Abstract
Perceptual learning, known to improve visual perception, demonstrates the plasticity of brain processes underlying vision. Early studies, using the backward-masked texture discrimination task (TDT), focused on the lack of generalizing learning to stimulus features, relating learning specificity to the selectivity of the brain networks involved in the visual task. Learning was found to be highly specific to the stimulus features, as expected from the processing selectivity found in early visual areas as well as to the task employed in training, pointing to top-down effects. More recent studies demonstrate the generalization of learning to untrained features under specifically designed training procedures. Here we suggest that transfer of learning takes place when the trained and untrained stimuli and task activate overlapping brain processes. We tested the effect of TDT learning, under conditions with and without visual adaptation, on the contrast detection (CD) of localized Gabor targets, either alone or backward masked (BM). At the TDT peripheral-target location, we found that the transfer of learning between TDT to CD and BM occurs under the TDT adaptation condition, but not under the no-adaptation condition, whereas at the TDT center-target location we found that transfer occurs for both conditions. Our results suggest that learning generalization across experimental conditions depends on overlapping neural processes within brain networks, here dominated by the inhibitory effects involved in adaptation and in spatiotemporal masking. Importantly, increased adaptation during training, due to increased stimulus consistency, enabled the transfer of learning to other tasks limited by sensory adaptation.
Keywords: Perceptual learning, Texture discrimination, TDT, Backward masking, Contrast detection, Collinear facilitation, Lateral masking
Subject terms: Neuroscience, Learning and memory, Visual system
Introduction
Perceptual learning has been established as a method for improving visual perception and processing. Early studies1–3, using the backward-masked (BM) texture discrimination task (TDT), focused on the specificity of learning to the trained stimulus. It was shown that in standard TDT training, learning is specific to the trained target’s retinal location, suggesting that visual learning occurs at a lower level of the visual cortex4. Surprisingly, recent TDT studies indicate that under some conditions, learning is generalized, and that performance gains at one learning location are transferred to another location5–7. A major condition that affects the transfer is repetition of trials8. In TDT, repetition of the same visual task leads to decreased performance within a training session due to adaptation9. In Harris et al.10–12 the experiments include two training conditions: the standard condition: all trials of the experiment include the standard stimuli, and a dummy condition, where trials are conducted without the texture target, and only with the background texture (see Fig. 3) added to reduce adaptation to the target orientation. The “dummy” trials, without a peripheral target, but with texture elements diagonally oriented toward the target, prevent sensory adaption13, thus leading to better performance10. Additional research5,9,14,15 has tried to determine the connection between perceptual learning and adaptation, as well as which condition can prevent this adaptation11. Studies2,3 have shown sleep to play a critical role in TDT learning16,17. Censor and Sagi14 demonstrated that sleep may help to recover from extensive exposure to the trained stimuli, possibly via synaptic normalization8,15,18.
Figure 3.
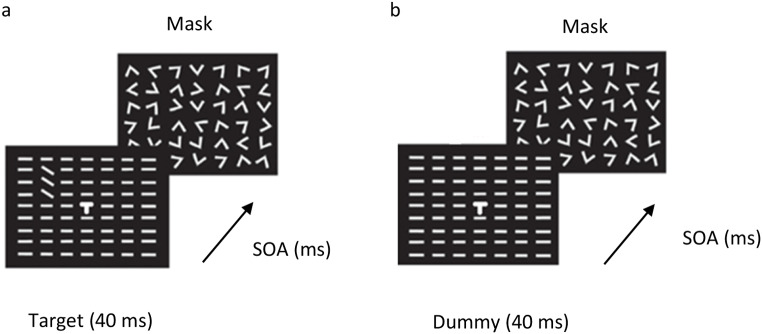
TDT: (a) Schematic illustration of the standard trials. The task is to discriminate between horizontal or vertical orientations of a peripheral target consisting of three diagonal lines surrounded by horizontal lines. After the target appears, a mask appears after a blank screen, known as the stimulus onset asynchrony (SOA), defined as the time from the appearance of the target until the mask appears. During the training, the SOA changes to form a psychometric curve used to estimate the threshold. (b) Schematic illustration of dummy trials. There is no texture target, and only the background stimuli appear. The dummy trials appear randomly between the test trials.
Plasticity of spatial interactions, suggested to underlie texture learning, was studied using localized contrast stimuli19,20. In these experiments, contrast-detection thresholds were measured for a foveally viewed, oriented Gabor target, when flanked by oriented high-contrast Gabor patches. Polat & Sagi20 showed that target visibility depends on the target-flankers’ spatial separations; thresholds increased (suppression) at small separations and decreased (facilitation) when flankers were separated from the target by three Gabor wavelengths (3λ). These effects were found to depend on the temporal delay between the target and the flankers21,22; when the flankers appear after the target (BM) with short stimulus onset asynchrony (SOA), the facilitation is replaced by suppression23(masking), whereas longer SOAs show no effect. It is assumed that masking is caused by excitatory and inhibitory interactions within neural networks responding to the visual stimuli. Thus, the masking effect is determined by the spatial and temporal parameters of these interactions24,25.
