Abstract
Background
We aimed to assess perioperative changes in fibrinogen in the cerebrospinal fluid (CSF), their association with markers of blood–brain barrier breakdown and neuroinflammation, and their association with postoperative delirium severity.
Methods
We conducted a secondary analysis of the Interventions for Postoperative Delirium-Biomarker 2 (IPOD-B2, NCT02926417) study, a prospective observational cohort study. We included 24 patients aged >21 yr undergoing aortic aneurysm repair. CSF samples were obtained before (n=24) and after surgery (n=13), with some participants having multiple postoperative samples. Our primary outcome was the perioperative change in CSF fibrinogen. Delirium was assessed using the Delirium Rating Scale-Revised-98.
Results
CSF fibrinogen increased after surgery (P<0.001), and this was associated with an increase in CSF/plasma albumin ratio (β=1.09, 95% CI 0.47–1.71, P=0.004). The peak change in CSF fibrinogen was associated with the change in CSF interleukin (IL)-10 and IL-12p70. The peak change in CSF fibrinogen was associated with the change in CSF total tau (β=0.47, 95% CI 0.24–0.71, P=0.002); however, we did not observe an association with postoperative delirium severity (incidence rate ratio = 1.20, 95% CI 0.66–2.17, P=0.540).
Conclusions
Our preliminary findings support the hypothesis that fibrinogen enters the brain via blood-brain barrier disruption, promoting neuroinflammation and neuronal injury. However, we did not observe an association between cerebrospinal fluid fibrinogen and peak delirium severity in this limited cohort.
Keywords: anaesthesia, cerebrospinal fluid, delirium, fibrinogen, neurocognitive disorders, surgery
Postoperative delirium is a common neurological complication in patients undergoing major surgery.1 It has been associated with longer hospital stays, greater mortality, and higher costs incurred by the health system.2 However, no pharmacological treatment has definitively shown benefit in the prevention of postoperative delirium. A key reason for this is the lack of proven therapeutic targets, which is underpinned by the absence of a proper understanding of the pathophysiology.3,4
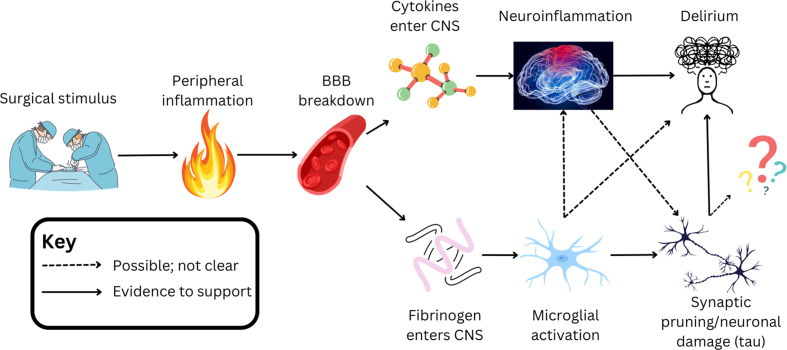
Recent studies have suggested that neuroinflammation, secondary to peripheral inflammation induced by a surgical stimulus, plays a pivotal role in postoperative delirium.4, 5, 6, 7 Inflammatory cytokines may promote delirium directly, or indirectly through facilitating access of deliriogenic agents into the brain via a breakdown of the blood–brain barrier (BBB). One candidate for such an agent is fibrinogen.
Fibrinogen is a blood coagulation factor produced in the liver and is the protein substrate for the final step of the coagulation cascade, where it is cleaved by thrombin to fibrin. Fibrinogen from the blood passes across the disrupted BBB, where it is deposited in the brain.8 Pathological deposition of fibrinogen has been noted in patients with Alzheimer's disease,9 multiple sclerosis,10 traumatic brain injury,11 and COVID-19.12 Fibrinogen has also been detected in the cerebrospinal fluid (CSF) and correlates with biomarkers of BBB dysfunction and damage.13 Fibrinogen concentrations in the CSF also correlate with multiple sclerosis and Alzheimer's disease progression.14, 15, 16, 17
Breakdown of the BBB is associated with delirium incidence,18,19 and our group has previously shown a dose–response relationship between BBB breakdown and delirium severity.20 BBB permeability was also associated with CSF concentrations of the inflammatory cytokine interleukin (IL)-6, linking neuroinflammation with delirium. Accompanying correspondence by Terrando and Akassoglou highlighted the need for further exploration of a potential role for fibrinogen as a pathophysiological link between neuroinflammation, BBB breakdown, and delirium.21
Herein, we assess evidence for a correlation between CSF fibrinogen and postoperative delirium severity, and relationships with BBB integrity and neuroinflammation. Our primary aim was to determine whether perioperative increases in CSF fibrinogen are associated with disruption of the BBB (as measured by the CSF:plasma albumin ratio (CPAR), sometimes referred to as Q-Alb). Secondary aims included determining whether perioperative increases in CSF fibrinogen are correlated with increases in inflammatory cytokines or biomarkers of neuronal damage and synaptic dysfunction, and whether there is a dose–relationship with delirium severity.
