls. NS indicates not significant.
In alignment with animal-level studies, significant alterations in bile acids and their metabolic pathways were observed in CAD patients (Supplementary Fig. 9A, B). Although the total plasma bile acid levels were not significantly different (Fig. 8D), a marked decrease in the proportion of SBAs was observed in CAD patients (Fig. 8E). Specifically, per cent CA and glycocholic acid (GCA) was markedly elevated in CAD sera, while taurochenodeoxycholic acid (TCDCA), the major PBAs, was unchanged (Fig. 8F). Conversely, per cent representation of DCA and hyodeoxycholic acid (HDCA) were lower in CAD plasma, while glycodeoxycholic acid (GDCA), glycohyodeoxycholic acid (GHDCA) and β-hyodeoxycholic acid (β-HDCA) were not significantly changed (Fig. 8G). Furthermore, the negative correlations between plasma DCA and HDCA levels and monocytes EphA2 expression were observed (Fig. 8H). These findings, in conjunction with the phenotypes observed with ALW-II-41-27 intervention, suggest a potential link between the beneficial effects of inactivating EphA2 on atherosclerosis and the modulation of bile acid profiles in humans.
Discussion
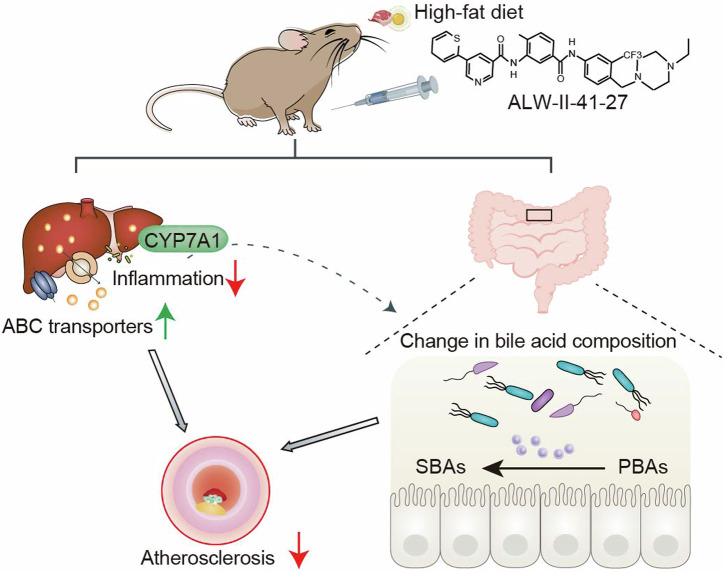
Despite advancements in therapeutic strategies, a substantial reduction in cardiovascular mortality has not been realized. Our review of the literature indicates that the genetic and pharmacological inhibition of EphA2, a cell-surface receptor tyrosine kinase, had a synergistic effect with atorvastatin in the treatment of atherosclerosis by mitigating macrophage inflammation6. Furthermore, EphA2 has been implicated in promoting intestinal inflammation, potentially leading to impaired barrier function31. This reminded us that ALW-II-41-27, as a specific inhibitor of EphA2, may influence the composition of the gut microbiota and its metabolites. However, the direct impact of ALW-II-41-27 on gut microbiome, along with its role in atherosclerosis, have not been fully explored. In the present study, we demonstrate that ALW-II-41-27 leads to a reduction in atherosclerotic plaque formation and an enhancement of plaque stability, accompanied by the changed gut microbiota composition and bile acids levels in apoE−/− mice (Fig. 9). We further found the higher monocytes expression of EphA2 and the lower circulating levels of DCA and HDCA in CAD patients. To our knowledge, this study is the first to delineate the direct correlation between EphA2 expression and bile acids levels, and reveal the novel roles of ALW-II-41-27 and its underlying mechanisms in the amelioration of atherosclerosis.
Fig. 9. A model depicting use of ALW-II-41-27 to attenuate atherosclerosis by targeting hepatic cholesterol transport and gut microbiota.
