Abstract
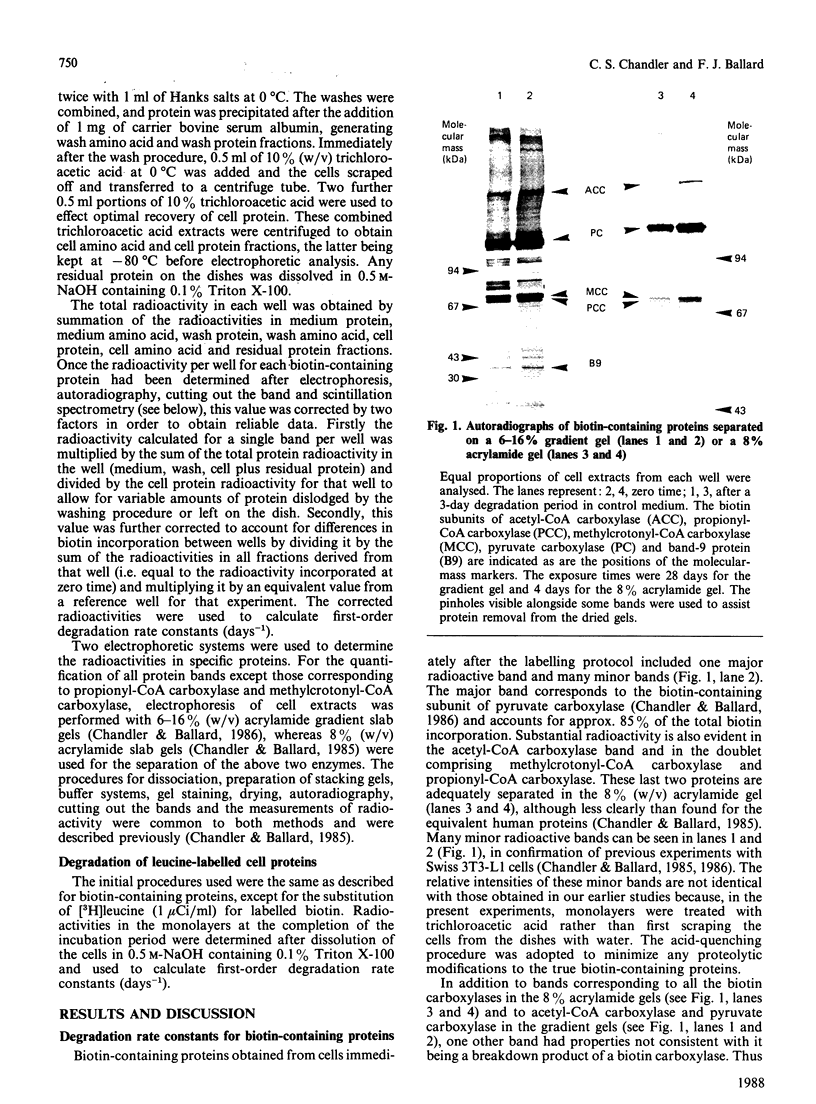
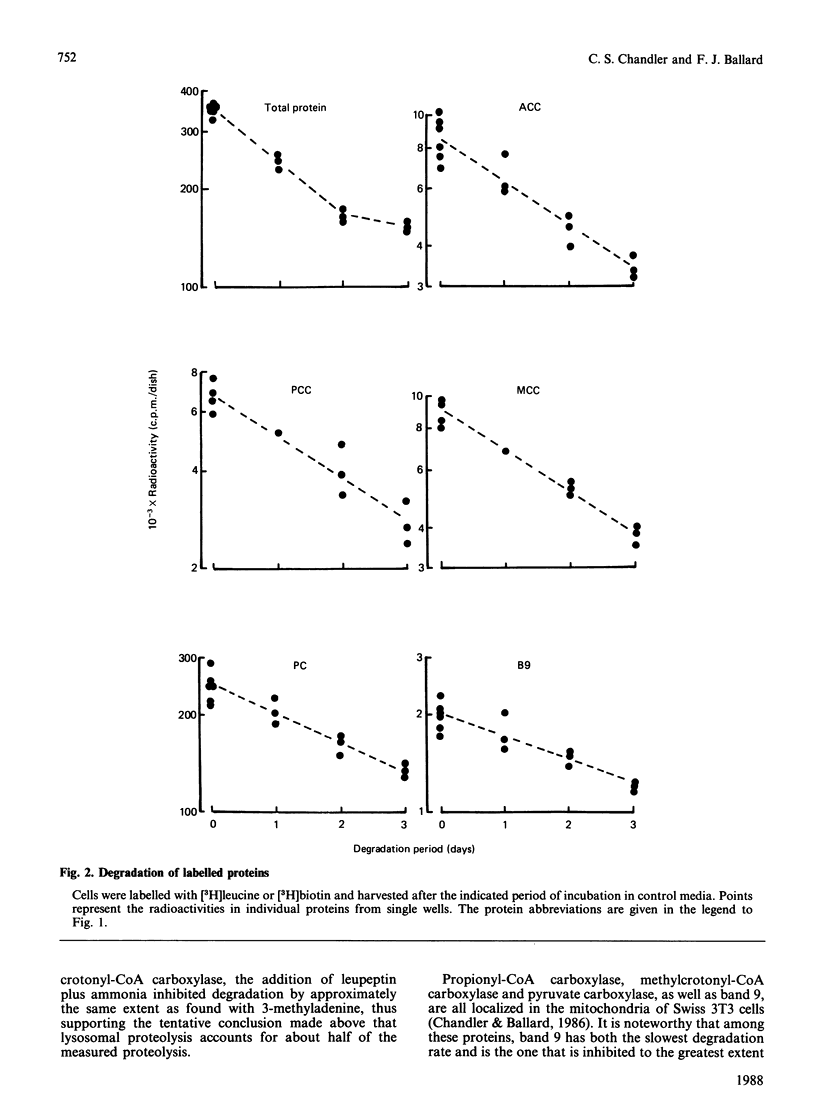
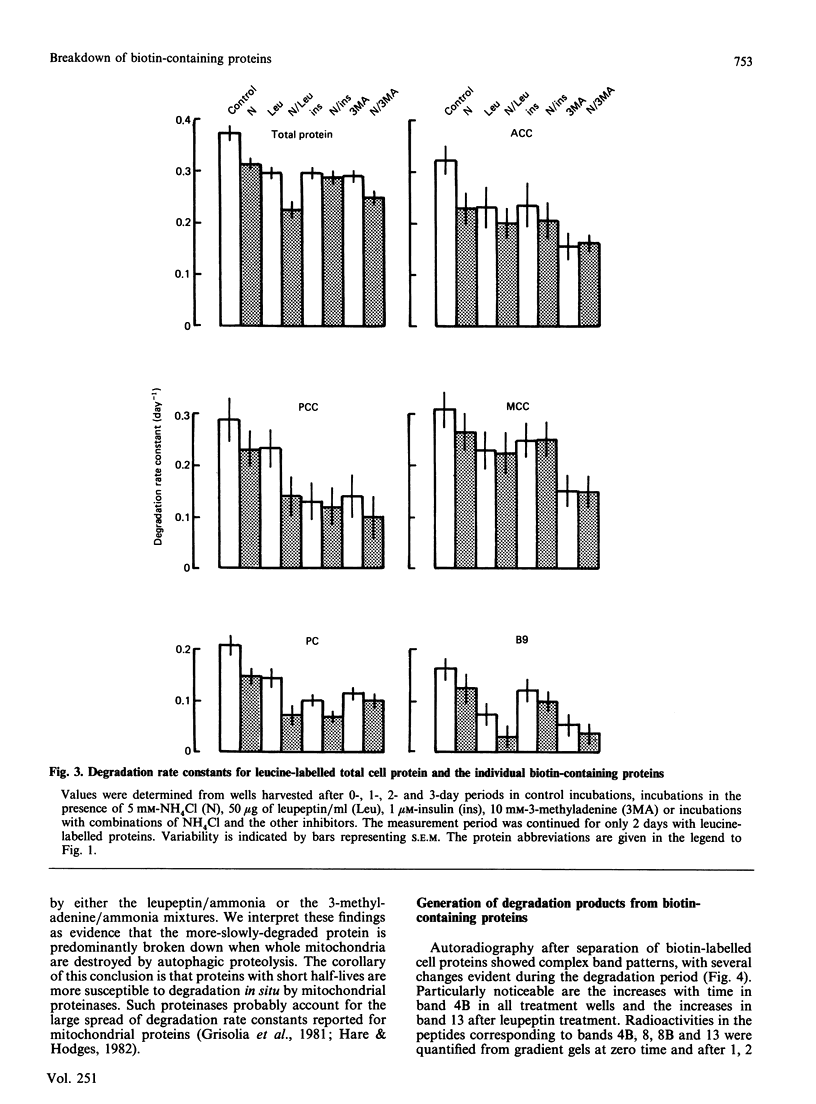
1. Degradation rate constants for individual biotin-labelled proteins were measured in Swiss 3T3-L1 adipocytes that had been incubated with inhibitors of autophagy or of lysosomal proteolysis. 2. Inhibitory effects produced by 10 mM-3-methyladenine and a combination of 5 mM-NH4Cl and leupeptin (50 micrograms/ml) were approximately equal. The inclusion of NH4Cl did not significantly enhance the responses to 3-methyladenine, suggesting that autophagy was already maximally inhibited. 3. The extent of inhibition by 3-methyladenine or by the NH4Cl/leupeptin mixture was similar for the cytosolic enzyme acetyl-CoA carboxylase and for the three mitochondrial carboxylases. This inhibition averaged 50%. The breakdown rate of a more-stable 38 kDa biotin-containing mitochondrial protein was more responsive to the inhibitory agents. These results are best explained by mitochondrial proteolysis occurring via a combination of the degradation of whole mitochondria within autophagic vacuoles, supplemented by the selective intramitochondrial breakdown of more labile proteins. 4. A number of intermediate products in the degradation of biotin-containing proteins were detected. Differences in the patterns of radioactivity between these peptides after incubation of cells in the presence of inhibitors of the breakdown process provided evidence that some peptides were produced before autophagy, others as a result of intralysosomal inhibition, while at least one was associated with intramitochondrial proteolysis.
