Abstract
Background
The use of Mechanical Circulatory Support (MCS) devices in cardiogenic shock (CS) is growing. However, the recent trends in using different MCS modalities and their outcomes in acute myocardial infarction associated CS (AMI-CS) are unknown.
Methods
The national readmission database (2016–2020) was used to identify AMI-CS requiring MCS. Cohorts were stratified as ECMO compared to Impella. Propensity score matching (PSM) was used to remove confounding factors. Pearson's x2 test was applied to matched cohorts to compare outcomes. We used multivariate regression and reported predictive margins for adjusted trend analysis.
Results
Among 20,950 AMI-CS hospitalizations requiring MCS, 19,628 (93.7 %) received Impella vs 1322 (6.3 %) were placed only on ECMO. ECMO group was younger (median age: 61 vs. 68 years, p < 0.001) and had a lower comorbidity burden. On propensity-matched cohorts (N 742), the ECMO cohort had higher adverse events, including mortality (51.6 % vs. 41.5 %), sudden cardiac arrest (SCA) (40.9 % vs. 31.8 %), acute stroke (9.2 % vs. 4.6 %) and major bleeding (16 % vs 12.2 %) [p < 0.05]. However, comparing ECPELLA (ECMO + Impella) to Impella alone, mortality (46.2 % vs. 39.4 %) and SCA (44 % vs. 36.4 %) rates were similar, though major bleeding was higher (18.2 % vs. 9.8 %). From 2016 to 2020, mortality trends for AMI-CS in the U.S. showed no significant change (p-trend: 0.071).
Conclusion
Despite advances in MCS modalities, the overall mortality rate for AMI-CS remains unchanged. ECMO use without LV unloading showed higher mortality and adverse events compared to Impella. Prospective studies are needed to verify these findings.
Keywords: LVAD, ECMO, Cardiogenic Shock, Myocardial Infarction, Impella, Mechanical Circulatory Support
Graphical abstract
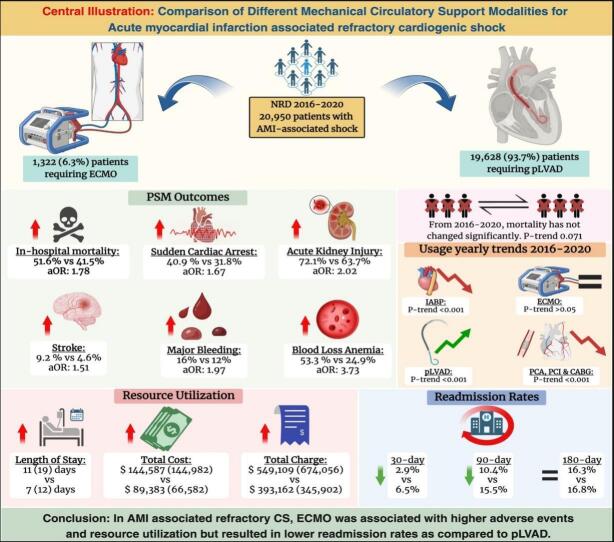
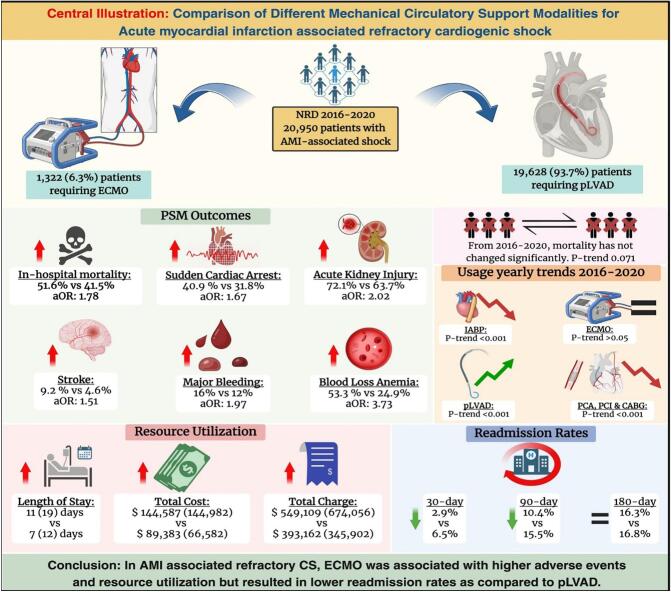
Central illustration.
Highlights
-
•
In the absence of LV unloading, the ECMO utilization in AMI-CS had higher in-hospital mortality, adverse events (major bleeding, stroke), and resource utilization in the index hospitalization.
-
•
The use of ECPELLA had similar rates of in-hospital mortality and SCA compared to Impella alone, but overall rates of bleeding events continued to be high.
-
•
From 2016-2020, AMI-CS related mortality has not changed significantly (p-trend>0.05).
1. Introduction
Cardiogenic shock (CS) is the leading cause of death for patients with acute myocardial infarction (AMI) who reach the hospital alive [1,2]. About 40,000 to 50,000 people with AMI develop CS per year in the US, and it corresponds to approximately 5 % to 10 % of all AMI patients. AMI complicated by CS (AMI-CS) has an early mortality of around 40 % and increases to 50 % in one year [2].
There has been an increased use of percutaneous mechanical circulatory support (MCS) devices in treating patients with AMI complicated by CS to improve outcomes [2]. The most frequently used percutaneous MCS devices include intra-aortic balloon pumps (IABP) and micro axial left ventricular assist devices (Impella) [3]. The IABP increases coronary artery blood flow and reduces left ventricular afterload via timed diastolic inflation and systolic deflation [2]. The percutaneous left ventricular assist device (pLVAD) is placed across the aortic valve into the LV and delivers blood directly from the LV to the proximal aorta [3]. Studies have shown that LVAD (e.g., Impella devices) provides more hemodynamic support as measured by cardiac output (2.5 L–5.5 L/min) compared to IABP (0.8 L–1.0 L/min) [2]. IABP-Shock II trial did not show a benefit of IABP plus optimal medical therapy (PCI or CABG) use versus optimal medical therapy alone on 30-day Mortality or one-year Mortality [4]. IMPRESS trial has shown similar short-term and long-term mortality outcomes in patients with IABP and Impella [5]. PROTECT II trial showed improved outcomes at 90 days with Impella use compared to IABP [6].
Recently, there has been an increase in the use of veno-arterial extracorporeal membrane oxygenation (VA-ECMO), which provides cardio-pulmonary support for patients with CS [5]. However, the ECLS-SHOCK trial did not show any mortality benefit with early utilization of VA-ECMO vs. standard medical therapy in patients with AMI complicated with CS with a planned early revascularization [7].
There is a lack of real-world data on Impella and VA-ECMO outcomes in AMI complicated by CS. We aim to study the trends in the utilization of commonly used MCS modalities and outcomes of Impella devices compared to VA-ECMO in patients hospitalized with AMI-CS undergoing revascularization during the index hospitalization.
2. Method
2.1. Study design and population
The Nationwide Readmission Database (NRD) from 2016 to 2020 was utilized for this study. NRD is maintained by the Agency for Healthcare Research and Quality (AHRQ) and provides data on roughly 35 million weighted hospitalizations [8]. It is a nationally representative administrative database of the United States comprising discharge and readmission records of 62.2 % of all hospitalizations. International Classification of Disease, Tenth Edition, Clinical Modification (ICD-10-CM), was used to identify patients admitted with cardiogenic shock complicated by AMI. ICD-10 Procedural Coding System (ICD-10-PCS) codes were used to identify venoarterial ECMO (VA-ECMO) & Impella devices. The ICD-10 codes used to identify the study population and primary and secondary outcomes of the study are included in Supplemental Table 1 (Table S1). Trend analysis for in-hospital mortality, utilization of different MCS modalities, and interventions during the index hospitalizations were obtained from the entire study population.