Methods
Interventions for Postoperative Delirium Biomarker-2 cohort
The Interventions for Postoperative Delirium Biomarker-2 (IPOD-B2, NCT02926417) study was a prospective observational cohort study conducted in the USA, which enrolled participants aged >21 yr without dementia undergoing elective open thoracoabdominal aortic aneurysm repair or thoracic endovascular aortic aneurysm repair under general anaesthesia and requiring the insertion of a spinal drain that was predicted to stay in for ≥2 postoperative days. This study received ethics approval from the University of Wisconsin–Madison Institutional Review board (2015-0960). The details of this cohort have been published elsewhere.22,23 We used the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines to report the findings.24
Outcomes
Here, we report secondary outcomes of the IPOD-B2 study: the perioperative change in CSF fibrinogen and the association of CSF fibrinogen with biomarkers of BBB breakdown (measured by the CPAR), neuroinflammation (CSF cytokines), neuronal injury (neurofilament light [NfL], total tau, phospho-tau [ptau]), and amyloid pathology (amyloid beta ratio [AβR; Aβ42:Aβ40]). We also analysed the association of CSF fibrinogen and cytokines with delirium severity. We have previously shown a relationship between some CSF biomarkers of neuronal injury (NfL, tau) and delirium severity in this cohort.25
Cerebral spinal fluid analysis
The preoperative (baseline) CSF samples were collected when the spinal drain was inserted before surgery for vascular surgery as previously reported.25 The timing of postoperative sample collection varied by when the drain was being used clinically for CSF pressure relief. In some enrolled participants a drain was not placed, and not all participants who had a drain placed before surgery ultimately required postoperative CSF to be drained. Some participants who required repeated CSF access also had multiple CSF samples taken for research. Plasma samples were taken at the same time as CSF samples. The ‘peak’ (maximal) postoperative result for CSF samples refers to the highest measured postoperative concentration. Samples were centrifuged at 3000 rpm for 10 min and then stored at –80°C. CSF fibrinogen and cytokines were assayed via enzyme-linked immunoassay according to manufacturer's standard protocol (Abcam (Cambridge, UK) ab108841; 1:200 dilution). CSF and plasma albumin were measured with immunoturbidimetry using a cobas instrument (Roche Diagnostics, Penzberg, Germany). Samples were also sent to the University of Gothenburg for amyloid beta, NfL, and tau analysis using an ultrasensitive single-molecule analysis (Simoa) machine (Quanterix, Billerica, MA, USA).
Delirium assessment
Delirium severity was measured using the Delirium Severity Score-Revised-98 (DRS). Delirium (yes/no) was diagnosed using the 3-min Diagnostic Confusion Assessment Method (3D-CAM). If the patient was intubated at the time of assessment, the CAM for the ICU was used.26,27 Participants were assessed for delirium twice a day while they were inpatient in the hospital by physicians, nurses, and research coordinators trained specifically to administer these assessments. Training involved learning the standard operating procedures for each assessment, observation, practice sessions, and supervised assessments with comparison of scores between the trainee and an expert. In addition, assessments were reviewed on a weekly basis with one of the study physicians to ensure consistent scoring over time.
Sample size justification
The IPOD-B2 study was powered to observe differences in frontoparietal feedback effective connectivity using resting state electroencephalogram recordings. Based on a hypothesized 20% difference in change from baseline in feedback connectivity between groups, a standard deviation of 20% observed in previous work with propofol,28 and our local 33% incidence of delirium, we required 38 subjects (13 delirious and 25 controls) to achieve 80% power with type I error rate alpha of 5% based on a two-sample t-test. However, the study was stopped prematurely owing to the principal investigator (RDS) leaving the University of Wisconsin and the project being superseded by the Interventions for Postoperative Delirium-Biomarker 3 (IPOD-B3) cohort that used evoked responses to understand feedback connectivity.29 As the analyses presented here are secondary outcomes of the IPOD-B2 study, an a priori power analysis was not used to determine sample size. Given the small sample size, we are limited to observing large effects in the data. For example, if we consider only paired samples from 13 participants, three predictors, 80% power, and alpha of 0.05 in a fixed effects model, we would only observe effects with a Cohen's f2 >0.76. Of note, we previously have observed large-size effects on BBB disruption using this dataset.20
Statistical analysis
Visual inspection of raw CSF biomarker histograms demonstrated strong positive skew, so we log10-transformed all biomarkers before analysis. Change scores were calculated as the log10 postoperative value minus the log10 baseline value. For all fixed effect analyses, the change value refers to the peak postoperative (maximal) change from baseline. As our analysis was exploratory, we excluded outliers for all models. We defined outliers as data points with a Cook's distance greater than four times the mean Cook's distance. Wilcoxon signed rank tests were used to compare biomarker concentrations in two groups if the data were paired. A P-value of 0.05 threshold for statistical significance was used in all analyses.