Administration of ALW-II-41-27 upregulates hepatic cholesterol transporters and downregulates inflammatory genes. In addition, ALW-II-41-27 induces hepatic CYP7A1 expression, and increases BSH and HSDH activity by gut microbiota remodeling, resulting in the changes in bile acid composition. All these changes ultimately result in the inhibition of atherosclerosis.
The influence of anti-atherosclerotic drugs (such as metformin, statins, aspirin and puerarin) on human gut microbiota adds another level of complexity to current studies32, which is especially worth investigating in light of the recent advances in leveraging the microbiota’s potential for treatment of human diseases. It is currently well-known that gut microbiota takes part in mediating the efficacy and toxicity of some drugs33. For example, irinotecan, an anti-cancerous drug commonly used for treating colon cancer, exhibited the gastrointestinal toxicity, which could be explained by the fact that irinotecan caused the unfavorable modification by gut microbial β-glucuronidases34. ALW-II-41-27, traditionally recognized as an anti-cancer drug11–13, has been demonstrated to cause the amelioration of inflammation and modulation of cholesterol transport through its pleiotropic effects. These previously underappreciated mechanisms may elucidate the influence of this drug on the outcomes of other non-targeted diseases, including CAD. Using the apoE−/− model of atherogenesis induced by HFD, we observed the increased atherosclerotic lesion burden and plaque instability, while these changes were restored after ALW-II-41-27 treatment, which underscoring its potential therapeutic significance in CAD outcome.
In the present study, significant alterations in gut microbiome and metabolomic profiles were observed following the administration of the EphA2 inhibitor ALW-II-41-27. Specifically, ALW-II-41-27 was found to remodel the gut microbiome by increasing the abundance of genera such as Eggerthella, Enterococcus, Akkermansia, Clostridium, Lactobacillus and Thermococcus. Notably, exposure to ALW-II-41-27 resulted in heightened bile acid-modification taxonomies and increased plasma concentrations of SBAs, including LCA, DCA, and GUDCA. Although we did not evaluate the activity of BSH and HSDH, the increased genus Eggerthella, Enterococcus, Clostridium, Lactobacillus and Escherichia, to our knowledge, has been identified as a significant contributor to production of LCA and DCA from PBAs by utilizing hydrolase or 7α-dehydrogenase. In addition, our finding of increased Verrucomicrobia in ALW-II-41-27-injected group versus the controls requires further exploration. Akkermansia muciniphila, the sole member of the Verrucomicrobia family, has been implicated in the potential regression of atherosclerosis35; at the same time, several species within the Prevotella phylum were showed to be increased in patients with carotid artery plaque32. These observations potentially serve as another protective mechanisms underlying ALW-II-41-27 against atherosclerosis. Hence, the therapeutic implications of correcting dysbiosis through restoration of key bacterial species via ALW-II-41-27 administration hold clinical promise in microbial dysbiosis-induced atherosclerosis.
The interplay between EphA2 and bile acids has been explored in human patients, both with and without CAD. Metabolomics analysis revealed higher levels of DCA and HDCA in CAD patients, which were found to be negatively correlated with plaque area. Based on this, we focused on exploring the role of bile acids in ALW-II-41-27’s mitigation of atherosclerosis. Bile acids exert an important effect on host lipid metabolism, glucose/insulin metabolism, as well as inflammation though interaction with a variety of host nuclear receptors including Takeda G-protein receptor 5 (TGR5) and the farnesoid X receptor (FXR). The activation of FXR by specific BA metabolites varies in potency, with CDCA being the most potent, followed by DCA, LCA, and CA36. Despite minimal differences in plasma total bile acid content and species between groups, LCA and DCA was increased in per cent. Conversely, per cent CDCA, recognized as the most potent natural agonist for FXR, was found to be decreased in mice treated with ALW-II-41-27. This suggests that the altered total bile acid levels may not stimulate FXR-mediated signaling in the liver and intestine of mice subjected to ALW-II-41-27 treatment. Actually, ALW-II-41-27 treatment enhanced the expression of cholesterol transporters, implying that the reduction in hepatic cholesterol content may be due to ALW’s ability to facilitate cholesterol efflux. Notably, LCA and DCA have been identified as the most potent bile acid species in activating TGR5, succeeded by CDCA and CA. TGR5 is expressed in various cell types that are known to suppress inflammatory processes, including macrophages, enteric neurons, and epithelial cells. A multitude of studies have corroborated the anti-inflammatory effects of TGR5 upon DCA and LCA treatment18. Based on these findings, we hypothesize that the reduction in atherosclerosis may be attributed to ALW-II-41-27’s capacity to facilitate cholesterol efflux and activate TGR5. Further research is warranted to elucidate the roles of ALW-II-41-27 in atherogenesis, particularly using mice deficient in cholesterol transporters or TGR5. Several studies have demonstrated that DCA and LCA, as the most potent ligands for the TGR5 bile acid receptor, ameliorate atherosclerosis by suppressing the activation of the NLRP3 inflammasome and NF-κB signaling pathways, thereby reducing the production of pro-inflammatory cytokines37,38. Overall, our findings suggest that restoration of SBAs, either direct supplementation or potentially through restoration of bile acids-modification bacteria, may mitigate lipid accumulated effects observed in HFD-treated models, as does blockage of EphA2.