However, for our outcomes analysis, cohorts were created based on the types of MCS modalities, such as Impella devices and ECMO, in patients undergoing PCI during the index hospitalization. Patients who did not undergo percutaneous coronary intervention (PCI) (N 129,783) and underwent coronary artery bypass grafting (CABG) (N 22,910) were excluded from the analysis to limit the bias from lack of revascularization or CABG-related procedural adverse events. Similarly, patients receiving only IABP were excluded due to its transient use in cases of lower-severity cardiogenic shock. Subsequently, we classified the patient population into two groups: AMI-CS patients who received MCS with either Impella alone or placed only on ECMO. For our subgroup analysis, we compared the Impella cohort to ECMO with LV unloading by the Impella (ECPELLA) cohort. This step was taken to evaluate the impact of LV unloading with concomitant use of Imepella in ECMO patients.
Individual cases were identified using the unique identifier code. The number of days to intervention/procedure and length of stay (LOS) variables were used to calculate the readmission day of the same patient population. Data were used in its totality for analysis at index admission. As NRD is annualized, and only patients admitted within the same calendar year could be identified, we sequentially included the first 11-month, 9-month, and 6-month data from each year to ensure all patients have 30, 90, and 180-day follow-ups, respectively. Observations with a cell count <11 were not reported as per HCUP reporting guidelines.
2.2. Baseline characteristics
We identified adult patients (age ≥ 18 years) who were admitted between 2016 and 2020 with AMI-CS. Baseline patient characteristics (i.e., age, sex, and patient comorbidities) were analyzed. Hospital characteristics analyzed included bed size, teaching status, and urban-rural designation.
2.3. Study outcomes
The primary outcome was the difference in in-hospital Mortality between AMI-CS patients receiving Impella, ECMO, or ECPELLA. Secondary outcomes included other complications during the index hospitalization: acute kidney injury (AKI), sudden cardiac arrest (SCA), cardiac tamponade, acute stroke, major bleeding, acute liver injury or respiratory complication (respiratory failure or need for intubation); Length of stay, adjusted total charges; Propensity-matched 30, 90 and 180-day readmission rates; Trends of AMI-CS related mortality, utilization of different MCS modalities, and interventions for AMI. The definitions of study outcomes are provided in Supplementary Table S2.
2.4. Statistical analysis
Descriptive statistics were used to summarize continuous and categorical variables. Categorical variables were expressed as percentages and frequencies and compared using Pearson's x2 test. After assessing the distribution of data with histogram analysis (Supplementary Fig. S1), continuous variables were compared using the independent sample t-test analysis (for normally distributed) or the Mann-Whitney U test (Wilcoxon rank sum test) for non-parametric distribution. Patient demographics, comorbidities, and study outcomes were compared between Impellaand ECMO cohorts. The frequency of missing values was summarized, and Little's MCAR (missing completely at random) was used to screen for missing data patterns. A non-significant p-value (P > 0.05) represented randomly missing, while a significant p-value (P < 0.05) indicated missing not at random (MNAR) [9]. Data was complete except for randomly missing data patterns in the following variables; “Primary Expected Payer” missing N 38 (0.18 %), “Admission Status” missing N 29 (0.14 %), and “Median Household Income” missing N 324 (1.5 %). As the overall, randomly missing data was less than <2 %, we marked it missing and excluded it from the analysis.
After handling missing data, unadjusted and adjusted odds ratios were analyzed for in-hospital outcomes using univariate and multivariate logistic regression for study cohorts. We measured the adjusted odd ratios of in-hospital outcomes with a p-value significance <0.05. We utilized univariate screening for building the regression model; p-value <0.2 was used as cut off for the covariates to be included in the final multivariate regression model [10]. The multicollinearity among independent variables was assessed by measuring the variance inflation factor (VIF) and tolerance (1/VIF). VIF >5 and tolerance value <0.2 were used as a significant correlation marker among independent variables [11]. Covariates included in multivariate regression are listed in Supplementary Table S4. Our Propensity Score Matching (PSM) used the same multivariate regression model. After multivariate regression, the Mahalanobis distance matching was used with the propensity score caliper set at (0.2) to create matched cohorts. Pearson's x2 test was applied to the matched cohorts to compare outcomes. Furthermore, a graphical box plot demonstrating the balance of matching variables for both cohorts is presented in Supplementary Fig. S2. The matching variables (demographics, disease severity, mortality risk, and 15 different baseline comorbidities) used in the PSM module are listed in Supplementary Table S3. A similar propensity score matching (PSM) model was performed on 30-, 90-, and 180-day readmission analyses to calculate readmission rates on matched cohorts, respectively. Index hospitalizations alive at discharge were retained for readmission analysis to avoid mortality readmission bias. Using combined data from all years, we used a multivariable logistic regression model described above to obtain predictive margins for the adjusted trends over the years; the year was included as an independent variable. Unadjusted Trend analysis was performed using the Cochran-Armitage test for binary outcomes and the Jonckheere-Terpstra test or Cuzick's test for ordered categorical or continuous variables, given the non-parametric distribution of the study population. Total cost was adjusted for national inflation and merged with cost-charge ratio (CCR) NRD files. All analyses were conducted using appropriate stratifying, clustering, and weighting samples provided by Healthcare Cost and Utilization Project regulations. Stata v. 18 software (Stata Corp, College Station, TX) was used for all statistical analyses [12]. We used Biorender for the central illustration (Fig. 1) [13].
Fig. 1.
Central illustration.
3. Results
3.1. Demographic and baseline characteristics
A retrospective analysis was conducted on a cohort of 20,950 hospitalizations for Acute Myocardial infarction complicated by cardiogenic shock (AMI-CS) requiring different mechanical circulatory support modalities. The majority of patients (93.7 %) underwent mechanical circulatory support with Impella (N: 19,629), while only 1322 (6.3 %) underwent support with Extracorporeal Membrane Oxygenation (ECMO). Patients requiring Impella are significantly older, with a median age of 68 years (Interquartile range, IQR:17 years) compared to a median age of 61 years (IQR: 15 years) in patients placed on ECMO (p < 0.001). Notably, there is a difference in the insurance status between the two groups, with most Impella requiring patients having Medicare (59.2 % vs. 39.8 %) while a higher proportion of ECMO-requiring patients having Medicaid (14.3 % vs 8 %) and private insurance (37.7 % vs 23.4 %) (p < 0.001). Hospital characteristics also differ between the two groups, with higher proportions of ECMO patients being treated at large (87.8 % vs. 65.5 %, p < 0.001), non-profit private (83.1 % vs 74.8 %, p < 0.001), metropolitan-teaching (92.3 % vs 80.8 %, p < 0.001) hospitals. Interestingly, more Impella patients were treated in small metropolitan areas with at least 1 million residents (44.1 % vs 30.5 %, p < 0.001). A higher number of ECMO-requiring patients are transferred from other hospitals (24.4 % vs 12.2 %, p < 0.001), likely reflecting the need for specialized care in this group. ECMO group also demonstrates a higher risk of mortality, as evidenced by a greater percentage of patients in the extreme likelihood of dying (93.9 % vs. 87.9 %, p < 0.001) category and higher severity of illness as shown by a larger proportion of patients falling into extreme loss of function (93.1 % vs. 88.4 %, p: 0.001) category in the All-Patient Refined Diagnosis Related Groups (APRDRG).
The prevalence of various comorbidities also differs between the two groups; Impella patients have a higher prevalence of comorbidities like diabetes melilites (45.8 % vs. 38.3 %, p < 0.001), hyperlipidemia (56.5 % vs. 47 %, p < 0.001), hypertension (57.6 % vs. 43.7 %, p < 0.001), smoking history (24.4 % vs. 18.1 %, p < 0.001), CKD stage ≥3 (31 % vs. 19.3 %, p < 0.001), End Stage Renal Disease (ESRD) (8.1 % vs. 5.5 %, p: 0.014), history of Myocardial Infarction (14.5 % vs. 11.2 %, p < 0.001, previous Coronary Artery Bypass Graft (CABG) surgery (6 % vs. 3.9 %, p < 0.001), prior Implantable Cardioverter Defibrillator (ICD) (2.5 % vs 1.4 %, p: 0.041), pulmonary disease (15.4 % vs 9 %, p < 0.001) and pulmonary hypertension (7.9 % vs 5.3 %, p: 0.026).
