Abstract
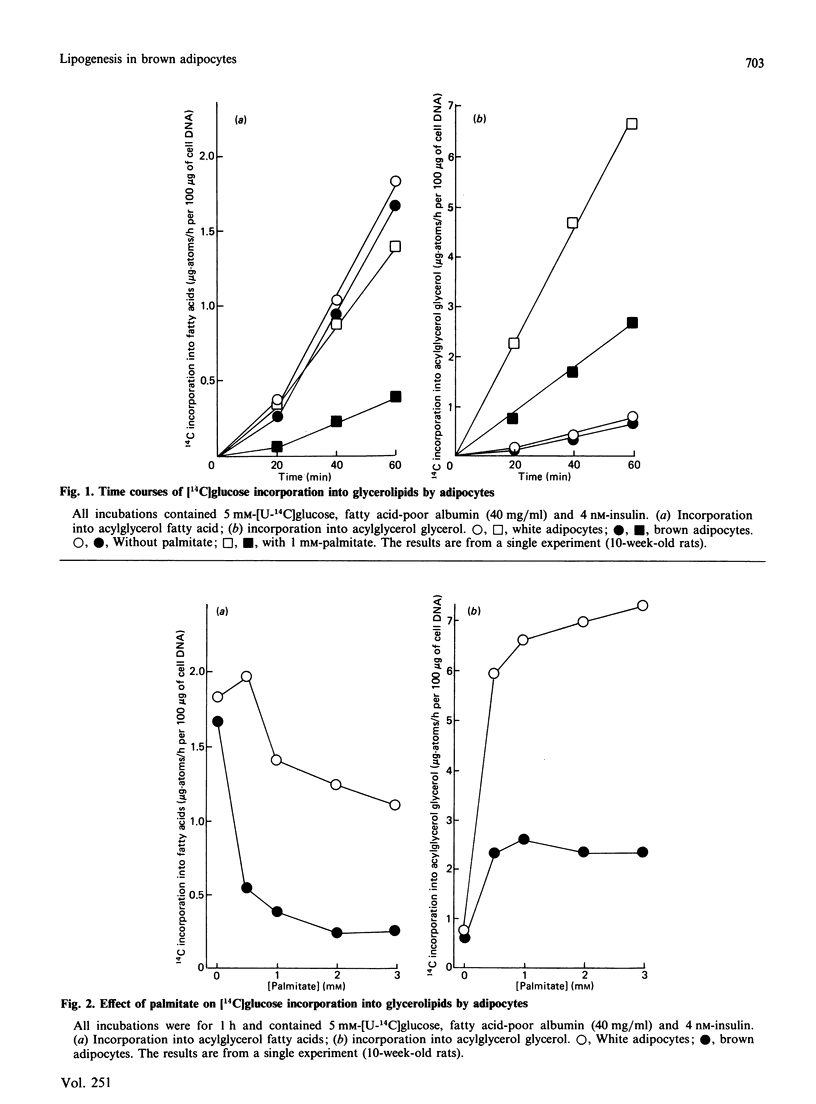
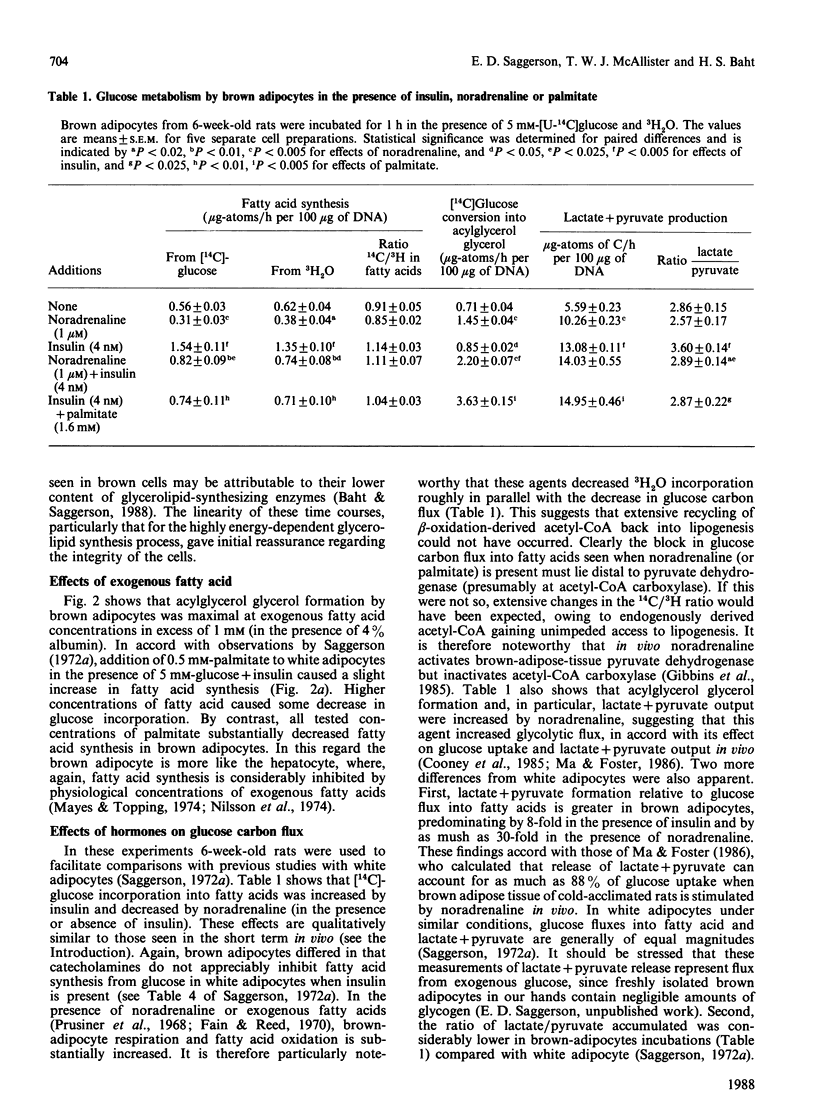
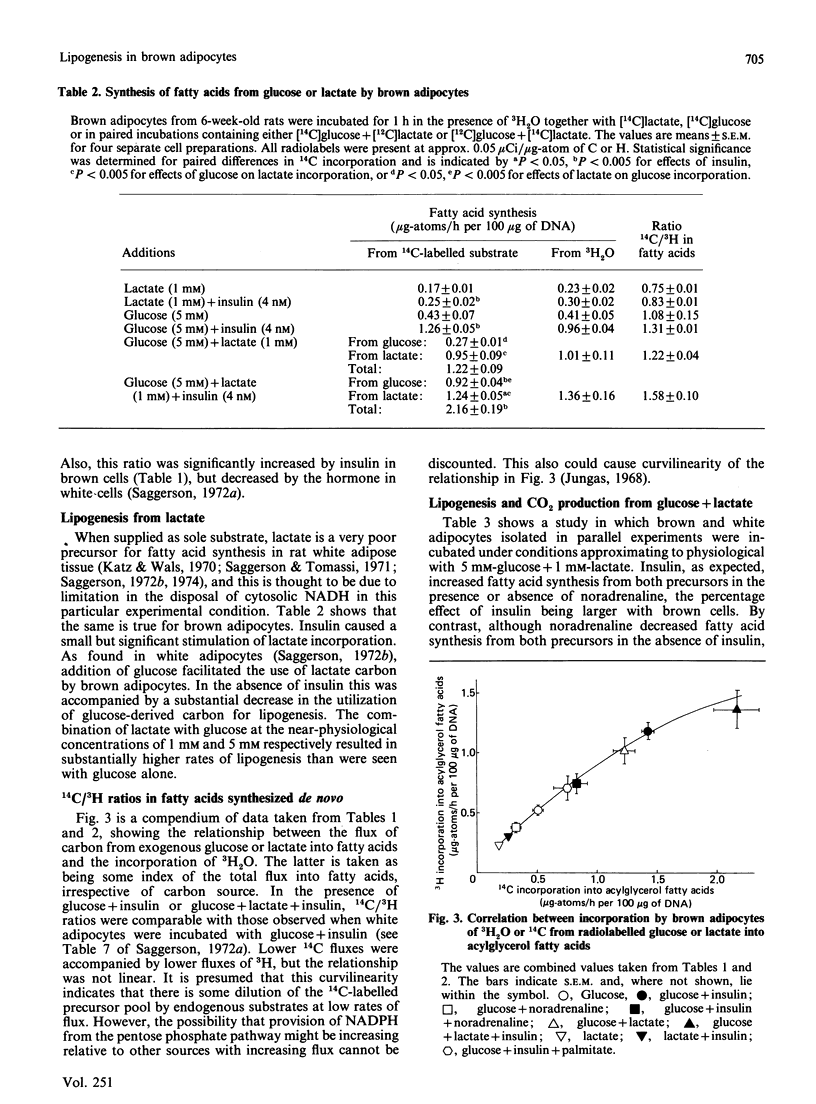
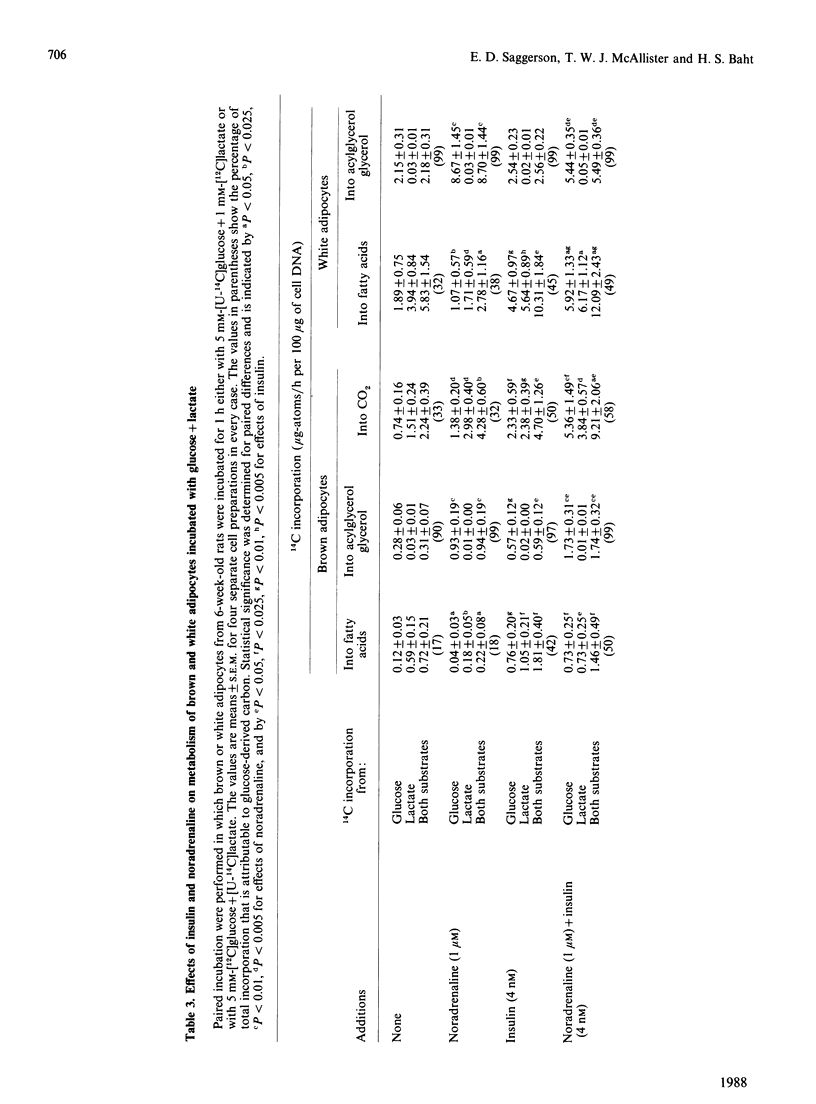
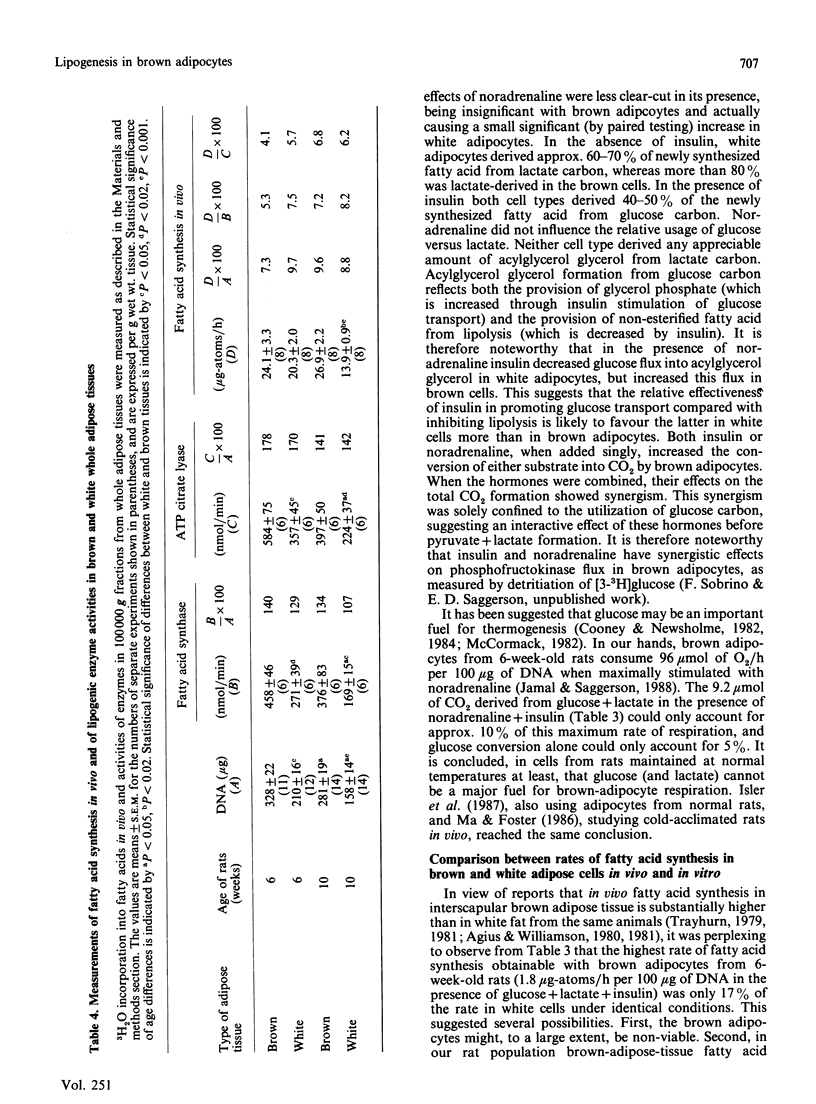
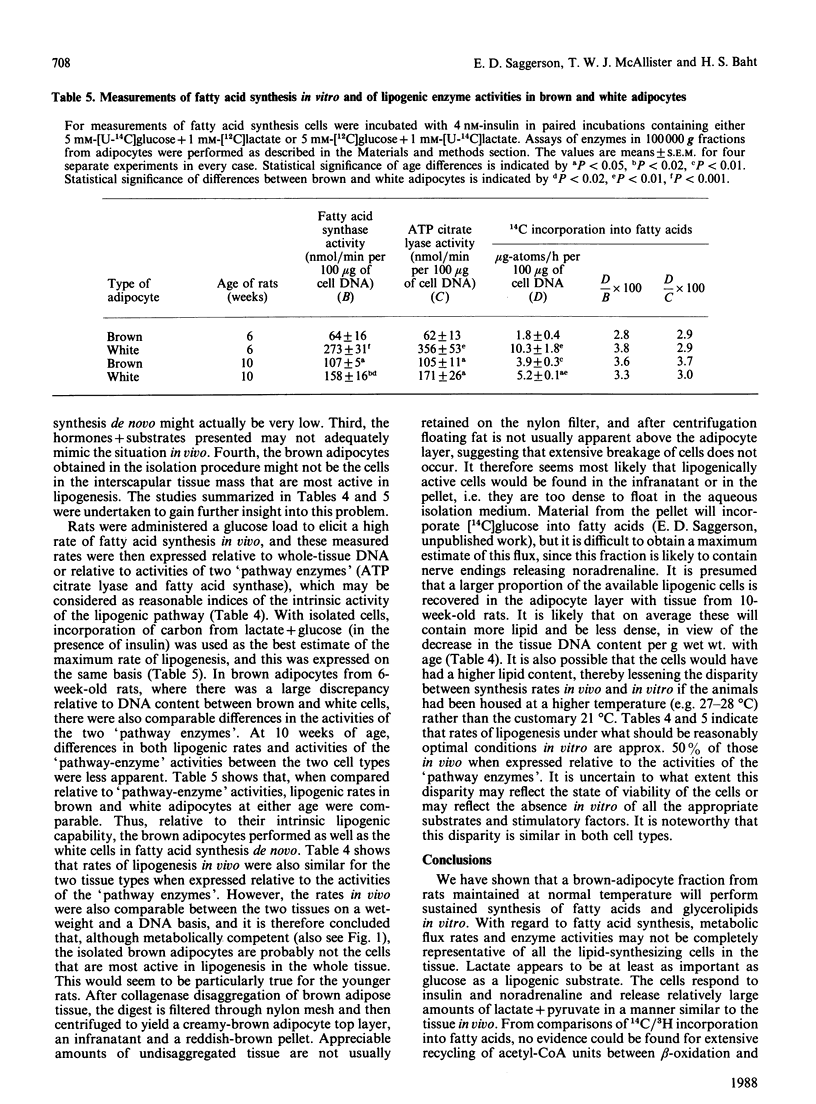
1. Brown adipocytes were isolated from the interscapular depot of male rats maintained at approx. 21 degrees C. In some experiments parallel studies were made with white adipocytes from the epididymal depot. 2. Insulin increased and noradrenaline decreased [U-14C]glucose incorporation into fatty acids by brown adipocytes. Brown adipocytes differed from white adipocytes in that exogenous fatty acid (palmitate) substantially decreased fatty acid synthesis from glucose. Both noradrenaline and insulin increased lactate + pyruvate formation by brown adipocytes. Brown adipocytes converted a greater proportion of metabolized glucose into lactate + pyruvate and a smaller proportion into fatty acids than did white adipocytes. 3. In brown adipocytes, when fatty acid synthesis from [U-14C]glucose was decreased by noradrenaline or palmitate, incorporation of 3H2O into fatty acids was also decreased to an extent which would not support proposals for extensive recycling into fatty acid synthesis of acetyl-CoA derived from fatty acid oxidation. 4. In the absence of glucose, [U-14C]lactate was a poor substrate for lipogenesis in brown adipocytes, but its use was facilitated by glucose. When brown adipocytes were incubated with 1 mM-lactate + 5 mM-glucose, lactate-derived carbon generally provided at least 50% of the precursor for fatty acid synthesis. 