We used linear regression with the ordinary least squares estimator in cases where the dependent variable was a biomarker concentration. The use of change scores as the dependent variable is known to be susceptible to regression to the mean, hence we used multivariable models to predict the postoperative change values. These models use the postoperative value as the dependent (outcome) variable, and control for the corresponding baseline biomarker value. Our coefficient estimates correspond to the change in the biomarker concentration when the regressor increases by 1 unit, holding other regressors at their reference value. We used likelihood ratio tests to compare the more complex models with the simpler models.
Delirium severity was treated as a count variable. We aimed to use the simplest distribution to model the DRS score (the Poisson distribution), so long as the assumptions were met. We tested model assumptions and model fit using scaled stimulated residuals.30 If the scaled residuals suggested overdispersion, we used a negative binomial model. The incidence rate ratio (IRR) was used as the effect estimate from these models.
We conducted both fixed effects and mixed effects analyses for our multivariable models examining fibrinogen and delirium severity. Fixed effect models used the peak DRS value for each patient as the dependent variable and the peak (maximal) change in the fibrinogen as the independent variable (plus age as a covariable). Mixed effect models used all available data for patients (peak postoperative or otherwise), with a random intercept for each patient.
We plotted conditional predictions for multivariable models, which we defined as the predicted values for the model conditional on the values of a specified regressor, while holding unspecified regressors at their mean values. All analyses were conducted in R, via RStudio (Version 2023.06.1; R Foundation for Statistical Computing, Vienna, Austria). The ‘DHARMa’ package was used to simulate standardised residuals to assess the fit of count models.31 The ‘marginaleffects’ package was used to plot conditional predictions.32
Results
Patient characteristics
In total, 32 participants undergoing thoracoabdominal aneurysm repair with anticipated spinal drain placement were enrolled in the IPOD-B2 study. Of these, a spinal drain was ultimately placed in 26 participants, of which 24 had postoperative delirium assessments. Of these, 16/24 underwent open repair and 8/24 participants underwent endovascular repair. A total of 13 participants had both baseline and postoperative CSF fibrinogen data (their spinal drain was also accessed for a clinical indication after surgery), and delirium assessments (Supplementary Fig. S1). Of these, 10/13 underwent open and 3/13 underwent endovascular repairs. The characteristics of the cohort are shown in Supplementary Table S1. The incidence of postoperative delirium was 16/24 in those with preoperative CSF data (15/16 underwent open repair and 1/16 underwent endovascular repair), and 10/13 in those with preoperative and postoperative CSF data (all 10 underwent open repair). Participants with postoperative delirium generally had greater surgical risk and experienced greater intraoperative blood loss. The timeline of postoperative DRS scores in delirious and non-delirious participants is shown in Supplementary Fig. S2.
Perioperative change in CSF fibrinogen and blood–brain barrier permeability
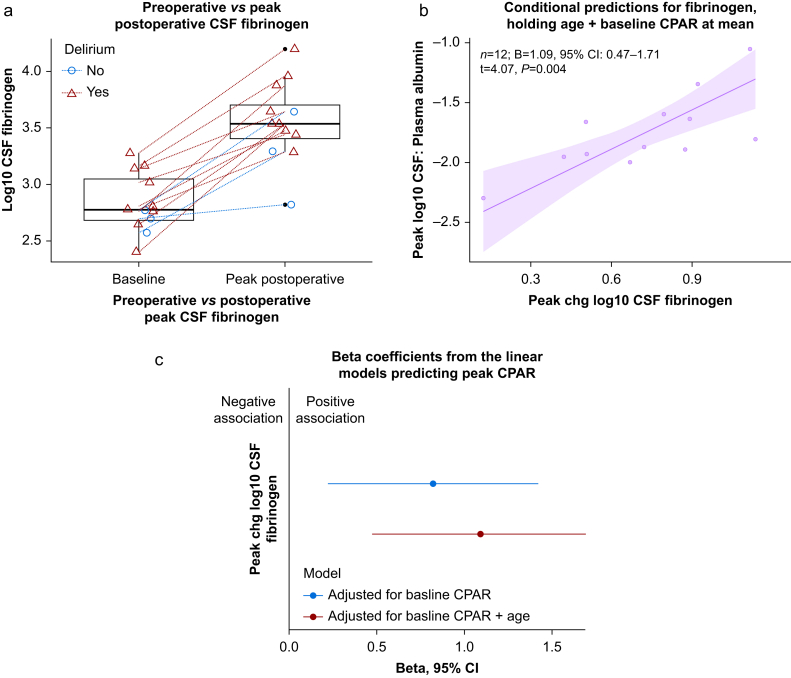
We analysed the change in CSF fibrinogen for each participant. For the 8/13 total participants with only one postoperative CSF sample, this represents the only available postoperative sample. We observed an increase in CSF fibrinogen after surgery (n=12; median 601.2 vs 3445.8 ng ml−1, Wilcoxon signed rank test, V=91, P<0.001; Fig. 1a).