Full text
PDF

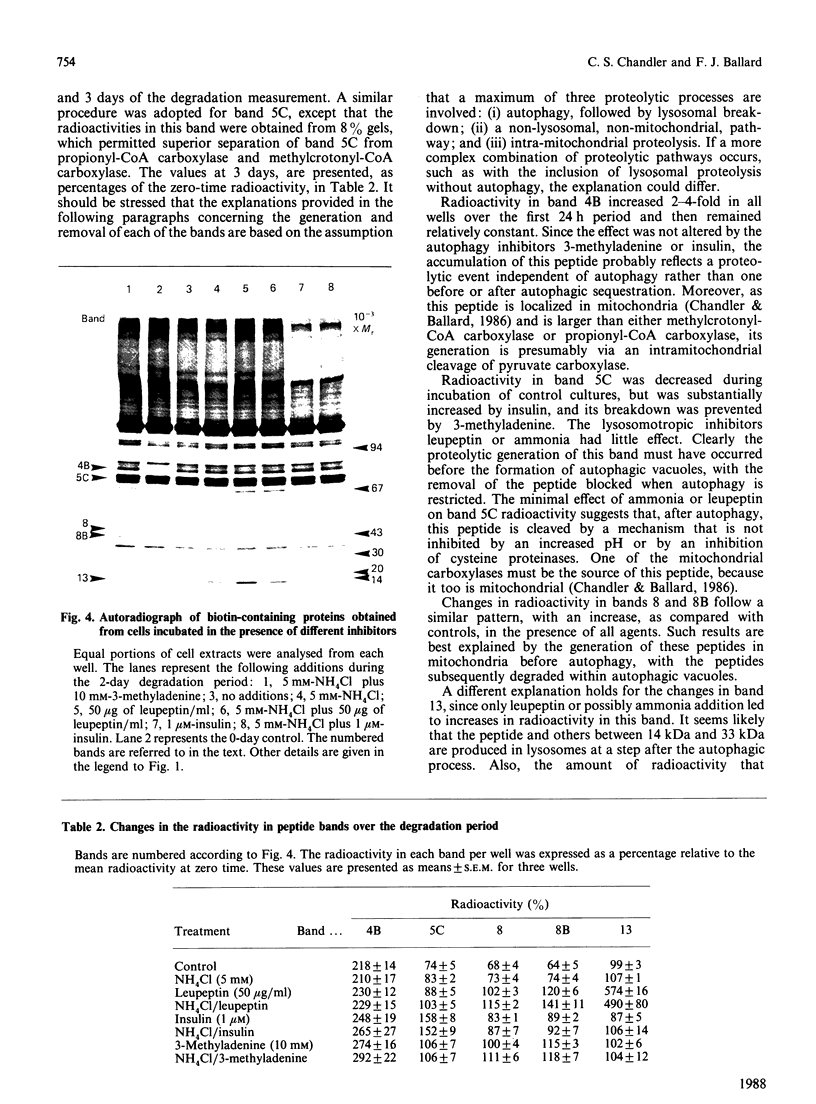

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Bourret L., Dice J. F. Regulation of catabolism of microinjected ribonuclease A requires the amino-terminal 20 amino acids. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2166–2170. doi: 10.1073/pnas.80.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard F. J., Wong S. S., Knowles S. E., Partridge N. C., Martin T. J., Wood C. M., Gunn J. M. Insulin inhibition of protein degradation in cell monolayers. J Cell Physiol. 1980 Nov;105(2):335–346. doi: 10.1002/jcp.1041050216. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Fairhurst D., Cookson E. J. Turnover of skeletal muscle glycogen phosphorylase. Biomed Biochim Acta. 1986;45(11-12):1619–1625. [PubMed] [Google Scholar]
- Bigelow S., Hough R., Rechsteiner M. The selective degradation of injected proteins occurs principally in the cytosol rather than in lysosomes. Cell. 1981 Jul;25(1):83–93. doi: 10.1016/0092-8674(81)90233-6. [DOI] [PubMed] [Google Scholar]
- Chandler C. S., Ballard F. J. Distribution and degradation of biotin-containing carboxylases in human cell lines. Biochem J. 1985 Dec 1;232(2):385–393. doi: 10.1042/bj2320385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C. S., Ballard F. J. Inhibition of pyruvate carboxylase degradation and total protein breakdown by lysosomotropic agents in 3T3-L1 cells. Biochem J. 1983 Mar 15;210(3):845–853. doi: 10.1042/bj2100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C. S., Ballard F. J. Multiple biotin-containing proteins in 3T3-L1 cells. Biochem J. 1986 Jul 1;237(1):123–130. doi: 10.1042/bj2370123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft D. V., Goss N. H., Chandramouli N., Wood H. G. Purification of biotinidase from human plasma and its activity on biotinyl peptides. Biochemistry. 1985 May 7;24(10):2471–2476. doi: 10.1021/bi00331a012. [DOI] [PubMed] [Google Scholar]
- Dice J. F., Chiang H. L., Spencer E. P., Backer J. M. Regulation of catabolism of microinjected ribonuclease A. Identification of residues 7-11 as the essential pentapeptide. J Biol Chem. 1986 May 25;261(15):6853–6859. [PubMed] [Google Scholar]
- Grisolía S., Timoneda J., Hernández-Yago J., Soler J., De Arriaga M. D., Wallace R. Intracellular degradation of mitochondrial enzymes. Acta Biol Med Ger. 1981;40(10-11):1407–1418. [PubMed] [Google Scholar]