Later research has suggested that the predominant aspect of the backward masked TDT learning is location-specific temporal learning26. Accordingly, TDT training may be considered as a task that improves temporal resolution and processing speed27 at the target location. Indeed, Censor et al.28 using TDT training combined with EEG recording, showed that the temporal resolution of the target and mask-evoked potentials is correlated with TDT thresholds. Texture target- and mask-evoked potentials with lower SOA stimuli were initially integrated, but differentiable after training.
In this study, we used TDT training to explore how learning can be generalized to other visual functions. Based on the literature reviewed above, we assume that inhibitory processes triggered by pattern adaptation and spatial-temporal context (masking) reduce learning efficiency. Thus, visual texture learning is limited by visual processes that are not specific to texture stimuli, possibly also involved in backward masking (BM). More specifically, we suggest that the improved temporal resolution reported in previous studies reduces temporal masking, including BM, by reducing inhibitory interactions within networks of early vision. We addressed this issue by testing the effect of the TDT training using different adaptation levels, on contrast detection (CD) and on backward masking (BM). In the experiments described next, there were two adaptation levels in TDT training, taking advantage of the earlier studies showing reduced adaptation in TDT with interleaved dummy trials10–12.
Methods
Participants
Thirty-two participants with normal or corrected-to-normal vision participated in the experiments. The age of the participants was between 18 and 30 years (23.6 ± 2.9, average ± STD).
All participants passed a full optometric eye exam and had fully corrected vision when required. The participants were divided into 5 experimental groups (see Table 1).
Table 1.
Study groups.
| Group number | Number of participants | Training type | Pre/Post Tasks |
|---|---|---|---|
| 1 | 5 | Dummy | No |
| 2 | 5 | Dummy | BM & CD |
| 3 | 7 | Standard | No |
| 4 | 6 | Standard | BM & CD |
| 5 | 9 | No training (control) |
BM (9 participants) CD (6 participants) |
The study protocol was approved by the Internal Review Board (IRB) of Bar-Ilan University.
Informed consent was obtained from all participants.
All experimental protocols were performed following the guidelines provided by the committee approving the experiments.
The participants received financial compensation for their participation. Participants were recruited using electronic advertisements and direct recruitment.
Apparatus
The stimuli were presented at a viewing distance of 100 cm, via a windows-PC computer on a EIZO 24-inch FHD with a 100 Hz refresh rate, using custom software for the psychophysical experiments (PSY) developed by Yoram S. Bonneh (2004). The screen resolution was 1920 × 1080 pixels and subtended a visual angle of 29° × 17° (~ 65 pixels/degree); gamma correction was applied. The mean monitor screen display luminance was 40 cd/m2 when using contrast detection and BM (Gabor patches). The black screen in the TDT training was 0.3 cd/m2. All experiments were conducted in a dark environment: the only ambient light came from the monitor. Participants responded using the keyboard spacebar or the computer mouse.
Stimuli and task
All experiments and training were performed under binocular conditions.
Two of the five groups completed pre- and post-tests with training in between (dummy or standard training). The control group, however, completed the pre- and post-tests without any training:
Pre/post-testing 1: Backward masking
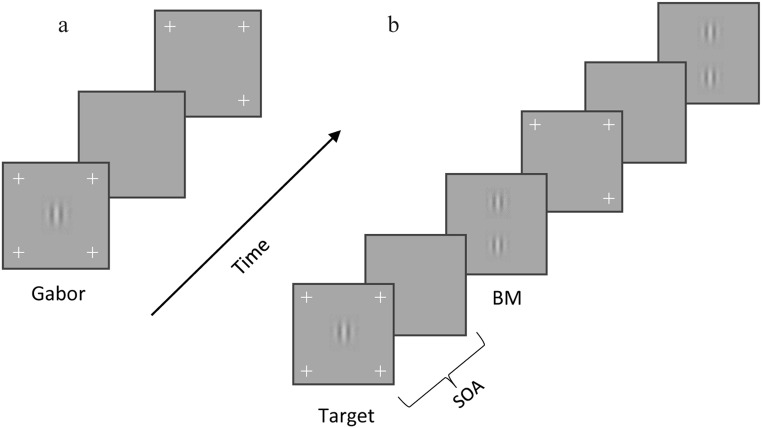
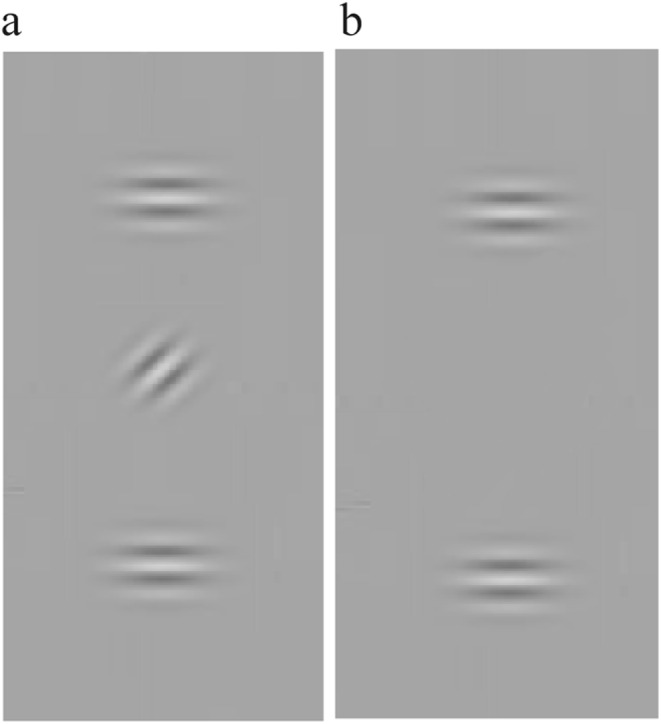
The test consists of 2 parts: The first one required measuring the contrast detection of a Gabor target at the screen center (the fixation point). The stimulus was a vertically oriented Gabor patch (GPs, Fig. 1a), 4.6 cpd, 40ms duration. The Gabor contrast threshold was measured using an adaptive method (staircase). The target contrast increased by 0.1 log unit after one error and decreased by 0.1 log unit following three consecutive correct responses (3-down 1-up using the adaptive staircase procedure). In the second part, the target presentation was followed by two high-contrast (60%) collinear flankers (backward masking, BM, Fig. 1b) with 4λ target-flanker separation and 40ms duration. The SOA, defined as the time between the onsets of the target and flankers, was 80, 120,160,180, or 240 milliseconds (ms). There were 50 trials for each SOA, totaling 300 trials, including the no-mask baseline test.