5. Both insulin and noradrenaline increased [U-14C]glucose conversion into CO2 by brown adipocytes (incubated in the presence of lactate) and, in combination, stimulation of glucose oxidation by these two agents showed synergism. Rates of 14CO2 formation from glucose by brown adipocytes were relatively small compared with maximum rates of oxygen consumption by these cells, suggesting that glucose is unlikely to be a major substrate for thermogenesis. 6. Brown adipocytes from 6-week-old rats had considerably lower maximum rates of fatty acid synthesis, relative to cell DNA content, than white adipocytes. By contrast, rates of fatty acid synthesis from 3H2O in vivo were similar in the interscapular and epididymal fat depots. Expressed relative to activities of fatty acid synthase or ATP citrate lyase, however, brown adipocytes synthesized fatty acids as effectively as did white adipocytes. It is suggested that the cells most active in fatty acid synthesis in the brown adipose tissue are not recovered fully in the adipocyte fraction during cell isolation. Differences in rates of fatty acid synthesis between brown and white adipocytes were less apparent at 10 weeks of age.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Rolls B. J., Rowe E. A., Williamson D. H. Increased lipogenesis in brown adipose tissue of lactating rats fed a cafeteria diet. The possible involvement of insulin in brown adipose tissue hypertrophy. FEBS Lett. 1981 Jan 12;123(1):45–48. doi: 10.1016/0014-5793(81)80016-6. [DOI] [PubMed] [Google Scholar]
- Agius L., Williamson D. H. Lipogenesis in interscapular brown adipose tissue of virgin, pregnant and lactating rats. The effects of intragastric feeding. Biochem J. 1980 Aug 15;190(2):477–480. doi: 10.1042/bj1900477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agius L., Williamson D. H. The utilization of ketone bodies by the interscapular brown adipose tissue of the rat. Biochim Biophys Acta. 1981 Oct 23;666(1):127–132. doi: 10.1016/0005-2760(81)90098-9. [DOI] [PubMed] [Google Scholar]