- Hare J. F., Hodges R. Turnover of mitochondrial matrix polypeptides in hepatoma monolayer cultures. J Biol Chem. 1982 Nov 10;257(21):12950–12953. [PubMed] [Google Scholar]
- Hopgood M. F., Knowles S. E., Ballard F. J. Degradation of microinjected glycolytic enzymes in L6 myoblasts. Biomed Biochim Acta. 1986;45(11-12):1603–1610. [PubMed] [Google Scholar]
- KOIVUSALO M., ELORRIAGA C., KAZIRO Y., OCHOA S. Bacterial biotinidase. J Biol Chem. 1963 Mar;238:1038–1042. [PubMed] [Google Scholar]
- Katznelson R., Kulka R. G. Degradation of microinjected methylated and unmethylated proteins in hepatoma tissue culture cells. J Biol Chem. 1983 Aug 25;258(16):9597–9600. [PubMed] [Google Scholar]
- Kreis T. E., Winterhalter K. H., Birchmeier W. In vivo distribution and turnover of fluorescently labeled actin microinjected into human fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3814–3818. doi: 10.1073/pnas.76.8.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. J., Gaskell M. J., Earl R., Billett E. E., Mangiapane H. E., Fernig D., Doherty F. J. Intracellular protein catabolism: evidence for sequestration of proteins into an intermediate-filament fraction before lysosomal degradation. Biomed Biochim Acta. 1986;45(11-12):1591–1602. [PubMed] [Google Scholar]
- Reznick A. Z., Rosenfelder L., Shpund S., Gershon D. Identification of intracellular degradation intermediates of aldolase B by antiserum to the denatured enzyme. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6114–6118. doi: 10.1073/pnas.82.18.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. W., Rechsteiner M. C. Microinjection studies on selective protein degradation: relationships between stability, structure, and location. Biomed Biochim Acta. 1986;45(11-12):1611–1618. [PubMed] [Google Scholar]
- Rote K. V., Rechsteiner M. Degradation of microinjected proteins: effects of lysosomotropic agents and inhibitors of autophagy. J Cell Physiol. 1983 Jul;116(1):103–110. doi: 10.1002/jcp.1041160116. [DOI] [PubMed] [Google Scholar]
- Slot L. A., Lauridsen A. M., Hendil K. B. Intracellular protein degradation in serum-deprived human fibroblasts. Biochem J. 1986 Jul 15;237(2):491–498. doi: 10.1042/bj2370491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Ohashi M. Purification and identification of intermediate catabolic products in the in vivo degradation of pig liver phosphofructokinase. J Biol Chem. 1986 Sep 25;261(27):12455–12461. [PubMed] [Google Scholar]