Figure 1.
Pre/post-testing 1- backward masking (BM): (a) The first part of the experiment. A Gabor patch appears at the center. The procedure involves the two-alternative contrast detection forced-choice method. The participant determines in which interval the Gabor appears. (b) The second part of the experiment. Backward masking: the target appears before two collinear flankers with a different SOA (80, 120, 160, 200, and 240 ms). The participant’s task is to determine in which interval the target appears.
The two-temporal alternative forced-choice (2tAFC) method was employed, with the two intervals, 240ms each, separated by 800ms. The participant’s task was to report the interval at which the target appeared. An auditory signal indicated an incorrect response.
Pre/Post-testing 2: contrast detection at the center and periphery
The target consisted of a Gabor patch oriented at 45°, the same as the target in the TDT training. At 7λ, above and below the target, two horizontal high-contrast (90%) flankers were presented (Fig. 2) to reduce the spatial and temporal uncertainty of the target. The target appeared at one of three locations (randomized across trials); at the center of the screen (fixation point) or in the upper left quadrant at 4° from the fixation (the NW direction, the location of the texture target in TDT), or 4° away, in the lower right quadrant (the SE direction). The chosen location, upper left and lower right, are symmetric and have equal sensitivity29. The participants were asked to keep fixating at the center during each trial. The contrast amplitude of the target was 6, 8, or 12% at the center and 16, 24, or 32% at the periphery. The target’s contrast (3 levels) and spatial location (2 levels) were randomized across trials. The stimuli were presented for 90ms, 20 trials/condition, totaling 120 trials. A Yes/No paradigm was used to test different randomly presented locations without prior cues. The hit and false alarm rates were used to calculate the sensitivity (d’) for each condition.
Figure 2.
Pre/post-testing 2-contrast detection at the center vs. the periphery: A yes–no paradigm. The stimuli consist of a slant (45 degrees) Gabor patch target, whereas above and below the Gabor there are 2 constant orthogonal high-contrast (90%) flankers. The target and the orthogonal flankers are separated by 7λ. The stimuli appear randomly at 3 locations (mixed-by-trial): center, 4 degrees upper left or 4 degrees lower right, while the participant continuously looks at the center. The contrast amplitude of the target is changed randomly to 6, 8, and 12% at the center and 16, 24, and 32% at the periphery. (a) An example of central target appearances. (b) An example of central target disappearances.
In both experiments, the parameters and the experimental conditions were chosen after dedicated pilot trials, with parametrization aided by previous studies.
TDT
TDT training
Stimuli
The TDT stimulus (Fig. 3a) was based on and was nearly identical to the original one used by Karni and Sagi (1991). In both standard (adaptation) and dummy (reduced- adaptation) conditions, there was a 3 × 1 diagonal bar target array embedded in the background (a 19 × 19 array of horizontal short lines, 0.5°×0.035°, spaced 0.72° apart, and with 0.05° jitter), which appeared at 4° from fixation, in the upper left quadrant (NW). The central target was the letter “L” or “T”. The stimulus duration was 40ms. A mask of 100ms, composed of randomly oriented ˅ patterns, appeared after a blank interval. Performance was measured as a function of the SOA interval (Stimulus Onset Asynchrony: 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, 260, 300, 400, 500, 600, 700, and 800ms). The SOA was randomized across trials. In the dummy condition, 50% of the trials consisted of background texture only, without the target (i.e., ‘dummy’ trials; see Fig. 3b). Dummy trials were randomly interleaved with the regular trials. There were 18 target trials per SOA, with 18 dummy trials per SOA added in the dummy condition. Overall, there were 306 trials in the standard condition, and 612 trials in the dummy condition.