In addition, the effect of ALW-II-41-27 on other metabolites likewise indicated that ALW-II-41-27 possesses anti-atherosclerotic effects. For example, indole-3-acetic acid, as the gut microbiota-dependent metabolites, has been demonstrated to inhibit inflammatory responses, oxidative stress, and modulate metabolic homeostasis39,40. Several studies have shown that Akkermansia muciniphila is capable of metabolizing tryptophan into indole and indole-3-acetic acid41,42. Further studies are needed to explore whether the regression of atherosclerosis by ALW-II-41-27 resulted from the increased levels of Akkermansia or the corresponding indole-3-acetic acid.
Intestinal inflammation induced by HFD caused profound changes in the gut microbiota15,43. Our previous work has shown that the silencing EphA2 lead to the downregulation of NF-κB signaling phosphorylation in atherosclerotic plaque macrophages, as well as in peritoneal macrophages and liver6. Consistently, our current study showed that blockage of EphA2 activation using ALW-II-41-27 inhibited the aforementioned inflammation in colon via dampening NF-κB and iNOS-mediated signals, suggesting that suppression of colonic inflammation may be the additional protective effect of EphA2 inhibitor.
Further studies are required to address several gaps and limitations exist in our study. Initially, while we have demonstrated plaque regression induced by ALW-II-41-27, the efficacy of this compound may be constrained by its intrinsic physiological characteristics, despite the highly specific inhibitory effect of ALW-II-41-27 on the EphA2 kinase. So the generation of EphA2-knockout mice is necessary to substantiate our findings. Secondly, exactly how the microbiota and metabolome fine-tuned by ALW-II-41-27 affects atherosclerosis warrants additional investigations. Thirdly, the regulatory roles of EphA2 inhibition on the gut microbiota and bile acids metabolism in CAD patients requires further validation. Considering the ethical concerns surrounding the current utilization of ALW-II-41-27 in human, dasatinib, an inhibitor targeting reducing EphA2 phosphorylation and kinase activity44, may hold promise as a candidate for exploring the inhibitory effects of EphA2. Lastly, although our study has confirmed that ALW-II-41-27 can regulate microbiota and bile acid levels, the mechanisms underlying its pleiotropic effects remain largely elusive and need to be further elucidated to understand.
In conclusion, our study provides valuable insights into the impact of ALW-II-41-27 on atherosclerosis, the gut microbiome, and thus bile acids production, and EphA2 can be targeted by novel therapeutic approaches for promoting cardiometabolic health and stabilized the atherosclerotic plaques.
Methods
Admission of patients
For the present study, a total of 30 patients without non-CAD and 30 patients with CAD were recruited from the cardiovascular department of Sichuan Provincial People’s Hospital. The research protocol was reviewed and approved by the Ethics Committee of the University of Electronic Science and Technology of China. All participants provided written informed consent prior to their inclusion in the study. The diagnosis of CAD was ascertained through a combination of clinical symptoms, electrocardiogram (ECG), myocardial markers, and coronary angiography. Exclusion criteria were defined to include individuals with any concurrent infectious diseases, heart failure (NT-proBNP levels > 1200 pg/mL), renal dysfunction (eGFR < 60 mL/min per 1.73 m2) or malignancy. Blood samples were collected in the morning subsequent to the performance of coronary angiography. Each sample was divided into aliquots and cryopreserved at −80 °C for subsequent analysis.