Fig 1.
The change in CSF fibrinogen. (a) Preoperative to postoperative increase in CSF fibrinogen; lines connect each participant; n=12; median 601.2 vs 3445.8 ng ml−1, Wilcoxon signed rank test, V=91, P<0.001. Red triangles/lines represent delirious participants, and blue circles/lines are those without delirium at any time point. Horizontal scatter is added to the data points to facilitate visual interpretation. (b) Model-implied conditional predictions for peak CPAR based on change in CSF fibrinogen, holding age and baseline CPAR at their means. A Gaussian-family linear model is used, with n=12 (one outlier excluded per Cook's distance). The shaded region represents the 95% confidence interval for the predictions of peak CPAR conditional on the value for fibrinogen. More details of the linear model are in Supplementary Table S2. (c) The β coefficients and their 95% confidence intervals adjusted for baseline CPAR, and additionally adjusted for age (Supplementary Table S2). One outlier was excluded based on Cook's distances. CPAR, CSF:plasma albumin ratio; CSF, cerebrospinal fluid.
We observed a positive association between the change in CSF fibrinogen and CPAR after adjusting for age and baseline CPAR (n=12; β=1.09, 95% confidence interval [CI] 0.47–1.71, P=0.004; Supplementary Table S2). Conditional predictions are shown in Fig. 1b and c shows the coefficient estimates. The postoperative time course of CSF fibrinogen and CPAR is shown in Supplementary Fig. S3.
Association of change in CSF fibrinogen with CSF cytokines
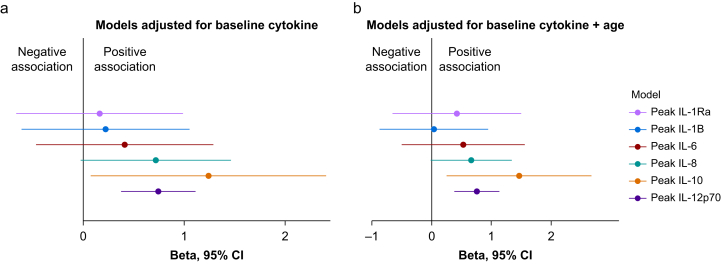
We analysed the association between the change in CSF fibrinogen and the change in CSF IL-1Ra, IL-1β, IL-6, IL-8, IL-10, and IL-12p70 to test the link between fibrinogen leakage and neuroinflammation. Adjusting for age, we observed an association between the change in fibrinogen and the change in IL-10 (β=1.46, 95% CI: 0.25–2.68, P=0.023) and IL-12p70 (β=0.76, 95% CI 0.38–1.13, P=0.002; Fig. 2, Supplementary Table S3). We did not observe evidence of an association between the change in IL-1Ra, IL-1β, IL-6, or IL-8 and the change in fibrinogen, in unadjusted or adjusted analyses (Fig. 2, Supplementary Table S3).
Fig 2.
The association between the change in CSF fibrinogen and CSF cytokines. (a) The β coefficients using the change in CSF fibrinogen to predict the change for each cytokine, controlling for the baseline cytokine concentration. (b) The β coefficients for each cytokine additionally adjusted for patient age. Based on Cook's distances, two outliers were excluded for IL-1Ra and IL-8, one outlier was excluded for IL-6 and IL-12p70, and no outliers were excluded for IL-1β and IL-10. Full details of the models are provided in Supplementary Table S3. CSF, cerebrospinal fluid; IL, interleukin.