Tasks
The participants performed two tasks (“double tasks”) on the same stimulus. The first task: In the center there is an “L” or “T” target; the aim of the central target is to keep the participant fixated on the center of the screen during the task. The participant’s task was to report the letter seen. The second task: The participant reported whether the three diagonal bars were arranged horizontally or vertically.
Responses were provided by two key pressings: the first one was for the “L” or “T” task (left click “L”, right click “T”); the second response was for the peripheral target (left click “horizontal”, right click “vertical”). Auditory feedback indicated an incorrect response for the fixation (T/L) task only (no feedback was provided for the peripheral horizontal/vertical texture task).
Before training, the participants conducted a practice session. The aim of this part was to familiarize participants with the experimental paradigm. The practice phase was like the actual training, but it gradually increased in difficulty. There are three stages: first, the participant performed the double tasks of the T/L task and the V/H task without masks. The number of trials was adjusted for each participant until it reached 100% correct in both tasks (not exceeding ~ 30 trials). In the next stage, there were 10 additional trials in which the mask stimulus was presented with a high SOA (800 ms). For dummy trials, participants performed as in the standard trials: a T/L response and a V/H response, guessing the second response in the absence of a detected texture target. The third stage was very similar to stage two, but with faster SOA trials added (40–800 ms, as in the full training). In both last stages, the participant had to reach 95% correct on both tasks, before starting the training.
The participants performed the training consisting of four sessions separated by 2–10 days.
Data and statistical analysis
One-way, Two-way, and Three-way mixed ANOVA were performed to test the effect of 1, 2, or 3 nominal variables (such as group, pre/post-test, or session) on continuous outcomes. Specifically, linear mixed-effect models were performed, and ANOVA was performed on the resulting models. Post hoc analysis was performed as pairwise comparisons defined by linear contrasts. P-values were adjusted for multiple testing using the Benjamini Hochberg False Discovery rate (FDR) procedure. The normality of residuals and the homogeneity of variance assumptions were assessed graphically using diagnostic plots. P values less than 0.05 were considered statistically significant. The normality of residuals and the homogeneity of the variance assumptions were assessed graphically using diagnostic plots.
Results
TDT training
First, we compared learning curves obtained under the standard (adaptation) condition and under the dummy (reduced-adaptation) condition; the results are presented in Fig. 4. The group average daily thresholds show the typical improvements between days, along with some typical scattering of the participants’ data, in agreement with previous studies. The results demonstrate the effectiveness of the reduced-adaptation condition, showing reduced thresholds when dummy trials are added (Fig. 4, blue curves vs. orange curves), though with similar learning rates (as in Harris and Sagi, 2015).
Figure 4.
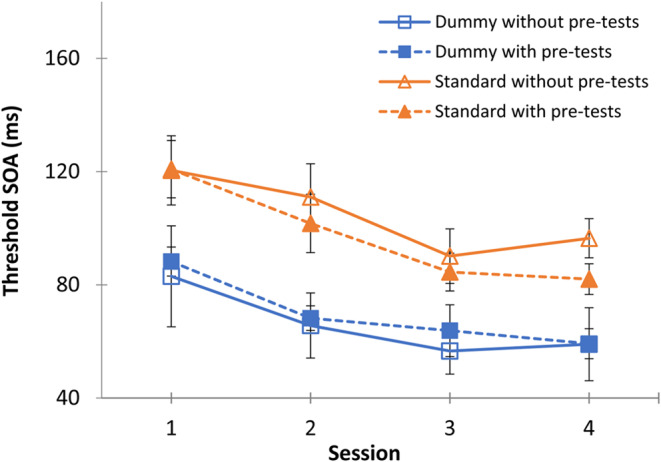
Comparison of the average daily threshold between standard and dummy training with and without pre-tests. The blue and orange dashed line represents the group learning curves for dummy (n = 5) and standard (n = 6) training with pre-tests training, respectively. The blue and orange continuous lines represent the group learning curves for dummy (n = 5) and standard (n = 7) training without pre-tests training, respectively. Error bars represent the standard error.
The learning curves under the dummy condition are significantly lower than under the standard condition, suggesting that less adaptation took place. A three-way ANOVA was performed to test the training, pre/post-test, and sessions. There was no significant three-way interaction (F (3,57) = 0.17, p = 0.91). However, there was a significant effect of the training type that revealed a significant difference between the ‘dummy’ and the ‘standard’ groups during all 4 days of training (F (1,19) = 15.57, p = 0.0009), without any significant interactions between the conditions or pre-tests. In addition, we observed a significant improvement between the sessions (F (3,57) = 21.55, p < 0.0005).