However, other comorbidities like prior Percutaneous Coronary Intervention (PCI), presence of a permanent pacemaker, Obstructive Sleep Apnea (OSA), hypothyroidism, pneumonia, liver disease, heart failure, and COVID-19 are not statically different between the two cohorts (p > 0.05). Interestingly, some comorbidities have a higher prevalence in the ECMO group, including baseline Right Ventricular Failure (RVF) (7.1 % vs 3.1 %, p < 0.001) and a history of any cardiac arrhythmias (71.8 % vs 64.6 %, p < 0.001). Baseline characteristics and comorbidities are shown in Table 1.
Table 1.
Baseline characteristics and comorbidities comparison in hospitalizations with acute myocardial infarction associated cardiogenic shock undergoing Mechanical Circulatory Support (MCS) with pLVAD vs ECMO.
| Impella N = 19,628 |
ECMO N = 1322 |
P-value | |
|---|---|---|---|
| Age: Median (IQR) | 68 (17) | 61 (15) | <0.001 |
| Indicator of sex | |||
| Male | 13,879 (70.7 %) | 953 (72.1 %) | 0.464 |
| Female | 5750 (29.3 %) | 369 (27.9 %) | |
| Insurance type | |||
| Medicare | 11,593 (59.2 %) | 512 (38.9 %) | <0.001 |
| Medicaid | 1757 (9 %) | 188 (14.3 %) | |
| Private insurance | 4587 (23.4 %) | 497 (37.7 %) | |
| Self-pay | 911 (4.6 %) | 69 (5.3 %) | |
| Other | 674 (3.4 %) | 46 (3.5 %) | |
| Type of admission | |||
| Non-elective | 18,663 (95.2 %) | 1248 (94.4 %) | 0.335 |
| Elective | 936 (4.8 %) | 74 (5.6 %) | |
| Bed size of the hospital | |||
| Small | 1852 (9.4 %) | 36 (2.7 %) | <0.001 |
| Medium | 4911 (25 %) | 125 (9.5 %) | |
| Large | 12,866 (65.5 %) | 1160 (87.8 %) | |
| Control/Ownership of the Hospital | |||
| Government, Non-Federal | 2002 (10.2 %) | 175 (13.2 %) | <0.001 |
| Private, non-profit | 14,683 (74.8 %) | 1098 (83.1 %) | |
| Private, invest-own | 2943 (15 %) | 49 (3.7) | |
| Teaching status of urban hospitals | |||
| Metropolitan non-teaching | 3100 (15.8 %) | 94 (7.1 %) | <0.001 |
| Metropolitan teaching | 15,858 (80.8 %) | 1220 (92.3 %) | |
| Non-metropolitan hospital | 670 (3.4 %) | <11 (0.6 %) | |
| Hospital urban-rural designation | |||
| Large metropolitan areas with at least 1 million residents | 10,310 (55.5 %) | 911 (58.9 %) | <0.001 |
| Small metropolitan areas with <1 million residents | 8648 (44.1 %) | 403 (30.5 %) | |
| Micropolitan areas | 632 (3.2 %) | <11 (0.4 %) | |
| Admission day of the week | |||
| Mon – Fri | 14,560 (74.2 %) | 982 (74.3 %) | 0.950 |
| Sat – Sun | 5068 (25.8 %) | 340 (25.7 %) | |
| Transfer flag indicating a combination of discharges involving same day events | |||
| Not a transfer or other same-day stay | 15,766 (80.3 %) | 792 (59.9 %) | <0.001 |
| A transfer involving two discharges from different hospitals | 2389 (12.2 %) | 322 (24.4 %) | |
| Same-day stay involving two discharges from different hospitals | 601 (3.1 %) | 84 (6.4 %) | |
| Same-day stay involving two discharges at the same hospitals | 542 (2.8 %) | 49 (3.7 %) | |
| Same-day stay involving three or more discharges at the same or different hospitals | 330 (1.7 %) | 74 (5.6 %) | |
| Median household income national quartile for patient ZIP Code | |||
| 0-25th percentile | 6063 (31.4 %) | 281 (21.6 %) | <0.001 |
| 26th to 50th percentile | 5596 (29 %) | 361 (27.8 %) | |
| 51st to 75th percentile | 4679 (24.2 %) | 320 (24.6 %) | |
| 76th to 100th percentile | 2989 (15.5 %) | 338 (26 %) | |
| A combined record involving rehab transfer | |||
| Not a combined record or a combined record not involving rehabilitation, evaluation, or other aftercare | 19,141 (97.5 %) | 1272 (96.2 %) | 0.088 |
| Combined record involving transfer to rehabilitation, evaluation, or other aftercare | 487 (2.5 %) | 50 (3.8 %) | |
| Patient's State is the same as the Hospital's State | |||
| Non-resident | 152 (8.5 %) | 38 (16.3 %) | 0.015 |
| Resident | 1638 (91.5 %) | 197 (83.7 %) | |
| All Patient Refined DRG: Risk of Mortality Subclass | |||
| Minor likelihood of dying | 47 (0.2 %) | <11 (0.5 %) | <0.001 |
| Moderate likelihood of dying | 363 (1.8 %) | 12 (0.9 %) | |
| Major likelihood of dying | 1970 (10 %) | 61 (4.6 %) | |
| Extreme likelihood of dying | 17,247 (87.9 %) | 1242 (93.9 %) | |
| All Patient Refined DRG: Severity of Illness Subclass | |||
| Minor loss of function | 47 (0.2 %) | <11 (0.2 %) | 0.001 |
| Moderate loss of function | 85 (0.4 %) | 12 (0.9 %) | |
| Major loss of function | 2151 (11 %) | 77 (5.9 %) | |
| Extreme loss of function | 17,343 (88.4 %) | 1230 (93.1 %) | |
| Comorbidities | |||
| Diabetes | 8991 (45.8 %) | 507 (38.3 %) | <0.001 |
| Hyperlipidemia | 11,111 (56.6 %) | 621 (47 %) | <0.001 |
| Hypertension | 11,329 (57.7 %) | 578 (43.7 %) | <0.001 |
| Smoking Status | 4796 (24.4 %) | 240 (18.1 %) | <0.001 |
| CKD Stage over 3 | 6082 (31 %) | 255 (19.3 %) | <0.001 |
| ESRD | 1588 (8.1 %) | 72 (5.5 %) | 0.014 |
| Prior CVA | 145 (0.7 %) | 35 (2.7 %) | <0.001 |
| Prior MI | 2855 (14.5 %) | 148 (11.2 %) | 0.023 |
| Prior PCI | 3017 (15.4 %) | 228 (17.2 %) | 0.229 |
| Prior CABG | 1187 (6 %) | 51 (3.9 %) | 0.022 |
| Prior Defibrillator | 486 (2.5 %) | 18 (1.4 %) | 0.041 |
| Prior Permanent Pacemaker | 326 (1.7 %) | 17 (1.3 %) | 0.454 |
| OSA | 1430 (7.3 %) | 80 (6.1 %) | 0.238 |
| Pulmonary disease | 3032 (15.4 %) | 119 (9 %) | <0.001 |
| Pulmonary Hypertension | 1548 (7.9 %) | 70 (5.3 %) | 0.026 |
| RV Heart Failure | 602 (3.1 %) | 94 (7.1 %) | <0.001 |
| Hypothyroid | 1833 (9.3 %) | 106 (8 %) | 0.266 |
| Anemia | 1022 (5.2 %) | 39 (3 %) | 0.007 |
| Pneumonia | 2248 (11.5 %) | 165 (12.5 %) | 0.441 |
| Liver disease | 551 (2.8 %) | 51 (3.9 %) | 0.124 |
| Malnutrition | 1509 (7.7 %) | 250 (18.9 %) | <0.001 |
| Heart failure | 13,918 (70.9 %) | 905 (68.5 %) | 0.206 |
| Arrhythmias | 12,687 (64.6 %) | 948 (71.8 %) | <0.001 |
| COVID-19 | 76 (0.4 %) | <11 (0.2 %) | 0.437 |
Abbreviations: CKD: Chronic Kidney Disease; ESRD: End Stage Renal Disease; CVA: Cerebrovascular Accident; MI: Myocardial Infarction; PCI: Percutaneous Coronary intervention; CABG: Coronary Artery Bypass Graft; OSA: Obstructive Sleep Apnea; RV: Right Ventricular; COVID-19: Coronavirus Disease 19; <11: non-reportable per HCUP policy.