- Baht H. S., Saggerson E. D. Comparison of triacylglycerol synthesis in rat brown and white adipocytes. Effects of hypothyroidism and streptozotocin-diabetes on enzyme activities and metabolic fluxes. Biochem J. 1988 Mar 1;250(2):325–333. doi: 10.1042/bj2500325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley M. G., Rath E. A. Regulation of fatty acid synthesis and malonyl-CoA content in mouse brown adipose tissue in response to cold-exposure, starvation or re-feeding. Biochem J. 1987 Apr 15;243(2):437–442. doi: 10.1042/bj2430437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. The biochemistry of an inefficient tissue: brown adipose tissue. Essays Biochem. 1985;20:110–164. [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Lipogenesis in rat hepatocytes. J Biol Chem. 1974 Apr 10;249(7):2028–2036. [PubMed] [Google Scholar]
- Cooney G. J., Caterson I. D., Newsholme E. A. The effect of insulin and noradrenaline on the uptake of 2-[1-14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett. 1985 Sep 2;188(2):257–261. doi: 10.1016/0014-5793(85)80383-5. [DOI] [PubMed] [Google Scholar]
- Cooney G. J., Newsholme E. A. The maximum capacity of glycolysis in brown adipose tissue and its relationship to control of the blood glucose concentration. FEBS Lett. 1982 Nov 8;148(2):198–200. doi: 10.1016/0014-5793(82)80807-7. [DOI] [PubMed] [Google Scholar]
- DOLE V. P. Effect of nucleic acid metabolites on lipolysis in adipose tissue. J Biol Chem. 1961 Dec;236:3125–3130. [PubMed] [Google Scholar]