Next, we determined whether the learning curves are affected by the pre-tests (BM and CD). A three-way ANOVA analysis was performed to test the effect of groups. The results indicated that under both conditions, dummy and standard, there was no significant difference between groups that either performed and did not perform the pre-testing of BM and CD (mean ± SE, day 1, day 2, day 3, day 4; standard: without pre-tasks: 120.42 ± 12.22; 111 ± 11.73; 90.14 ± 9.6; 96.42 ± 6.91; with pre-tasks: 120.83 ± 10.13; 101.66 ± 10.32; 84.5 ± 6.67; 82 ± 5.42; dummy: without pre-tasks: 83 ± 17.84; 65.6 ± 11.51; 56.6 ± 8.15; 59 ± 12.88; with pre-tasks: 88.2 ± 5.15; 68.2; 63.8 ± 9.14; 59.2 ± 5.28; the interaction between the pre-test and sessions, (F(3,57) = 0.51, p = 0.67)).
Backward masking
A two-way ANOVA was performed to determine whether training affects the performance of the BM and CD tests. The pre-test BM thresholds of all training groups (dummy, standard, and control) were similar; the differences did not reach statistical significance (the interaction between training type and SOA, F (8,68) = 0.44; p = 0.88). In addition, each group showed a significant reduction of detection thresholds with increasing SOA (SOA main effect F (4,68) = 29.27; p < 0.0005).
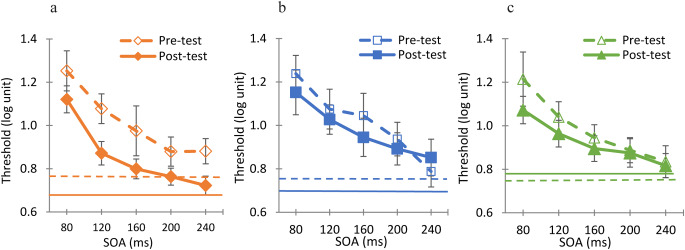
Next, a three-way ANOVA was performed to test the effect of TDT training on the BM thresholds (pre- vs. post-test) under the two training conditions: dummy and standard. We compared the results to the results of the control group that participated in the pre- and post-test sessions without any training in between. The results are presented in Fig. 5, showing a large and significant improvement in thresholds for the standard training group (Fig. 5a), but only small effects and non-significant for the dummy training (Fig. 5b) and the control groups (Fig. 5c) (F (2,153) = 3.89, p = 0.02).
Figure 5.
Effects of TDT training on BM, 3 groups: (a) standard (n = 6), (b) dummy (n = 5), and (c) control (n = 9). The mean group results are shown pre-test (dashed curves) and post-test (continuous curves) training. The horizontal lines represent the contrast detection threshold in the absence of masks. Error bars represent the standard error. A large improvement occurred after standard TDT training, whereas other groups showed only moderate improvements.
In addition, a three-way ANOVA was performed to compare the no-mask contrast detection thresholds between the groups pre- and post-test. There was no significant interaction (F (10,143) = 0.6, p = 0.81). The contrast detection threshold after standard training decreased by 16% (mean ± S.E.; n = 6: pre-test 0.78 ± 0.04 ms; post-test 0.65 ± 0.01 ms, post hoc analysis, p = 0.21). After dummy training there was a smaller improvement, 9% (mean ± S.E.; n = 5: pre-test 0.77 ± 0.01 ms; post-test 0.70 ± 0.06 ms, post hoc analysis, p = 0.66), whereas the control group thresholds showed a small (4%) reduction in threshold (mean ± S.E; n = 9: pre-test 0.75 ± 0.05 ms; post-test 0.78 ± 0.04 ms, post hoc analysis, p = 0.37). None of the groups showed a significant improvement.
These results suggest improved temporal resolution, implying a faster processing speed, after TDT training in the standard group, manifested by the larger reduction of the masking effect at the short SOAs (the 80-120ms slope).
Next, we tested the effect of the location specificity of the TDT training on CD.