IQR = Interquartile Range (P75-P25).
3.2. Outcomes of unmatched and propensity-matched cohorts of LVAD compared to ECMO in acute MI associated cardiogenic shock
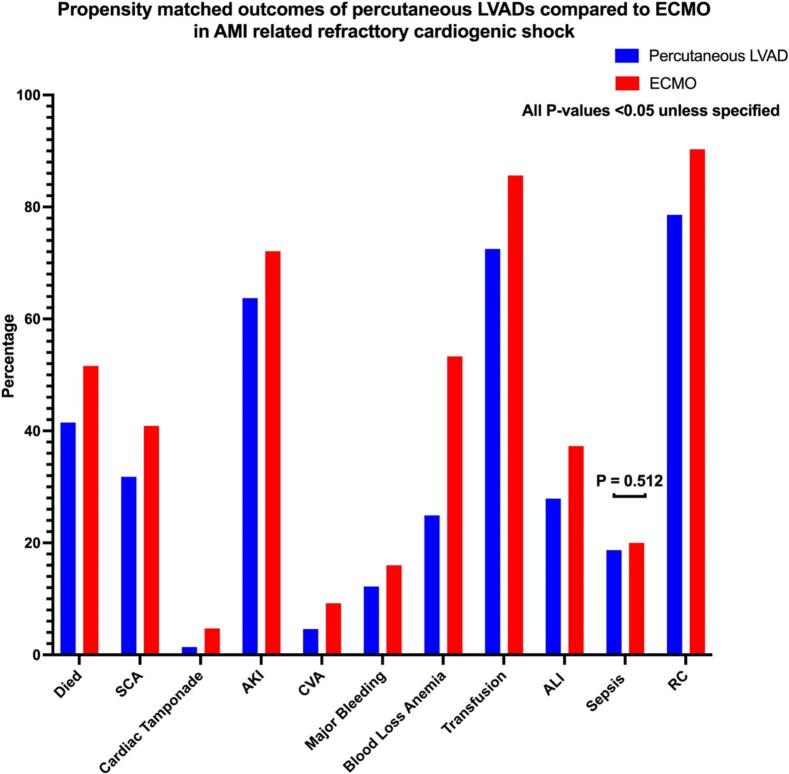
ECMO cohort has a higher in-hospital mortality rate both in the crude outcomes (52.3 % vs 44 %, p < 0.001) and after propensity matching (51.6 % vs 41.5 %, p < 0.001). ECMO group also has a higher incidence of adverse events. In crude analysis, ECMO-requiring patients have a higher incidence of sudden cardiac arrest (SCA) (40.3 % vs. 26.2 %, p < 0.001), cardiac tamponade (4.8 % vs. 1.6 %, p < 0.001), acute kidney injury (AKI) (73.7 % vs. 59.4 %, p < 0.001), acute stroke (8.4 % vs. 4.6 %, p < 0.001), major bleeding (15.6 % vs. 7.3 %, p < 0.001), blood loss anemia (53.9 % vs. 24 %, p < 0.001), need for transfusions (86.1 % vs. 70.5 %, p < 0.001), acute liver injury (ALI) (38.1 % vs. 22.4 %, p < 0.001), sepsis (19.9 % vs 16.3 %, p: 0.015) and respiratory complications (90.8 % vs 77 %, p < 0.001).
On a propensity-matched cohort (N = 742), most of the crude differences remain significant, including a higher incidence in the ECMO group of SCA (40.9 % vs. 31.8 %, p < 0.001), cardiac tamponade (4.7 % vs. 1.4 %, p < 0.001), AKI (72.1 % vs. 63.7 %, p < 0.001), acute stroke (9.2 % vs. 4.6 %, p < 0.001), major bleeding (16 % vs. 12.2 %, p: 0.037), blood loss anemia (53.3 % vs. 24.9 %, p < 0.001), need for blood transfusion (85.6 % vs. 72.5 %, p < 0.001), ALI (37.3 % vs. 27.9 %, p < 0.001) and respiratory complications (90.3 % vs 78.6 %, p < 0.001). Interestingly, the incidence of sepsis, found to be significantly higher in the ECMO group on crude analysis (19.9 % vs 16.3 %, p < 0.001), was non-significant after propensity matching (p > 0.05). Crude and propensity-matched outcomes are shown in Table 2 and Fig. 2.
Table 2.
Crude and Propensity Matched In-Hospital Outcomes of different MCS modalities in Acute Myocardial Infarction associated cardiogenic shock.
| Outcomes | Crude Outcomes |
Propensity Match Outcomes |
||||
|---|---|---|---|---|---|---|
| Impella N = 19,628 |
ECMO |
P value | Impella |
ECMO |
P value | |
| N = 1322 |
N = 742 |
N = 742 |
||||
| N (%) | N (%) | N (%) | N (%) | |||
| Died during hospitalization | 8647 (44 %) | 690 (52.3 %) | <0.001 | 308 (41.5 %) | 383 (51.6 %) | <0.001 |
| SCA | 5150 (26.2 %) | 533 (40.3 %) | <0.001 | 236 (31.8 %) | 304 (40.9 %) | <0.001 |
| Cardiac Tamponade | 318 (1.6 %) | 63 (4.8 %) | <0.001 | 10 (1.4 %) | 35 (4.7 %) | <0.001 |
| AKI | 11,667 (59.4 %) | 974 (73.7 %) | <0.001 | 473 (63.7 %) | 536 (72.1 %) | <0.001 |
| Acute Stroke | 898 (4.6 %) | 111 (8.4 %) | <0.001 | 34 (4.6 %) | 68 (9.2 %) | <0.001 |
| Major Bleeding | 1439 (7.3 %) | 206 (15.6 %) | <0.001 | 91 (12.2 %) | 119 (16 %) | 0.037 |
| Blood Loss Anemia | 4714 (24 %) | 713 (53.9 %) | <0.001 | 185 (24.9 %) | 396 (53.3 %) | <0.001 |
| Transfusions | 13,830 (70.5 %) | 1139 (86.1 %) | <0.001 | 539 (72.5 %) | 636 (85.6 %) | <0.001 |
| ALI | 4395 (22.4 %) | 504 (38.1 %) | <0.001 | 207 (27.9 %) | 277 (37.3 %) | <0.001 |
| Sepsis | 3193 (16.3 %) | 263 (19.9 %) | 0.015 | 139 (18.7 %) | 149 (20 %) | 0.512 |
| RC | 15,115 (77 %) | 1201 (90.8 %) | <0.001 | 584 (78.6 %) | 671 (90.3 %) | <0.001 |
Abbreviations: ECMO: Extracorporeal Membrane Oxygenation; SCA: Sudden Cardiac Arrest; AKI: Acute kidney Injury; ALI: Acute Liver Injury; RC: Respiratory complications.
Fig. 2.
Propensity-matched outcomes of percutaneous LVADs compared to ECMO in AM-related refractory cardiogenic shock.