- Fain J. N., Reed N. A mechanism for hormonal activation of lipolysis and respiration in free brown fat cells. Lipids. 1970 Feb;5(2):210–219. doi: 10.1007/BF02532471. [DOI] [PubMed] [Google Scholar]
- Fain J. N., Reed N., Saperstein R. The isolation and metabolism of brown fat cells. J Biol Chem. 1967 Apr 25;242(8):1887–1894. [PubMed] [Google Scholar]
- Ferré P., Burnol A. F., Leturque A., Terretaz J., Penicaud L., Jeanrenaud B., Girard J. Glucose utilization in vivo and insulin-sensitivity of rat brown adipose tissue in various physiological and pathological conditions. Biochem J. 1986 Jan 1;233(1):249–252. doi: 10.1042/bj2330249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbins J. M., Denton R. M., McCormack J. G. Evidence that noradrenaline increases pyruvate dehydrogenase activity and decreases acetyl-CoA carboxylase activity in rat interscapular brown adipose tissue in vivo. Biochem J. 1985 Jun 15;228(3):751–755. doi: 10.1042/bj2280751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Rath E. A., Verrinder T. R. Fatty acid synthesis in liver and adipose tissue of normal and genetically obese (ob/ob) mice during the 24-hour cycle. Biochem J. 1975 Aug;150(2):167–173. doi: 10.1042/bj1500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isler D., Hill H. P., Meier M. K. Glucose metabolism in isolated brown adipocytes under beta-adrenergic stimulation. Quantitative contribution of glucose to total thermogenesis. Biochem J. 1987 Aug 1;245(3):789–793. doi: 10.1042/bj2450789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal Z., Saggerson E. D. Factors influencing the altered thermogenic response of rat brown adipose tissue in streptozotocin-diabetes. Biochem J. 1988 Jan 15;249(2):415–421. doi: 10.1042/bj2490415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungas R. L. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry. 1968 Oct;7(10):3708–3717. doi: 10.1021/bi00850a050. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A. Effect of phenazine methosulfate on lipogenesis. J Biol Chem. 1970 May 25;245(10):2546–2548. [PubMed] [Google Scholar]
- Katz J., Wals P. A. Lipogenesis from lactate in rat adipose tissue. Biochim Biophys Acta. 1974 Jun 26;348(3):344–356. doi: 10.1016/0005-2760(74)90214-8. [DOI] [PubMed] [Google Scholar]
- Ma S. W., Foster D. O. Uptake of glucose and release of fatty acids and glycerol by rat brown adipose tissue in vivo. Can J Physiol Pharmacol. 1986 May;64(5):609–614. doi: 10.1139/y86-101. [DOI] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes P. A., Topping D. L. Regulation of hepatic lipogenesis by plasma free fatty acids: simultaneous studies on lipoprotein secretion, cholesterol synthesis, ketogenesis and gluconeogenesis. Biochem J. 1974 Apr;140(1):111–114. doi: 10.1042/bj1400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G., Denton R. M. Evidence that fatty acid synthesis in the interscapular brown adipose tissue of cold-adapted rats is increased in vivo by insulin by mechanisms involving parallel activation of pyruvate dehydrogenase and acetyl-coenzyme A carboxylase. Biochem J. 1977 Sep 15;166(3):627–630. doi: 10.1042/bj1660627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G. The regulation of fatty acid synthesis in brown adipose tissue by insulin. Prog Lipid Res. 1982;21(3):195–223. doi: 10.1016/0163-7827(82)90009-1. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Lindberg O. The brown fat cell. Int Rev Cytol. 1982;74:187–286. doi: 10.1016/s0074-7696(08)61173-0. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Locke R. M. Thermogenic mechanisms in brown fat. Physiol Rev. 1984 Jan;64(1):1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- Nilsson A., Sundler R., Akesson B. Effect of different albumin-bound fatty acids on fatty acid and cholesterol biosynthesis in rat hepatocytes. FEBS Lett. 1974 Sep 1;45(1):282–285. doi: 10.1016/0014-5793(74)80862-8. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Cannon B., Ching T. M., Lindberg O. Oxidative metabolism in cells isolated from brown adipose tissue. 2. Catecholamine regulated respiratory control. Eur J Biochem. 1968 Dec;7(1):51–57. doi: 10.1111/j.1432-1033.1968.tb19572.x. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rath E. A., Beloff-Chain A., Hems D. A. Contribution of lactate carbon of fatty acid synthesis in adipose tissue of normal and genetically obese (ob/ob) mice. Biochem Soc Trans. 1975;3(4):513–515. doi: 10.1042/bst0030513. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Effects of altered dietary and hormonal conditions. Biochem J. 1970 Sep;119(2):221–242. doi: 10.1042/bj1190221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Lipogenesis in rat and guinea-pig isolated epididymal fat-cells. Biochem J. 1974 May;140(2):211–224. doi: 10.1042/bj1400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of acetate, pyruvate, lactate, palmitate, electron-acceptors, uncoupling agents and oligomycin. Biochem J. 1972 Aug;128(5):1069–1078. doi: 10.1042/bj1281069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Tomassi G. The regulation of glyceride synthesis from pyruvate in isolated fat cells. The effects of palmitate and alteration of dietary status. Eur J Biochem. 