Contrast detection at three locations
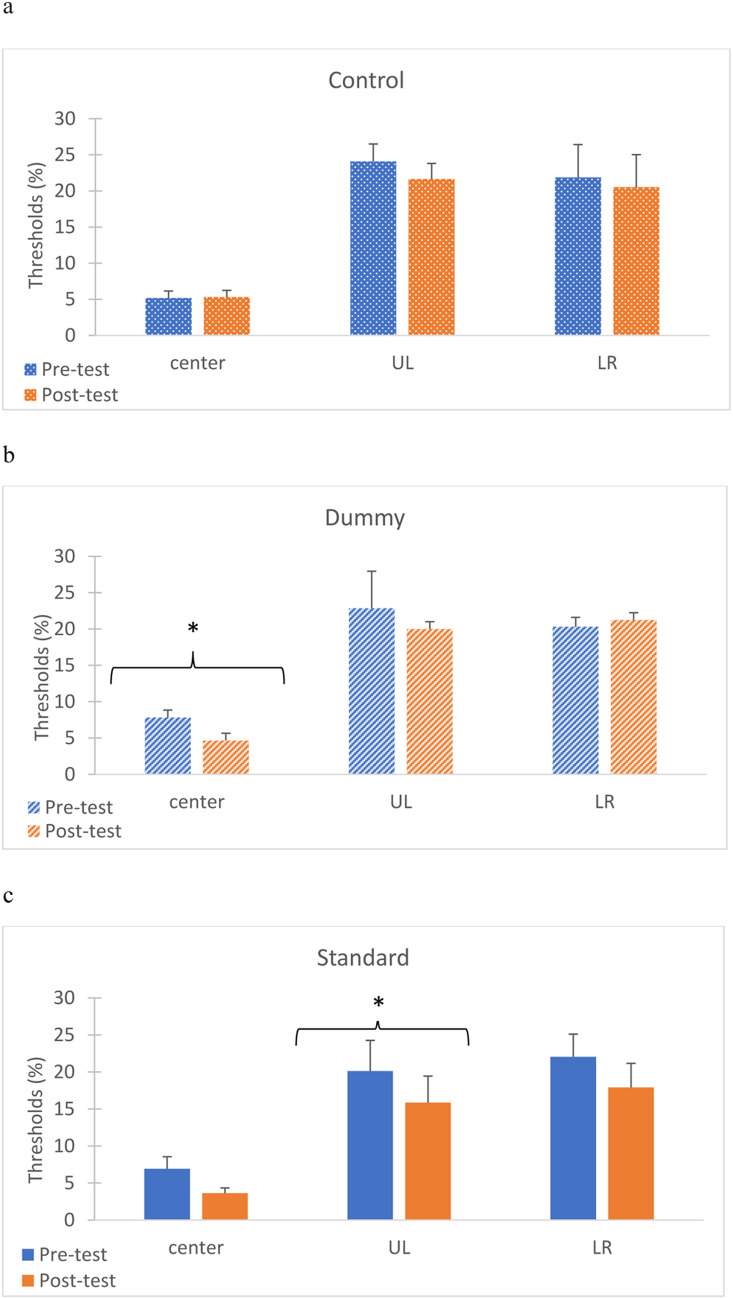
In TDT training the observers are presented with stimuli consisting of a large background texture, a small, localized texture target, and a foveal fixation target (see Fig. 3); thus, there are three stimulus regions and two tasks. To determine the main factors in TDT training affecting the transfer to CD, we measured the contrast sensitivity at 3 locations: the stimulus center (at the fovea), in the upper left (UL) quadrant (the texture-target location as in the TDT training), and in the lower right (LR) stimulus quadrant (no target in the TDT training but with the same eccentricity). We tested contrast sensitivity pre- and post-standard and dummy TDT training. In addition, we added a control group to test the sensitivity changes without TDT training. The sensitivity at 3 contrast levels was quantified using d’= z(hit) - z(fa). We used d’=1 to calculate the contrast threshold level. d’=1 is commonly used to define the threshold level24,30. Psychometric functions tend to be linear at around d’=131. Figure 6 presents the group averages of the contrast detection thresholds, defined as the contrast level at which d’=1; they were determined through linear interpolation between the two contrast levels above and below the threshold. In the control group that performed the tasks without training, there was no significant difference in any of the locations when comparing the two times that the experiment was conducted (paired t-test: center, p = 0.9; UL, p = 0.27; LR, p = 0.77). In the dummy group, there was a significant improvement after TDT training, but only at the center location (paired t-test: center, p = 0.03; UL, p = 0.3; LR, p = 0.79). In the standard group there was a significant improvement post-TDT training at the UL location, the location where the targets were presented during training, but no improvement at the center and LR locations (paired t-test: center, p = 0.09; UL, p = 0.036; LR, p = 0.3). Interestingly, both the standard and the dummy methods show similar threshold reductions in the central task (48% and 40%, respectively, compared with − 3% in the control condition); however, only the improvement in the dummy condition was statistically significant. This difference can possibly be explained by the difference in the number of tested T/L trials (central task); it is doubled in the dummy condition (both conditions had the same number of trials with the texture target, see the Methods).
Figure 6.
Comparison of the contrast detection thresholds at three different locations: center, Upper Left (UL), at the texture target location, and Lower Right (LR) in three different TDT training groups: (a) Control, no TDT, group (n = 6), (b) Dummy TDT (reduced-adaptation) group (n = 5), and (c) Standard TDT (adaptation) group (n = 6). Shown are detection thresholds (% contrast) pre-test (blue bars) and post-test (orange bars) training without TDT training (a), and with TDT (b, c). One asterisk denotes the significance (p < 0.05) of the average improvement in the pre- and post-test after dummy training at the center location, and after standard training at the UL location. Error bars represent the standard error.
Discussion
The aim of our study was to explore the effects of experimental conditions on perceptual learning by TDT training. We wanted to test the generalization of learning to other visual functions such as backward masking, and contrast detection. As observed in previous studies24,30,32, training in BM enhances the ability to visually perceive the stimulus under BM when using the same stimuli and task. Here, we tested the transfer between different types of BM paradigms by employing different stimuli and tasks.