3.3. Outcomes after multivariate regression analysis
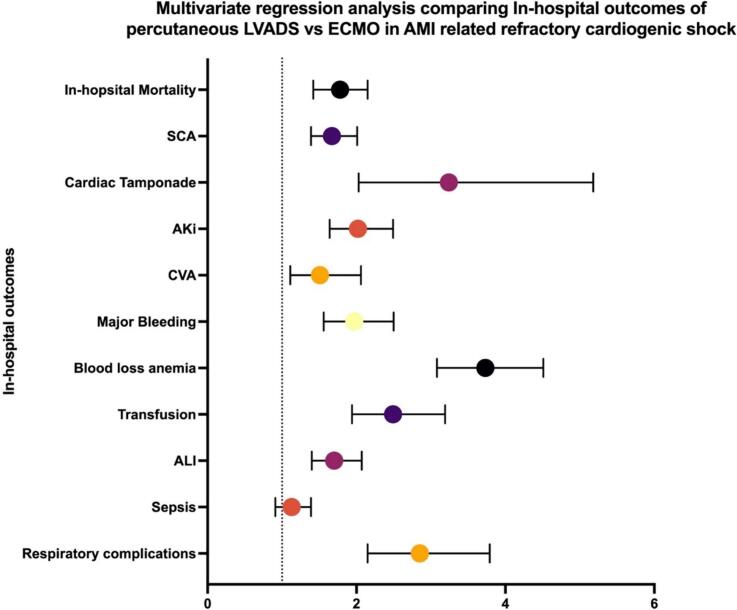
On a multivariate regression analysis adjusting the confounders, ECMO-requiring patients have significantly higher odds of in-hospital mortality (adjusted odds ratio, aOR: 1.78, 95 % CI: 1.47–2.15, p < 0.001). The risk of other complications is also significantly higher in acute MI associated cardiogenic shock requiring ECMO, including SCA (aOR: 1.67, 95 % CI: 1.39–2.01, p < 0.001), Cardiac Tamponade (aOR: 3.24, 95 % CI: 2.03–5.18, p < 0.001), AKI (aOR: 2.02, 95 % CI: 1.64–2.49, p < 0.001), CVA (aOR: 1.51, 95 % CI: 1.11–2.06, p < 0.001), major bleeding (aOR: 1.97, 95 % CI: 1.56–2.50, p < 0.001), blood loss anemia (aOR: 3.73, 95 % CI: 3.08–4.51, p < 0.001), need for transfusions (aOR: 2.49, 95 % CI: 1.94–3.19, p < 0.001), acute liver injury (aOR: 1.70, 95 % CI: 1.40–2.07, p < 0.001) and respiratory complications (aOR: 2.85, 95 % CI: 2.15–3.79, p < 0.001). Interestingly, the odds of sepsis are not statistically different between the two groups on multivariate regression, as seen similarly in propensity matching. Multivariate regression analysis is shown in Table 3 and Fig. 3.
Table 3.
Multivariate Regression Analysis Comparing In-Hospital Outcomes of ECMO with Impella in Acute Myocardial Infarction associated cardiogenic shock.
| In-hospital Outcomes | ECMO compared with Impella |
||
|---|---|---|---|
| aOR | 95 % CI | P-value | |
| Died during hospitalization | 1.78 | 1.47–2.15 | <0.001 |
| SCA | 1.67 | 1.39–2.01 | <0.001 |
| Cardiac Tamponade | 3.24 | 2.03–5.18 | <0.001 |
| AKI | 2.02 | 1.64–2.49 | <0.001 |
| Acute Stroke | 1.51 | 1.11–2.06 | <0.001 |
| Major Bleeding | 1.97 | 1.56–2.50 | <0.001 |
| Blood Loss Anemia | 3.73 | 3.08–4.51 | <0.001 |
| Transfusion | 2.49 | 1.94–3.19 | <0.001 |
| ALI | 1.70 | 1.40–2.07 | <0.001 |
| Sepsis | 1.13 | 0.91–1.39 | 0.272 |
| Respiratory Complications | 2.85 | 2.15–3.79 | <0.001 |
Abbreviations: ECMO: Extracorporeal Membrane Oxygenation; SCA: Sudden Cardiac Arrest; AKI: Acute kidney Injury; ALI: Acute Liver Injury.
IQR = Interquartile Range (P75-P25); CI: Confidence Interval.
Fig. 3.
Multivariate regression analysis comparing In-hospital outcomes of percutaneous LVADS vs ECMO in AMI-related refractory cardiogenic shock.
3.4. Subgroup analysis comparing Impella to concomitant use of ECMO with Impella
When comparing Impella to ECPELLA, the latter had higher major bleeding (18.2 % vs. 9.8 %%, p: 0.041), acute blood loss anemia (55.3 % vs. 29.6 %, p < 0.001), and need for transfusions (93.9 % vs. 83.3 %, p: 0.007). However, the rates of in-hospital mortality (46.2 % vs. 39.4 %, p: 0.192) and SCA (44 % vs. 36.4 %, p: 0.209) were similar as presented in Table 4.
Table 4.
Propensity Matched In-Hospital Outcomes of Impella compared to ECPELLA in Acute Myocardial Infarction associated cardiogenic shock.
| Outcomes | Propensity Match Outcomes |
||
|---|---|---|---|
| Impella |
ECPELLA (ECMO + Impella) |
P value | |
| N = 132 |
N = 132 |
||
| N (%) | N (%) | ||
| Died during hospitalization | 52 (39.4 %) | 61 (46.2 %) | 0.192 |
| SCA | 48 (36.4 %) | 58 (44 %) | 0.209 |
| Cardiac Tamponade | 1 (0.8 %) | 3 (2.3 %) | 0.314 |
| AKI | 99 (75 %) | 111 (84.1 %) | 0.067 |
| Acute Stroke | 13 (9.8 %) | 14 (10.6 %) | 0.839 |
| Major Bleeding | 13 (9.8 %) | 24 (18.2 %) | 0.041 |
| Blood Loss Anemia | 39 (29.6 %) | 73 (55.3 %) | <0.001 |
| Transfusions | 110 (83.3 %) | 124 (93.9 %) | 0.007 |
| ALI | 60 (45.5 %) | 69 (52.3 %) | 0.268 |
| Sepsis | 31 (23.5 %) | 42 (31.8 %) | 0.130 |
| RC | 116 (87.9 %) | 129 (97.7 %) | 0.002 |
Abbreviations: ECMO: Extracorporeal Membrane Oxygenation; SCA: Sudden Cardiac Arrest; AKI: Acute kidney Injury; ALI: Acute Liver Injury; RC: Respiratory complications.
3.5. Resource utilization of different modalities of MCS in patients with Acute MI-associated cardiogenic shock
ECMO cohort exhibit extended length of stay (LOS), with a median length of stay of 11 days (Interquartile Range; IQR: 19 days) compared to 7 days (IQR: 12 days) for the Impella group (p < 0.001). Moreover, the total cost of hospitalization is also higher in the ECMO group, with a median total cost of 144,587 USD (IQR: $144,982) as compared to 89,383 USD (IQR: $66,582) for the Impella-requiring patients (p < 0.001). Similar patterns also exist in adjusted total charge, indicating the financial burden associated with these mechanical circulatory support modalities. Resource Utilization of different modalities of MCS in patients with Acute MI associated cardiogenic shock is shown in Table 5.
Table 5.
Resource Utilization of Patients with Acute Myocardial Infarction Associated Cardiogenic Shock Receiving Different Modalities of Mechanical Circulatory Support.
| Resource Utilization | Impella |
ECMO |
P-value |
|---|---|---|---|
| Median (IQR) | Median (IQR) | ||
| Index Admission | |||
| LOS in days | 7 (12) | 11 (19) | <0.001 |
| Total Cost | $89,383 (66582) | $144,587 (144982) | <0.001 |
| Total Adjusted Charge | $393,162 (345902) | $549,109 (674056) | <0.001 |
Abbreviations: ECMO: Extracorporeal Membrane Oxygenation.