Association of change in CSF cytokines with delirium severity
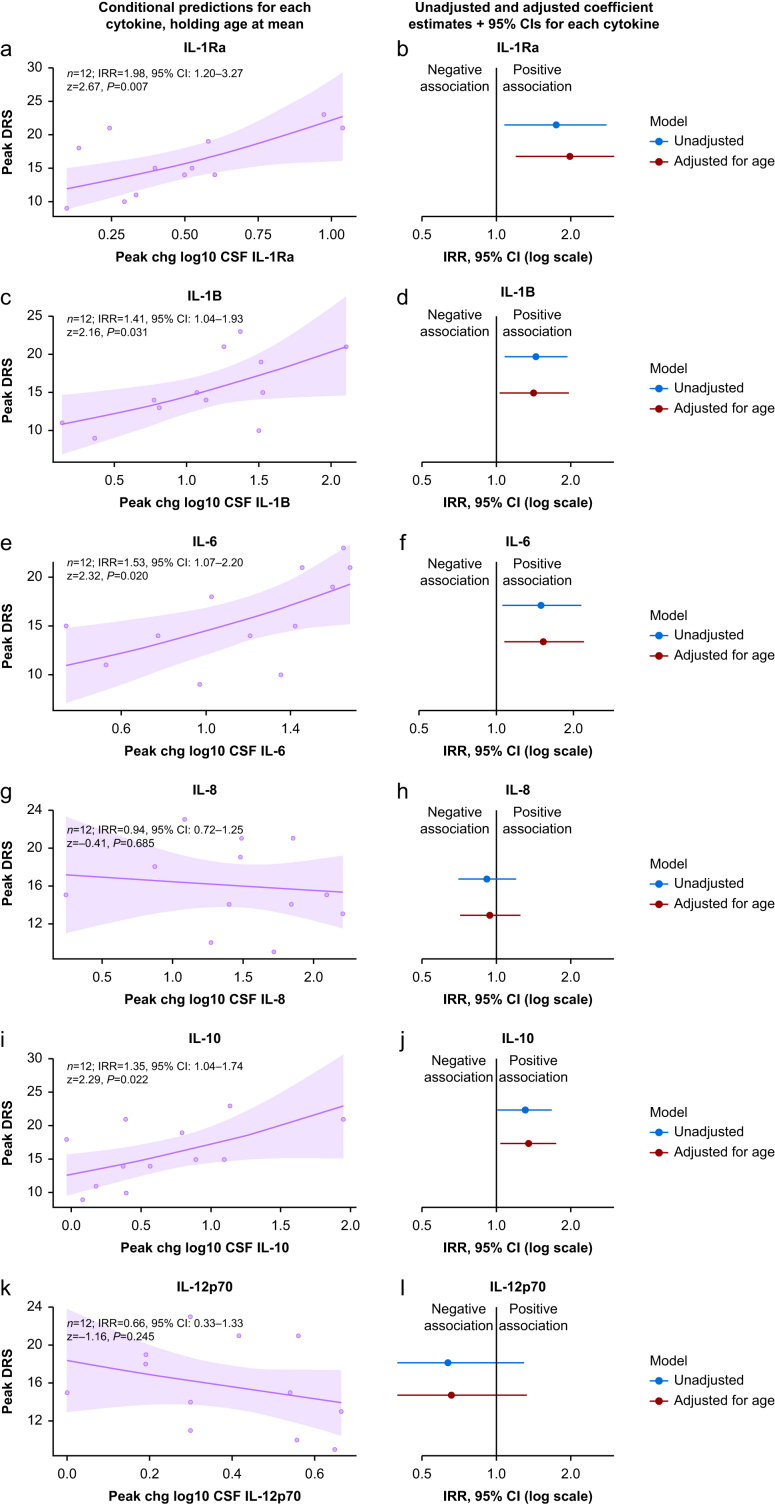
We did not observe evidence of overdispersion in any models assessing the relationship between the change in CSF cytokines and delirium severity (Supplementary Table S4), hence a Poisson-family linear model was used in all cases. In models adjusted for age, we observed evidence of an association of peak delirium severity and change in CSF IL-1Ra (n=12; IRR=1.98, 95% CI 1.20–3.27, P=0.007), IL-1β (n=12; IRR=1.41, 95% CI 1.04–1.93, P=0.031), IL-6 (n=12; IRR=1.53, 95% CI 1.07–2.20, P=0.020), and IL-10 (n=12; IRR=1.35, 95% CI 1.04–1.74, P=0.022) (Supplementary Table S5). We observed little to no evidence of an association between change in CSF IL-8 or IL-12p70 and peak delirium severity. Conditional predictions from the adjusted models and plots of the coefficient estimates from all models are shown in Fig. 3.
Fig 3.
The association of CSF cytokines with delirium severity. (Left; a, c, e, g, i, k) Conditional predictions for peak delirium severity based on CSF cytokines, holding age at its mean. Model coefficient estimates and 95% confidence intervals (CIs) printed within each plot. Shaded regions denote the 95% CI. All models are Gaussian-family linear models that adjust for age, with n=12 for all plots. (Right; b, d, f, h, j, l) Estimated incidence rate ratio for each cytokine from unadjusted models, and models adjusted for age. One outlier was excluded from all models, based on Cook's distances. Full details of these models are provided in Supplementary Table S5. CSF, cerebrospinal fluid; DRS, Delirium Severity Score-Revised-98.