Our results suggest that learning transfers between experimental conditions and tasks, depending on the experimental conditions, which, in our study, are mainly affected by the adaptation level. Increased adaptation, due to stimulus consistency, affects the transfer of learning to BM; this is possibly due to the inhibitory processes that are reduced by training. Based on earlier studies putting forward the idea that adaptation is implemented by long-term inhibition33, we suggest that by decreasing inhibition, we diminish adaptation, thus leading to improved performance. In our experiments, the adaptation level varied between the “standard” and “dummy” training methods, with adaptation reduced in the latter by inserting targetless stimuli. Consequently, the results show the transfer of TDT learning in the standard condition, but not in the dummy condition, to tasks that are limited by inhibitory processes, such as BM.
A critical determining factor in learning specificity is stimulus consistency. Harris et al. (2012)5, by showing constraints on stimulus variability and allowing for the generalization of learning, suggested a link between learning specificity and visual adaptation. Taken together with the above-mentioned suggestion linking adaptation and inhibition, we suggest that the standard TDT performance is limited by inhibition before training but less so after training; thus, there is generalization to BM.
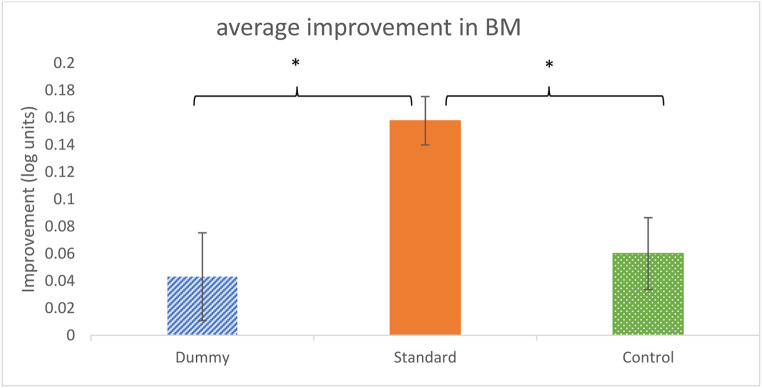
Importantly, we found that training with the backward masked TDT paradigm can improve performance on the classical backward masked contrast-detection task (BM). However, this transfer of learning occurs only with the ‘standard’ TDT paradigm, thought to be limited by temporal inhibition, such as in sensory adaptation. We suggest that the training-induced BM improvement results from reduced temporal inhibition. Thus, any visual function that is limited by temporal inhibition can potentially benefit from such training. Reduced temporal inhibition improves the rate at which visual information accumulates. Figure 7 summarizes the average threshold’s improvement (the post-test minus the pre-test) of all SOAs under BM, for each group session (one-way anova, F (2,12) = 6.99; p = 0.01). The main boost in processing the target’s thresholds occurs after standard training with the higher levels of adaptation: 43% improvement, compared with the dummy (reduced-adaptation group), with only 10% improvement (post hoc analysis, p = 0.01), or compared with the control group with 15% improvement (post hoc analysis, p = 0.03). Similarly, we investigated the variation in thresholds for non-BM conditions. Conversely, when comparing groups, there was a significant effect (one way ANOVA, F (2,17) = 4.3; p = 0.03). However, in post-hoc tests, no significant difference was found, which means that the effect was not strong enough. Thus, this selective effect cannot be explained by a general improvement in sensitivity (reduced thresholds) but instead by a reduced masking effect (BM).
Figure 7.
Improvement in the BM contrast detection thresholds (log units) in BM experiments after TDT training. Each bar represents the mean group threshold improvement (in log units) averaged across the tested BM SOAs. One asterisk indicates the significance (P < 0.05) of the average improvement pre- and post-test standard training, compared with the dummy and control groups. Error bars represent the standard error.
To summarize, learning generalization across experimental conditions largely depends on overlapping neural processes within brain networks. Here we suggest a reduction in slow inhibitory activity within the network that processes the trained stimuli to account for the results. By decreasing inhibition, we diminish adaptation, leading to improved performance. The modified inhibitory interactions may be specific to the mask stimulus34,35, although here the TDT mask practically covers all orientations. Enhanced contrast perception without masking can possibly be explained by the reduced inhibitory effects, combined with a more general, paradigm-independent training effect. Currently, the precise mechanisms underlying this training effect are unknown; however, we expect to find them at lower stages of visual processing, allowing transfer to other visual functions.
Author contributions
R.K.H. conducted the experiment, analyzed the data, and wrote the main manuscript; M.L., U.P and D.S. designed the experiment, reviewed the data, and edited the manuscript. All authors reviewed the manuscript. This work was written as part of the R.K.H’s Ph.D. studies at the School of Optometry and Vision Sciences, Bar-Ilan University, Ramat Gan, Israel.