LOS: Length of Stay; IQR = Interquartile Range (P75-P25).
3.6. Yearly trend of resource utilization of different MCS modalities in patients with acute MI associated cardiogenic shock
Significant differences exist in the trend of resource utilization between different MCS modalities in patients with acute MI-associated cardiogenic shock. From 2016 to 2020, Impella use was associated with decreasing median LOS from 8 days (IQR: 12 days) in 2016 to 7 days (IQR: 11 days) in 2020 (p-trend: 0.018). Interestingly, the total cost of hospitalization has continued to increase in the same time frame for Impella use. Total cost is up trending from $88,098 USD (IQR: $63,861) in 2016 to $92,502 USD (IQR: $69,577) in 2020 (p-trend: 0.001).
On the contrary, ECMO use is not only associated with higher resource utilization as compared to Impella use but also with the yearly trend of resource utilization not changing significantly (p-trend >0.05). The yearly trend of resource utilization of different MCS modalities in patients with Acute MI associated cardiogenic shock is shown in Table 6 & Fig. 4.
Table 6.
Yearly trend of resource utilization of different MCS modalities in patients with Acute MI Associated Cardiogenic Shock.
| Year | Length of Stay Yearly Trend |
Total Cost Yearly Trend |
||
|---|---|---|---|---|
| Impella |
ECMO |
Impella |
ECMO |
|
| Median (IQR) | Median (IQR) | Median USD (IQR) | Median USD (IQR) | |
| 2016 | 8 (12) | 11 (22) | 88,098 (63861) | 164,607 (138833) |
| 2017 | 8 (12) | 13 (17) | 87,960 (66735) | 150,033 (141999) |
| 2018 | 8 (12) | 10 (20) | 87,006 (62214) | 131,550 (125599) |
| 2019 | 7 (12) | 10 (16) | 91,176 (68544) | 140,695 (138701) |
| 2020 | 7 (11) | 14 (22) | 92,502 (69577) | 160,172 (181438) |
| P-trend | 0.0179 | 0.7620 | 0.0014 | 0.8665 |
Abbreviations: ECMO: Extracorporeal Membrane Oxygenation.
IQR = Interquartile Range (P75-P25).
Fig. 4.
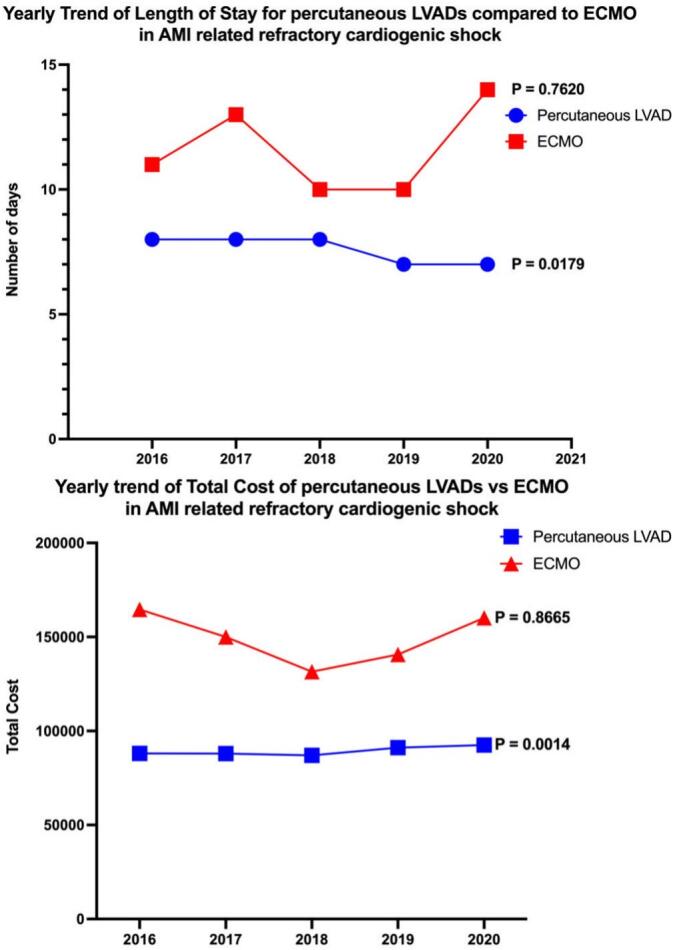
Yearly Trend of Length of Stay and Total Cost for percutaneous LVADs compared to ECMO in AM-related refractory cardiogenic shock.
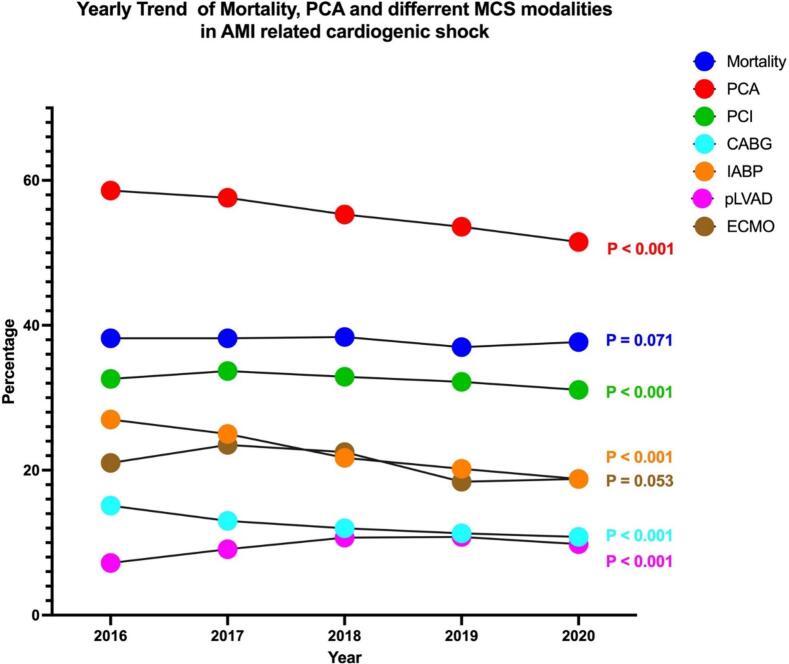
3.7. Yearly trend of mortality, coronary interventions, and utilization of MCS modalities in myocardial infarction associated cardiogenic shock
From 2016 to 2020, mortality has not changed significantly for acute MI-associated cardiogenic shock-related hospitalizations in the United States; it was 38.2 % in 2016 and 37.7 % in 2020 (p-trend: 0.071). The use of different coronary intervention modalities in index hospitalization has decreased in this cohort across the same period. From 2016 to 2020, there is a decreasing use of percutaneous coronary angiogram (58.6 % to 51.5 %, p-trend <0.001), Percutaneous Coronary Intervention (PCI) (32.6 % to 31.1 %, p-trend <0.001) and Coronary Artery Bypass Graft (CABG) surgery (15.1 % to 10.8 %, p-trend <0.001) in acute MI associated cardiogenic shock in index hospitalization.
The utilization of different MCS modalities has shown an interesting trend across the years. From 2016 to 2020, the use of Impella has increased (7.2 % to 9.8 %, p-trend <0.001) while Intra-Arterial Balloon Pump (IABP) use has decreased (27 % to 18.8 %, p-trend <0.001). Interestingly, ECMO use has not significantly changed across the years (p-trend >0.05). The yearly trend of mortality, different coronary interventions, and MCS modalities are shown in Fig. 5 and Supplementary Table S5.
Fig. 5.
Yearly Trend of Mortality, PCA, and different MCS modalities in AMI-related cardiogenic shock.
3.8. Readmission rates on propensity matched cohort for different modalities of MCS in Acute MI associated cardiogenic shock
Interestingly, on a propensity-matched cohort, Impella use is associated with higher rates of all-cause readmission at 30-day (6.5 % vs. 2.9 %, p < 0.001) and 90-day (15.5 % vs 10.4 %, p < 0.001) intervals as compared to ECMO-use in patients with acute MI associated Cardiogenic shock. At the same time, there was no significant difference in 180-day readmission rates (p > 0.05) as shown in Table 7.