Animal model and protocol
All animal experimental procedures were conducted with the approval of the Animal Use Subcommittee of the University of Electronic Science and Technology of China (Chengdu, China). Male 8-week-old apoE−/− mice on a C57BL/6 J genetic background were sourced from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The mice were housed four per cage at 25°C in a room under a 12-h light-dark cycle at the Laboratory Animal Research Centre. The apoE−/− mice were subjected to a 16-week HFD regimen, containing 15% fat and 1.2% cholesterol, after which they were randomized into two groups. One group received an intraperitoneal injection of ALW-II-41-27 at a dosage of 30 mg/kg once daily, while the vehicle group was administered an equivalent volume of saline solution for a 4-week treatment period. The selection of the 30 mg/kg/day dosage of ALW-II-41-27 was informed by previous studies demonstrating a significant reduction in pro-inflammatory cytokine levels, NF-κB phosphorylation, and tumor cell growth at this dosage11,21. At the conclusion of the treatment period, all mice were humanely euthanized using intraperitoneal anesthesia with 0.5% sodium pentobarbital (Sigma, USA). Subsequently, plasma, aortas, and other relevant tissues were harvested for further biochemical and histological analyses.
Atherosclerotic plaque analysis
To quantify lipid deposition in en face aortas and aortic root sections, the longitudinally dissected aortas and cryosections embedded in optimal cutting temperature (OCT) compound were subjected to staining with Oil Red O. For the assessment of necrotic core, collagen content, and specific protein distribution, aortic roots embedded in paraffin were sectioned and processed for hematoxylin and eosin (H&E), masson’s trichrome or immunofluorescence staining. For immunostaining, the following primary antibodies were applied: rat anti-F4/80 (abcam, ab6640; diluted 1:200), rabbit anti-CD68 (Cell Signaling Technology, 97778S; diluted 1:200), rabbit anti-α-smooth muscle actin (α-SMA) (Cell Signaling Technology, 19245S; diluted 1:400), rabbit anti-phospho-NF-κB p65 (Cell Signaling Technology, 3033S; diluted 1:1000), and rabbit anti-inducible nitric oxide synthase (iNOS) (diluted 1:400, ab178945). The sections were incubated with these antibodies overnight at 4 °C in a blocking solution. Primary rat antibodies were detected using Cy3-conjugated secondary antibodies, while rabbit antibodies were visualized with Alexa Fluor 488-conjugated secondary antibodies. Fluorescence imaging was conducted using a TCS SP8 confocal laser scanning microscope (LEICA, Germany). Quantitative analysis of the immunofluorescence was performed using ImageJ software.
Metagenomic sequencing and Untargeted metabolomics
The methodologies for metagenomic sequencing and untargeted metabolomics employed in this study were previously detailed in our research15. In summary, for metagenomic analysis, genomic DNA was extracted from freshly collected and immediately frozen fecal samples. Paired-end sequencing of the metagenome was conducted using an Illumina NovaSeq platform. The raw sequencing reads underwent quality control processing with Trimmomatic to ensure high-quality data. This involved the removal of reads with low-quality scores, ambiguous “N” bases, adapter contamination, and the presence of Illumina-specific DNA contaminants, as well as the trimming of low-quality terminal bases. The resulting clean reads constituted over 85% of the initial reads, ranging from 18.4 million to 21.7 million reads. Subsequently, PCoA was conducted at the genus level based on Bray-Curtis dissimilarity metrics. Using the HUMAnN2 software suite, post-quality control and host-removal sequences were aligned against UniRef90 protein database for sequence comparison, and reads that failed to align were filtered out. Subsequently, the relative abundance of each UniRef90 protein was quantified, and the relative abundance of bacteria was calculate based on the original counts. According to the correspondence between UniRef90 IDs and database IDs (KEGG and GO), the relative abundance of functions associated with each database was tallied. Ultimately, commencing from the relative abundance tables of the various databases, hierarchical clustering heat maps were generated for visualization, inter-group functional difference were assessed via LEfSe analysis.