Association of the change in CSF fibrinogen with neuronal biomarkers and delirium severity
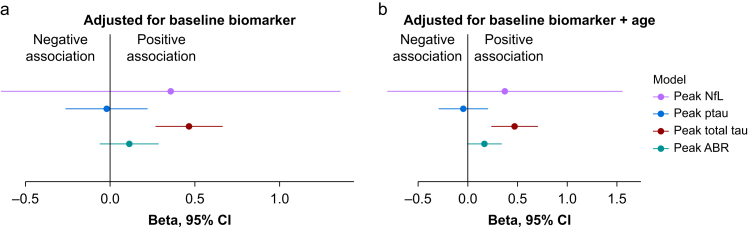
Our data showed a positive association between the change in CSF fibrinogen and change in the neuronal injury and synaptic dysfunction biomarker CSF total tau (β=0.47, 95% CI 0.24–0.71, P=0.002), after controlling for age and baseline total tau (Fig. 4, Supplementary Table S6). We did not observe an association with the change in AβR after adjusting for age and baseline AβR. Similarly, we did not observe an association between the change in fibrinogen and the change in the neuronal injury marker NfL or in ptau (Fig. 4, Supplementary Table S6).
Fig 4.
Association of the change in CSF fibrinogen with neuronal biomarkers. (a) The β coefficients using the change in CSF fibrinogen to predict CSF NfL, total tau, ptau, and AβR controlling for their baseline concentrations. (b) The β coefficients for CSF NfL, total tau, ptau, and AβR additionally adjusted for age. The coefficient point estimates and 95% confidence intervals are shown. One outlier was excluded based on Cook's distances in both models. Full details of these models are provided in Supplementary Table S6. AβR, amyloid beta ratio; CSF, cerebrospinal fluid; NfL, neurofilament light; ptau, phospho-tau.
We analysed the relationship between fibrinogen and delirium severity in a fixed effect model (using the change in fibrinogen and DRS value for each patient) and in a mixed effect model (using all available values for change in fibrinogen and DRS, and including a random intercept for each patient). In both models, there was no evidence of overdispersion (Supplementary Fig. S4), and the negative binomial model did not provide a better fit for the data (Supplementary Table S7); hence, the Poisson-family model was used.
In both the fixed effect and mixed effect models, we did not observe evidence of an association between change in CSF fibrinogen and peak delirium severity in the unadjusted analysis or after adjusting for age (Table 1).
Table 1.
Association of postoperative change in CSF fibrinogen with peak delirium severity, in linear fixed and mixed effect models. A Poisson-family model is used for both cases. AIC, Akaike information criterion; BIC, Bayesian information criterion; Chg, change; CI, confidence interval; Cook's D, Cook's distance; CSF, cerebrospinal fluid; IRR, incidence rate ratio; LRT, likelihood ratio test; No. Obs., number of observations. ∗Unadjusted model: Log-likelihood=–32.5; Deviance=10.7; AIC=68.9; BIC=69.9; No. Obs.=12; No. Obs. excluded (Cook's D)=1. Model adjusted for age: Log-likelihood=–31.9; Deviance=9.55; AIC=69.8; BIC=71.3; No. Obs.=12; No. Obs. excluded (Cook's D)=1; P-value from LRT comparing fit of two adjusted models =0.292. †Unadjusted model: Log-likelihood=–45.1; Deviance=13.7; AIC=96.1; BIC=98.3; No. Obs.=15; No. Obs. excluded (Cook's D)=2. Model adjusted for age: Log-likelihood=–44.7; Deviance=15.4; AIC=97.4; BIC=100; No. Obs.=15; No. Obs. excluded (Cook's D)=2; P-value from LRT comparing fit of two models =0.394. ‡P<0.05; P<0.01; P<0.001.