Table 7.
Readmission rates on propensity matched cohort.
| Readmission Rates on Propensity Matched Cohort | |||
|---|---|---|---|
| 30-day Readmission | Impella N = 680 |
ECMO N = 680 |
P-value |
| N (%) | N (%) | ||
| Readmits | 44 (6.5) | 20 (2.9) | 0.002 |
| 90-day Readmission | Impella N = 547 |
ECMO N = 547 |
P-value |
| N (%) | N (%) | ||
| Readmits | 85 (15.5) | 57 (10.4) | 0.012 |
| 180-day Readmission | Impella N = 375 |
ECMO N = 375 |
P-value |
| N (%) | N (%) | ||
| Readmits | 63 (16.8) | 61 (16.3) | 0.844 |
Abbreviations: ECMO: Extracorporeal Membrane Oxygenation.
4. Discussion
Our large retrospective nationwide study identified 20,950 patients with cardiogenic shock in acute myocardial infarction (AMI-CS) from 2016 to 2020 to evaluate the clinical outcomes of AMI-CS in patients receiving Impella when compared to ECMO devices. The key findings of our study are as follows: 1) The use of ECMO in AMI-CS was associated with significantly higher in-hospital mortality when compared with Impella devices. 2) There were significantly higher rates of complications such as sudden cardiac arrest, cardiac tamponade, stroke, and major bleeding in the ECMO group. 3) Further, the use of ECMO was associated with significantly greater length of hospital stay and total costs of hospitalization compared to Impella. 4) On trend analysis from 2016 to 2020, there has been a gradual decline in utilization of IABP, while the rate of utilization of Impella has shown a rising trend with no difference in the utilization rate of ECMO devices. 5) On readmission analysis, the Impella cohort had higher 30-day and 90-day all-cause readmission rates compared to ECMO devices.
Our study reports a significantly higher risk of in-hospital Mortality with ECMO compared with Impella devices. This difference in mortality could be attributed to greater severity of comorbidities and higher acuity of patients on ECMO as they are used for their superior hemodynamic support in the most critically ill patient population [14,15]. This is evident by a greater proportion of ECMO patients in all patients refined DRG (APDRG) subclasses of extreme loss of function and the extreme likelihood of dying. Lately, temporary LVAD devices have also been increasingly used as a de-escalation strategy for veno-arterial ECMO in CS patients, implying an improvement in their clinical condition during the gradation and hence reduced risk of Mortality with LVAD when compared with ECMO [16]. Furthermore, in our study, the use of ECPELLA for simultaneous LV unloading had similar rates of mortality and SCA, but the risk of bleeding continued to be high when compared with Impella alone. Landmark trials such as ISAR-SHOCK, PROTECT-II, and IMPRESS have reported similar mortality rates for Impella and IABP devices in critically ill patients with AMI-CS [6,17,18]. While most studies, including randomized controlled trials, have compared mortality outcomes of Impella with IABP in CS patients, comparative data on mortality outcomes with ECMO is limited in current literature. A large meta-analysis of 1866 patients evaluated the complication risk of ECMO for the treatment of cardiogenic shock and cardiac arrest and reported cumulative survival to hospital discharge rate of 20.8 to 65.4 % with considerable morbidity associated with ECMO devices [19]. Another meta-analysis evaluated the outcomes of ECMO in post-cardiotomy shock patients and reported pooled survival to hospital discharge rate of 34 %, pooled 1-year survival rate, and midterm survival rate of 24 % and 18 %, respectively [20]. Our study adds to the growing body of literature and raises concerns about the low survival outcomes and relatively high rate of complications post-ECMO.
The risk of cerebrovascular accidents is high with all mechanical circulatory support (MCS) devices. The increased risk of thrombosis due to the interaction of blood with non-biological surfaces, shear stress, thrombocytopenia due to destruction of platelets, consumption coagulopathy, and need for anticoagulation increases the risk of both ischemic and hemorrhagic CVA in MCS devices [20]. Our study reports significantly higher rates of CVA in ECMO compared with Impella devices. A meta-analysis published in 2013 reported an incidence rate of 3.3 % to 17.6 % for ischemic stroke and 1.6 % to 5.5 % for hemorrhagic stroke in ECMO devices in patients with CS [19]. Besides the common factors predisposing to stroke mentioned above, continuous retrograde flow of blood into the aorta, increased afterload, and subsequent left ventricular distention may also predispose to intracardiac stasis and thrombosis, increasing the risk of stroke in ECMO devices [21,22]. Cerebral hypoperfusion due to the mixing of oxygenated and deoxygenated blood in the aorta, a phenomenon that is referred to as “Harlequin's syndrome,” has also been hypothesized as contributory to cerebral ischemia in ECMO patients [15,20,23,24]. Studies have reported the rate of stroke in Impella devices between 2.4 and 6.3 % [25]. The lower risk of stroke with Impella compared to ECMO may be attributed to structural advancements in newer devices without a pigtail catheter that may mitigate the risk of intracardiac thrombus by facilitating insertion and repositioning in the left ventricle [26,27].
On trend analysis, our study reports a gradual decline in the use of IABP from 2016 to 2020. This could be explained by the 2012 IABP shock trial, which showed no significant 30-day mortality benefits with IABP in patients with AMI complicated by cardiogenic shock [4]. Currently, the use of IABP has been limited to a Class IIa recommendation by 2013 ACC/AHA guidelines in the management of refractory cardiogenic shock and is still widely used as a temporary support to hemodynamics in patients with fewer comorbidities due to simplicity of insertion and easy accessibility [28]. While the use of Impella has shown a gradual upward trend, according to our study, there has been no significant change in the rate of ECMO utilization devices. This trend of increasing Impella utilization may have followed randomized controlled trials that have shown greater hemodynamic support with Impella devices when compared with IABP in AMI-CS patients. However, it is important to note that despite the improvement in hemodynamic support, the studies failed to demonstrate significant mortality benefits up to 30 days from index admission [6,17,18]. The availability of newer Impella devices, such as Impella 5.0 and Impella 5.5, with greater duration of hemodynamic support, have also expanded their utilization in the management of cardiogenic shock in AMI patients [26].
The lower readmission rates seen in the ECMO cohort can likely be attributed to the fact that these patients were generally younger and had fewer comorbidities compared to those placed on Impella. Among those who survived their hospital stay and were discharged alive, they experienced fewer readmissions, possibly because they had a higher level of baseline functional status prior to index hospitalization.
4.1. Limitations
Our study has several limitations that must be considered before interpreting results. Given the retrospective nature of the study, there may be selection bias. Our data is administrative in nature and relies on ICD codes; hence, it may be subject to coding and documentation errors. Veno-arterial ECMO (VA-ECMO) ICD-10-PCS codes were first available in 2018, while the specific ICD-10 codes distinguishing venovenous (VV-ECMO) and VA-ECMO were not available before 2018. The presence of confounding bias due to unmeasured variables may have affected the outcomes of our study. Lack of patient-level data on the severity of comorbidities, treatment strategies adopted prior to insertion of mechanical circulatory devices, medication list, and operational or procedural techniques also limits our study. Furthermore, the severity of cardiogenic shock and baseline left ventricular ejection (LVEF) cannot be determined by available ICD codes, which could be an important effect modifier for presented outcomes; similarly, the duration of Impella or ECMO use is also unavailable. Our observational study results can only determine association; hence, causality cannot be established. We can determine only in-hospital outcomes, and the lack of data from ambulatory, emergency, and out-of-hospital cardiac events further limits our study. Nevertheless, the large size of the database and our ability to identify nationwide estimates empower our study and help us overcome these potential limitations.