1971 Nov 11;23(1):109–117. doi: 10.1111/j.1432-1033.1971.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Bowen N. L., Hems D. A. Synthesis of fatty acids in the perused mouse liver. Biochem J. 1974 Sep;142(3):611–618. doi: 10.1042/bj1420611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu T., Takahashi A. Stimulation of hypothalamic nuclei has differential effects on lipid synthesis in brown and white adipose tissue. Nature. 1980 Mar 6;284(5751):62–63. doi: 10.1038/284062a0. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer B. R., Summer G. K. A modified fluorometric micromethod for DNA. Clin Chim Acta. 1971 Apr;32(2):203–206. doi: 10.1016/0009-8981(71)90333-0. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Fatty acid synthesis in mouse brown adipose tissue. The influence of environmental temperature on the proportion of whole-body fatty acid synthesis in brown adipose tissue and the liver. Biochim Biophys Acta. 1981 Jun 23;664(3):549–560. doi: 10.1016/0005-2760(81)90132-6. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Fatty acid synthesis in vivo in brown adipose tissue, liver and white adipose tissue of the cold-acclimated rat. FEBS Lett. 1979 Aug 1;104(1):13–16. doi: 10.1016/0014-5793(79)81075-3. [DOI] [PubMed] [Google Scholar]
- Woodward J. A., Saggerson E. D. Effect of adenosine deaminase, N6-phenylisopropyladenosine and hypothyroidism on the responsiveness of rat brown adipocytes to noradrenaline. Biochem J. 1986 Sep 1;238(2):395–403. doi: 10.1042/bj2380395. [DOI] [PMC free article] [PubMed] [Google Scholar]