For the untargeted metabolomics approach, fecal supernatant from mouse samples was isolated through a sequence of steps including vortexing, ultrasonication, centrifugation, and filtration. A 20 µL aliquot of the resulting filtrate from each sample was reserved for quality control assessments, while the remainder was subjected to liquid chromatography-mass spectrometry (LC-MS) analysis. Chromatographic separation was achieved using a Thermo Ultimate 3000 system, and the electrospray ionization-tandem mass spectrometry (ESI-MSn) experiments were carried out on a Thermo Q Exactive Plus mass spectrometer. The spray voltages were set at 3.5 kV for positive mode and −2.5 kV for negative mode. The raw data underwent initial processing, which included peak identification, filtration, and alignment, yielding mass-to-charge ratio (m/z), retention time, and intensity values. Metabolite identification was initially confirmed by precise molecular weight determination and subsequently annotated by consulting the five most relevant databases as previously described. PCoA score plots were generated to illustrate the metabolic profile separation among the groups.
Antibiotic treatment and fecal microbiota transplantation studies
The protocols for antibiotic treatment and FMT were as previously described in our study15. In brief, fecal samples were obtained from mice with or without ALW-II-41-27 treatment. These samples were placed into tubes containing a freezing solution—sterile phosphate-buffered saline (PBS) supplemented with 12.5% glycerol—and subsequently homogenized. The homogenized fecal pellets were then stored at −80 °C until further use. Subsequently, 8-week-old apoE−/− mice were administered a daily gavage of a broad-spectrum antibiotic cocktail at the following concentrations: ampicillin 1 g/L, metronidazole 1 g/L, vancomycin 1 g/L, and neomycin 1 g/L, for a period of 2 weeks. Following the antibiotic treatment, the mice were randomized into two groups (n = 5 per group): the ALW-FMT group, which received fecal material from ALW-II-41-27 treated mice, and the Vehicle-FMT group, which received fecal material from untreated mice. FMT was carried out over the subsequent 2 weeks by oral gavage using the prepared fecal suspensions. After the completion of the 8-week HFD regimen, analyses of plasma lipid profiles and atherosclerotic plaque characteristics were conducted.
RT-qPCR
Total RNA extraction was carried out using the RNAiso Plus reagent (TaKaRa Biotechnology, Dalian, China). Reverse transcription was conducted at 37 °C for 15 minutes, followed by a denaturation step at 85 °C for 5 s, utilizing the Evo M-MLV RT Master Mix Kit (AG, Hunan, China). Quantitative real-time polymerase chain reaction (qPCR) was performed with the SYBR Green Pro Taq HS Premix (AG, Hunan, China) on a LightCycler 480 II system (Roche, Switzerland). The qPCR protocol consisted of an initial denaturation at 95 °C for 30 s, followed by 40 cycles of amplification at 95 °C for 15 s and 60 °C for 30 s. Each reaction was executed in duplicate to ensure technical reproducibility. The β-actin gene was employed as an endogenous reference for data normalization. Relative gene expression levels were calculated using the 2^-ΔΔCt method, a standard approach for quantifying the fold change in gene expression. Primer sequences for the target genes are detailed in Supplementary Table 2.
Statistical analysis
The Shapiro-Wilk test was used to determine the normal distribution of the data. Normally continuous variables were analyzed using Student’s t-test or one-way analysis of variance (ANOVA) of variance with Bonferroni’s test between two groups or among multiple groups, while non-normally distributed variables were performed using Mann-Whitney U test or Kruskal-Wallis H test with Dunn’s test. Pearson correlation analysis was used to evaluate the association between two continuous variables. The results were statistically analyzed using SPSS v13.0. Values of p < 0.05 (two tailed) were considered to be statistically significant.