| Fixed effect model: Peak change per patient∗ | Mixed effects model: All data points per patient† | |||||
|---|---|---|---|---|---|---|
| Characteristic | IRR | 95% CI | P-value‡ | IRR | 95% CI | P-value‡ |
| Unadjusted | ||||||
| Postoperative Chg Log 10 CSF fibrinogen | 1.38 | 0.82, 2.34 | 0.234 | 0.87 | 0.37, 2.08 | 0.760 |
| Adjusted for age | ||||||
| Postoperative Chg Log 10 CSF fibrinogen | 1.20 | 0.66, 2.17 | 0.540 | 0.71 | 0.28, 1.82 | 0.475 |
Association of baseline biomarkers with delirium severity
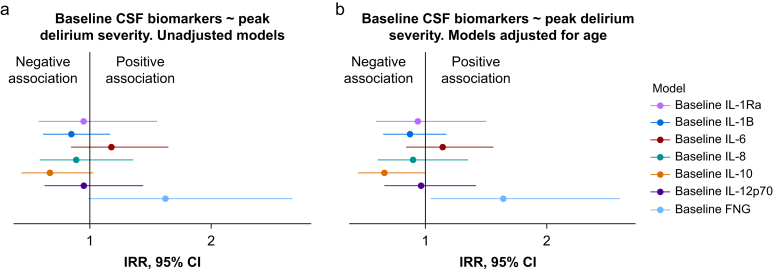
We analysed the relationship between baseline CSF biomarkers and peak postoperative delirium severity. Analysis of scaled residuals suggested overdispersion in our Poisson-family model and a negative binomial model provided a better fit to the data (Supplementary Tables S8 and S9). We observed evidence of an association between baseline CSF fibrinogen and delirium severity after adjusting for age (IRR=1.67, 95% CI 1.05–2.69, P=0.034; Fig. 5, Supplementary Table S10). We observed a negative association between baseline CSF IL-10 and peak delirium severity, after adjusting for age (IRR=0.65, 95% CI 0.42–1.00, P=0.041). We did not observe evidence of an association between baseline CSF concentrations of any other cytokine and peak delirium severity (Fig. 5, Supplementary Table S10).
Fig 5.
Association of baseline biomarkers with delirium severity. (a) The IRR for the baseline CSF concentration of each biomarker to predict delirium severity. (b) The IRR for each biomarker additionally adjusted for age. A negative binomial model was used for all biomarkers. The coefficient point estimates and 95% confidence intervals are shown. No outliers were excluded for IL-1Ra, whereas one outlier was excluded for IL-1β, IL-6, IL-8, 10, IL-12p70, and fibrinogen, based on Cook's distances. Full details of the models are provided in Supplementary Table S10. CSF, cerebrospinal fluid; IL, interleukin; IRR, incidence rate ratio.
Discussion
Our results suggest that CSF concentrations of fibrinogen increase in the perioperative period and correlate with markers of BBB disruption, inflammation and neuronal dysfunction. We demonstrated associations between the increase in fibrinogen and increases in CSF inflammatory cytokines IL-10 and IL-12p70, and with increases in the neuronal injury biomarker CSF total tau. However, in our small cohort that might be underpowered for many of our outcomes, we did not observe an association between the increase in CSF fibrinogen and delirium severity. Although the size of our cohort limits the strength our findings, our findings are consistent with a model where fibrinogen enters the CSF and promotes neuroinflammation and neuronal injury.
Our evidence that CSF fibrinogen correlates with CPAR is consistent with studies showing a correlation of CSF fibrinogen with platelet-derived growth factor receptor β (PDGFRβ),33 a marker of BBB dysfunction. Once inside the brain, the strong correlation between increases in CSF fibrinogen and CSF total tau suggests a possible role for fibrinogen in synaptic damage in postoperative delirium. Mouse models of Alzheimer's disease have shown that fibrinogen polarises microglia to a neurodegenerative and oxidative stress phenotype,34 resulting in loss of dendritic spines.35 Human studies have shown that plasma fibrinogen is correlated with CSF total tau in patients with Alzheimer's disease but not in healthy controls,15 and that increased plasma fibrinogen is independently associated with higher incident dementia risk.14
We have previously shown a correlation of CSF and plasma total tau and NfL with peak (maximal) delirium severity,7,25,36 suggesting a link between neuronal injury, synaptic alterations, and delirium. In the patient cohort analysed in this study, we identified correlations of fibrinogen with total tau, but not with NfL. It is possible that fibrinogen has an effect at the neuronal synapse that is independent of neuronal injury relating to postoperative delirium. Furthermore, a relationship between fibrinogen and other neuronal injury markers and delirium severity may exist, but the effect size is too small to be detected in our small cohort. Indeed, synaptic damage may correlate with delirium, but it may be a relatively small effect compared with inflammation, such as driven by IL-637 (as suggested by our data herein).
The lack of an observed association between the change in CSF fibrinogen and delirium severity may reflect our small sample size. It may also be explained by the observed associations with CSF cytokines; fibrinogen was associated with increases in IL-10 and IL-12p70, of which only IL-10 was associated with peak delirium severity. Intriguingly, IL-10 and IL-12/23p40 are jointly associated as predictors of β-amyloid load in patients with Alzheimer's disease and IL-10 suppresses microglia phagocytosis and worsens cognitive behaviour in Alzheimer's disease mice.38, 39, 40 As IL-10 is an anti-inflammatory cytokine limiting microglial functions with detrimental effects in Alzheimer's disease pathology, this may reflect a broad-based immune upregulation related to innate immune-mediated neurodegeneration. Meanwhile, increases in fibrinogen were not associated with increases in CSF IL-1Ra, IL-1β, or IL-6, all of which were individually associated with delirium. These proinflammatory cytokines have been implicated in neuronal dysfunction and apoptosis.3 Although our data point away from an association of fibrinogen with IL-1β and IL-6, neuroimmune processes are highly dynamic and the signals responsible for damaging the BBB likely precede subsequent CSF changes in proinflammatory cytokines, thus future efforts should address causality in the perioperative context.