Funding
Funding was provided by the Israel Science Foundation (ISF, Grant No. 2494/21).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.karni, A. & Sagi, D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. PNAS88, 4966–4970 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karni, A. & Sagi, D. The time course of learning a visual skill. Nature. 365, 250–252 (1993). [DOI] [PubMed] [Google Scholar]
- 3.Karni, A., Tanne, D., Rubenstein, B. S., Askenasy, J. J. M. & Sagi, D. Dependence on REM sleep of overnight improvement of a perceptual skill. Science.265, 679–682 (1994). [DOI] [PubMed]
- 4.Sagi, D. Perceptual learning in vision research. Vis. Res.51, 1552–1566 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Harris, H., Gliksberg, M. & Sagi, D. Generalized perceptual learning in the absence of sensory adaptation. Curr. Biol.22, 1813–1817 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Zhang, T., Xiao, L., Klein, S. A., Levi, D. M. & Yu, C. Decoupling location specificity from perceptual learning of orientation discrimination time. Vis. Res.50, 368–374 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Zhang, J. et al. Rule-based learning explains visual perceptual learning and its specificity and transfer. J. Neurosci.30, 12323–12328 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Censor, N. & Sagi, D. Benefits of efficient consolidation: short training enables long-term resistance to perceptual adaptation induced by intensive testing. Vis. Res.48, 970–977 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Ofen, N., Moran, A. & Sagi, D. Effects of trial repetition in texture discrimination. Vis. Res.47, 1094–1102 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Harris, H. & Sagi, D. Effects of spatiotemporal consistencies on visual learning dynamics and transfer. Vis. Res.109, 77–86 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Harris, H. & Sagi, D. Visual learning with reduced adaptation is eccentricity-specific. Sci. Rep.8, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris, H. et al. Perceptual learning in autism: over-specificity and possible remedies. Nat. Neurosci.18, 1–4 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Greenlee, M. W. & Magnussen, S. Interactions among spatial frequency and orientation channels adapted concurrently. Vis. Res.28, 1303–1310 (1988). [DOI] [PubMed] [Google Scholar]
- 14.Censor, N. & Sagi, D. Global resistance to local perceptual adaptation in texture discrimination. Vis. Res.49, 2550–2556 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Censor, N., Karni, A. & Sagi, D. A link between perceptual learning, adaptation and sleep. Vis. Res.46, 4071–4074 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Mednick, S., Nakayama, K. & Stickgold, R. Sleep-dependent learning: a nap is as good as a night. Nat. Neurosci.6, 697–698 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Mednick, S. C. et al. The restorative effect of naps on perceptual deterioration. Nat. Neurosci.5, 677–681 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Seitz, A. R. et al. Perceptual learning: policy insights from basic research to real-world applications. Policy Insights Behav. Brain Sci.10, 324–332 (2023). [Google Scholar]
- 19.Polat, U. Making perceptual learning practical to improve visual functions. Vis. Res.49, 2566–2573 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Polat, U. & Sagi, D. Lateral interactions between spatial channels: suppression and facilitation revealed by lateral masking experiments. Vis. Res.33, 993–999 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Polat, U., Sterkin, A. & Yehezkel, O. Spatio-temporal low-level neural networks account for visual masking. Adv. Cogn. Psychol.3, 153–165 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, R., Polat, U., Scalzo, F. & Bavelier, D. Reducing backward masking through action game training. J. Vis.10, 1–13 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Sterkin, A., Yehezkel, O., Bonneh, Y. S., Norcia, A. & Polat, U. Backward masking suppresses collinear facilitation in the visual cortex. Vis. Res.49, 1784–1794 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Lev, M. & Polat, U. Space and time in masking and crowding. J. Vis.15, 1–25 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Polat, U. & Sagi, D. Temporal asymmetry of collinear lateral interactions. Vis. Res.46, 953–960 (2006). [DOI] [PubMed] [Google Scholar]
- 26.Wang, R. & Cong, L. The classical TDT perceptual learning is mostly temporal learning. J. Vis.13, 1–9 (2013). [DOI] [PubMed]
- 27.Sterkin, A., Yehezkel, O. & Polat, U. Learning to be fast: Gain accuracy with speed. Vis. Res.61, 115–124 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Censor, N., Bonneh, Y., Arieli, A. & Sagi, D. Early-vision brain responses which predict human visual segmentation and learning. J. Vis.9, 12–12 (2009). [DOI] [PubMed]
- 29.Carrasco, M., Giordano, A. M. & McElree, B. Temporal performance fields: visual and attentional factors. Vis. Res.44, 1351–1365 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Schwiedrzik, C. M., Singer, W. & Melloni, L. Subjective and objective learning effects dissociate in space and in time. Proc. Natl. Acad. Sci.108, 4506–4511 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green, D. M. & Swets, J. A. Signal detection theory and psychophysics (Wiley, New York, 1966).
- 32.Lev, M. et al. Training improves visual processing speed and generalizes to untrained functions. Sci. Rep.4, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dealy, R. S. & Tolhurst, D. J. Is spatial adaptation an after-effect of prolonged inhibition? J. Physiol.241, 261–270 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schubo, A., Schlaghecken, F. & Meinecke, C. Learning to ignore the Mask in texture segmentation tasks. J. Exp. Psychol. Hum. Percept. Perform.27, 919–931 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Wolford, G., Marchak, F. & Hughes, H. Practice effects in Backward Masking. J. Exp. Psychol. Hum. Percept. Perform.14, 101–112 (1988). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.