5. Conclusion
In AMI-associated cardiogenic shock, ECMO was utilized in relatively younger patients with low comorbidity burden, but a higher severity of illness compared to other pLVAD. Our study concludes that utilization of ECMO in the absence of LV unloading in this cohort had significantly higher rates of in-hospital mortality, stroke, and other cardiovascular complications. Further, resource utilization, including length of hospital stay and total cost of hospitalizations, are significantly greater in the ECMO group. The use of Impella with ECMO for LV unloading had similar rates of short-term mortality and SCA but higher overall bleeding events when compared to Impella alone. Despite the advancement in MCS modalities, the AMI-CS related overall mortality has not changed from 2016 to 2020. This necessitates the need for prospective trials and a robust risk-benefit analysis to determine the optimum management strategy for this population.
CRediT authorship contribution statement
Shafaqat Ali: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Manoj Kumar: Data curation, Writing – original draft. Irisha Badu: Writing – original draft. Faryal Farooq: Writing – original draft. Thannon Alsaeed: Writing – review & editing. Muhammad Sultan: Writing – original draft. Lalitsiri Atti: Visualization. Sanchit Duhan: Visualization. Pratik Agrawal: Writing – review & editing. Vijaywant Brar: Writing – review & editing. Tarek Helmy: Writing – review & editing. Taher Tayeb: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2024.100468.
Contributor Information
Shafaqat Ali, Email: shafaqat231@gmail.com.
Pratik Agrawal, Email: Pratik.Agrawal@lsuhs.edu.
Vijaywant Brar, Email: Vijaywant.Brar@lsuhs.edu.
Tarek Helmy, Email: Tarek.Helmy@lsuhs.edu.
Taher Tayeb, Email: Taher.Tayeb@lsuhs.edu.
Appendix A. Supplementary data
Supplementary material
References
- 1.Hochman J.S. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107(24):2998–3002. doi: 10.1161/01.CIR.0000075927.67673.F2. [DOI] [PubMed] [Google Scholar]
- 2.Samsky M.D., Morrow D.A., Proudfoot A.G., Hochman J.S., Thiele H., Rao S.V. Cardiogenic shock after acute myocardial infarction: a review. Jama. 2021;326(18):1840–1850. doi: 10.1001/jama.2021.18323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amin A.P., Spertus J.A., Curtis J.P., Desai N., Masoudi F.A., Bach R.G., et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141(4):273–284. doi: 10.1161/CIRCULATIONAHA.119.044007. [DOI] [PubMed] [Google Scholar]
- 4.Thiele H., Zeymer U., Neumann F.-J., Ferenc M., Olbrich H.-G., Hausleiter J., et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N. Engl. J. Med. 2012;367(14):1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 5.Ouweneel D.M., Eriksen E., Sjauw K.D., van Dongen I.M., Hirsch A., Packer E.J., et al. Percutaneous mechanical circulatory support versus intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. J. Am. Coll. Cardiol. 2017;69(3):278–287. doi: 10.1016/j.jacc.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 6.O’Neill W.W., Kleiman N.S., Moses J., Henriques J.P., Dixon S., Massaro J., et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126(14):1717–1727. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 7.Thiele H., Zeymer U., Akin I., Behnes M., Rassaf T., Mahabadi A.A., et al. Extracorporeal life support in infarct-related cardiogenic shock. N. Engl. J. Med. 2023;389(14):1286–1297. doi: 10.1056/NEJMoa2307227. [DOI] [PubMed] [Google Scholar]
- 8.HCUP Nationwide Readmissions Database (NRD). Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality, Rockville, MD. 2016-2020. www.hcup-us.ahrq.gov/nrdoverview.jsp
- 9.Little R.J.A., Rubin D.B. 2nd ed. Wiley; New York: 2002. Statistical Analysis of Missing Data. [Google Scholar]
- 10.Harrell Frank E. vol. 608. Springer; New York: 2001. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. [Google Scholar]
- 11.Daoud J.I. Multicollinearity and regression analysis. J. Phys. Conf. Ser. 2017;949(1) [Google Scholar]
- 12.StataCorp. StataCorp LLC; College Station, TX: 2023. Stata Statistical Software: Release 18. [Google Scholar]
- 13.Created with BioRender.com. 2023.
- 14.Koerner M.M., Harper M.D., Gordon C.K., Horstmanshof D., Long J.W., Sasevich M.J., et al. Adult cardiac veno-arterial extracorporeal life support (VA-ECMO): prevention and management of acute complications. Ann. Cardiothorac. Surg. 2019;8(1):66–75. doi: 10.21037/acs.2018.12.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrams D., Combes A., Brodie D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J. Am. Coll. Cardiol. 2014;63(25_Part_A):2769-78 doi: 10.1016/j.jacc.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 16.Bhardwaj A., Salas De Armas I., Al Rameni D., Patel M., Akay M.H., Kar B., et al. IHJ Cardiovascular Case Reports (CVCR) 7(2) 2023. Use of Impella 5.5 in patients with cardiogenic shock as a bridge to decision, recovery, or destination therapy; pp. 61–64. [Google Scholar]
- 17.Seyfarth M., Sibbing D., Bauer I., Fröhlich G., Bott-Flügel L., Byrne R., et al. A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J. Am. Coll. Cardiol. 2008;52(19):1584–1588. doi: 10.1016/j.jacc.2008.05.065. [DOI] [PubMed] [Google Scholar]
- 18.Schrage B., Ibrahim K., Loehn T., Werner N., Sinning J.-M., Pappalardo F., et al. Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation. 2019;139(10):1249–1258. doi: 10.1161/CIRCULATIONAHA.118.036614. [DOI] [PubMed] [Google Scholar]
- 19.Cheng R., Hachamovitch R., Kittleson M., Patel J., Arabia F., Moriguchi J., et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann. Thorac. Surg. 2014;97(2):610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Ali J.M., Abu-Omar Y. Complications associated with mechanical circulatory support. Ann. Transl. Med. 2020;8(13):835. doi: 10.21037/atm.2020.03.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russo J.J., Aleksova N., Pitcher I., Couture E., Parlow S., Faraz M., et al. Left ventricular unloading during extracorporeal membrane oxygenation in patients with cardiogenic shock. J. Am. Coll. Cardiol. 2019;73(6):654–662. doi: 10.1016/j.jacc.2018.10.085. [DOI] [PubMed] [Google Scholar]
- 22.Truby L.K., Takeda K., Mauro C., Yuzefpolskaya M., Garan A.R., Kirtane A.J., et al. Incidence and implications of left ventricular distention during venoarterial extracorporeal membrane oxygenation support. ASAIO J. 2017;63(3):257–265. doi: 10.1097/MAT.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 23.Lawler P.R., Silver D.A., Scirica B.M., Couper G.S., Weinhouse G.L., Camp P.C. Extracorporeal membrane oxygenation in adults with cardiogenic shock. Circulation. 2015;131(7):676–680. doi: 10.1161/CIRCULATIONAHA.114.006647. [DOI] [PubMed] [Google Scholar]
- 24.Bisdas T., Beutel G., Warnecke G., Hoeper M.M., Kuehn C., Haverich A., et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann. Thorac. Surg. 2011;92(2):626–631. doi: 10.1016/j.athoracsur.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 25.Subramaniam A.V., Barsness G.W., Vallabhajosyula S., Vallabhajosyula S. Complications of temporary percutaneous mechanical circulatory support for cardiogenic shock: an appraisal of contemporary literature. Cardiol. Ther. 2019;8(2):211–228. doi: 10.1007/s40119-019-00152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zein R., Patel C., Mercado-Alamo A., Schreiber T., Kaki A. A review of the Impella devices. Interv. Cardiol. 2022;17 doi: 10.15420/icr.2021.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glazier J.J., Kaki A. The Impella device: historical background, clinical applications, and future directions. Int. J. Angiol. 2019;28(2):118–123. doi: 10.1055/s-0038-1676369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo N.M.O.E. 2018. Mechanical Circulatory Support in ST-Elevation Myocardial Infarction. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material