Supplementary information
Acknowledgements
This work was supported by the National Nature Science Foundation of China [grant number: 82470477], the Department of Science and Technology of Sichuan Province, China [grant numbers: 2023NSFSC1631, 2023YFS0116, 2022YFS0604] and the Medical Association of Sichuan, China [grant number: Q22066].
Author contributions
C.L. and T.T.L. designed the study. C.L. and D.L. performed in vivo animal experiments, including FMT. Q.W. performed metagenomics and metabolomics analysis. J.Z. and Y.X. performed clinical samples collection and analysis. T.T.L. performed all statistical analysis and wrote the manuscript. All authors revised and approved the manuscript. C.L. and D.L. contributed equally to this study.
Data availability
The metagenomic sequencing data are available in NCBI Sequence Read Archive (SRA) repository under accession number PRJNA1126993. The untargeted metabolomics data supporting the findings of this study are available upon reasonable request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Cong Lu, Dan Liu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41522-024-00585-7.
References
- 1.Talmor-Barkan, Y. et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat. Med.28, 295–302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernández-Friera, L. et al. Normal LDL-cholesterol levels are associated with subclinical atherosclerosis in the absence of risk factors. J. Am. Coll. Cardiol.70, 2979–2991 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Zhou, Y. & Sakurai, H. Emerging and diverse functions of the EphA2 noncanonical pathway in cancer progression. Biol. Pharm. Bull.40, 1616–1624 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Funk, S. D. et al. EphA2 activation promotes the endothelial cell inflammatory response: a potential role in atherosclerosis. Arterioscler Thromb. Vasc. Biol.32, 686–695 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finney, A. C. et al. EphA2 Expression Regulates Inflammation and Fibroproliferative Remodeling in Atherosclerosis. Circulation136, 566–582 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng, J. et al. Inhibition of EphA2 protects against atherosclerosis by synergizing with statins to mitigate macrophage inflammation. Biomed. Pharmacother.169, 115885 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Welch, C. L. et al. Localization of atherosclerosis susceptibility loci to chromosomes 4 and 6 using the Ldlr knockout mouse model. Proc. Natl Acad. Sci. USA98, 7946–7951 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang, Q. et al. Premature myocardial infarction novel susceptibility locus on chromosome 1p 34–36 identified by genomewide linkage analysis. Am. J. Hum. Genet.74, 262–271 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang, H. et al. EphA2 knockdown attenuates atherosclerotic lesion development in ApoE(-/-) mice. Cardiovasc Pathol.23, 169–174 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Wu, S. Y., Lopez-Berestein, G., Calin, G. A. & Sood, A. K. RNAi therapies: drugging the undruggable. Sci. Transl. Med.6, 240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amato, K. R. et al. Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. J. Clin. Invest124, 2037–2049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao, P. et al. Targeting the KLF5-EphA2 axis can restrain cancer stemness and overcome chemoresistance in basal-like breast cancer. Int J. Biol. Sci.19, 1861–1874 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, Y., Peng, Q. & Wang, L. EphA2 as a phase separation protein associated with ferroptosis and immune cell infiltration in colorectal cancer. Aging (Albany NY)15, 12952–12965 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres-Adorno, A. M. et al. Eicosapentaenoic acid in combination with EPHA2 inhibition shows efficacy in preclinical models of triple-negative breast cancer by disrupting cellular cholesterol efflux. Oncogene38, 2135–2150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo, T. et al. Deficiency of PSRC1 accelerates atherosclerosis by increasing TMAO production via manipulating gut microbiota and flavin monooxygenase 3. Gut Microbes14, 2077602 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masse, K. E. & Lu, V. B. Short-chain fatty acids, secondary bile acids and Indoles: gut microbial metabolites with effects on enteroendocrine cell function and their potential as therapies for metabolic disease. Front Endocrinol. (Lausanne)14, 1169624 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang, W. H. W., Li, D. Y. & Hazen, S. L. Dietary metabolism, the gut microbiome, and heart failure. Nat. Rev. Cardiol.16, 137–154 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha, S. R. et al. Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation. Cell Host Microbe27, 659–670.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, J. et al. Gut microbial metabolite hyodeoxycholic acid targets the TLR4/MD2 complex to attenuate inflammation and protect against sepsis. Mol. Ther.31, 1017–1032 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu, Q. et al. Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J. Clin. Invest.131, e142865 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng, L. et al. A novel EphA2 inhibitor exerts beneficial effects in PI-IBS in vivo and in vitro models via Nrf2 and NF-κB signaling pathways. Front Pharm.9, 272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansson, G. K., Libby, P. & Tabas, I. Inflammation and plaque vulnerability. J. Intern Med278, 483–493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warmbrunn, M. V. et al. Networks of gut bacteria relate to cardiovascular disease in a multi-ethnic population: the HELIUS study. Cardiovasc Res.120, 372–384 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusada, H., Arita, M., Tohno, M. & Tamaki, H. Bile salt hydrolase degrades β-lactam antibiotics and confers antibiotic resistance on Lactobacillus paragasseri. Front Microbiol.13, 858263 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin, Q. et al. Quinic acid regulated TMA/TMAO-related lipid metabolism and vascular endothelial function through gut microbiota to inhibit atherosclerotic. J. Transl. Med.22, 352 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dingemanse, C. et al. Akkermansia muciniphila and Helicobacter typhlonius modulate intestinal tumor development in mice. Carcinogenesis36, 1388–1396 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Leite, M. et al. Helicobacter Pylori targets the EPHA2 receptor tyrosine kinase in gastric cells modulating key cellular functions. Cells9, 513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiao, N. et al. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut67, 881–1891 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Song, Z. et al. Taxonomic profiling and populational patterns of bacterial bile salt hydrolase (BSH) genes based on worldwide human gut microbiome. Microbiome7, 9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long, S. L., Gahan, C. G. M. & Joyce, S. A. Interactions between gut bacteria and bile in health and disease. Mol. Asp. Med.56, 54–65 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Chen, Y. et al. EphrinA1/EphA2 promotes epithelial hyperpermeability involving in lipopolysaccharide-induced intestinal barrier dysfunction. J. Neurogastroenterol. Motil.26, 397–409 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, Z. H. et al. Puerarin alleviates atherosclerosis via the inhibition of Prevotella copri and its trimethylamine production. Gut, 331880 (2024). [DOI] [PubMed]
- 33.Koppel, N., Maini Rekdal, V. & Balskus, E. P. Chemical transformation of xenobiotics by the human gut microbiota. Science356, eaag2770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science330, 831–835 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, J., Lin, S., Vanhoutte, P. M., Woo, C. W. & Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe-/- mice. Circulation133, 2434–2446 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science284, 1362e5 (1999). [DOI] [PubMed] [Google Scholar]
- 37.Pols, T. W. et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab.14, 747–757 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi, Y. et al. TGR5 regulates macrophage inflammation in nonalcoholic steatohepatitis by modulating NLRP3 inflammasome activation. Front Immunol.11, 609060 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji, Y. et al. Anti-inflammatory and anti-oxidative activity of indole-3-acetic acid involves induction of HO-1 and neutralization of free radicals in RAW264.7 cells. Int J. Mol. Sci.21, 1579 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langan, D., Perkins, D. J., Vogel, S. N. & Moudgil, K. D. Microbiota-derived metabolites, indole-3-aldehyde and indole-3-acetic acid, differentially modulate innate cytokines and stromal remodeling processes associated with autoimmune arthritis. Int J. Mol. Sci.22, 2017 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin, J. et al. Dose-dependent beneficial effects of tryptophan and its derived metabolites on Akkermansia in vitro: a preliminary prospective study. Microorganisms9, 1511 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu, Z. et al. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct.12, 10184–10195 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Zhang, Y. et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome7, 116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang, J. et al. Cross-talk between EphA2 and BRaf/CRaf is a key determinant of response to Dasatinib. Clin. Cancer Res.20, 1846–1855 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The metagenomic sequencing data are available in NCBI Sequence Read Archive (SRA) repository under accession number PRJNA1126993. The untargeted metabolomics data supporting the findings of this study are available upon reasonable request from the corresponding author.