There is a paucity of CSF literature with which to compare our cytokine results as few studies have looked at changes in CSF cytokines over time. Though a consistent finding is elevated innate immune cytokines in the CSF in delirious compared with non-delirious patients in cross-sectional studies.41 Analysis of plasma is more widely reported. Casey and colleagues7 observed strong evidence of an association between postoperative day 1 increases in plasma IL-8 and delirium severity, but other cytokines (IL-1Ra, IL-1β, IL-6, or IL-10) did not achieve statistical significance with rigorous multiple comparison testing. Conversely, another study42 showed an association of plasma IL-6 with delirium (a finding also consistent across smaller studies43); however, they did not observe an effect for other cytokines (IL-1Ra was not tested).44
The discordance between our CSF results and those from plasma studies, especially for IL-1Ra, and IL-1β, has many possible explanations. Given delirium is thought to be mediated partly by ‘primed’ microglia exhibiting an exaggerated IL-1 response to inflammation,3 it is plausible that pathophysiological elevations of IL-1Ra and IL-1β in delirium are more likely to be detected in the CSF than in the plasma. Indeed, Cape and colleagues45 showed that the preoperative CSF:serum ratio of IL-1β was increased in those who went on to experience postoperative delirium. There is strong biological plausibility for an association of increases in IL-1β and IL-1Ra with delirium: mouse models suggest a damaging cerebral metabolic effect of IL-1β (via hypoglycaemia),46 and a sickness behaviour phenotype effect of IL-1.47
The association of baseline CSF fibrinogen with postoperative delirium is likely reflective of baseline vascular risk factors, given all patients were undergoing vascular surgery. Fibrinogen is not present in healthy brains, and participants in our vascular surgical cohort with more permeable BBBs at baseline likely reflect a sicker patient cohort who are at greater risk of delirium. Indeed, breakdown of the BBB has been hypothesised to be a pathophysiological link between vascular risk factors and Alzheimer's dementia,48 and vascular pathology is a comorbidity accelerating disease progression in multiple sclerosis.49
A strength of this study is our focus on delirium severity. Many studies of biomarkers in the field use a dichotomous delirium incidence, which sacrifices the power needed to study dose–response relationships. Another strength of this study is our analysis of perioperative change in CSF biomarkers. Many studies are capable of correlating outcomes with baseline CSF biomarkers (given the relative ease of obtaining CSF samples in consenting patients when the preoperative spinal anaesthetic is placed)45,50; however, postoperative samples are less widely available. The most important limitation of this study is the low number of participants. This is an ongoing issue for all studies that require postoperative CSF sampling,51 as they usually rely on patients needing CSF sampling for other indications. Therefore, we were conservative in fitting our statistical models, and report unadjusted and adjusted models. The study only recruited patients who underwent invasive vascular surgery. Although this was integral to the collection of CSF samples, it limits the generalisability of our results to other types of surgery. Other limitations include a relatively large number of analyses that did not control for multiple comparisons in this exploratory study, and the variable number of CSF samples that were collected for each patient. Moreover, the removal of outliers might have impacted our conclusions, hence the need for further work in larger samples that are more robust to individual data points.
Conclusions
Our preliminary findings in this limited cohort support important concepts underlying the pathology of delirium where fibrinogen enters the cerebral spinal fluid via blood-brain barrier disruption and promotes neuroinflammation and neurodegeneration. The correlation of fibrinogen with total tau in the cerebral spinal fluid suggests a role in synapse loss, in accordance with studies in Alzheimer's disease animal models. Fibrinogen might also contribute to some components of a neuroinflammatory response, a putative driver of delirium. However, we did not observe an association of fibrinogen with delirium severity. Given the limited size of our cohort, the potential role of fibrinogen in delirium will require further evaluation in larger patient cohorts.
Authors’ contributions
Designed the study in consultation: RS, RL, RP
Supplied the assays and managed biofluids analysis: HZ, KB
Data analysis: TP
Drafted the manuscript: TP
Critical feedback on the manuscript: KA, NT, AMF, RP, HZ, KB, FI, DK, KK, JT
Funding
Swedish Research Council (#2022-01018 and #2019-02397 to HZ); European Union’s Horizon Europe research and innovation programme (101053962 to HZ); Swedish State Support for Clinical Research (#ALFGBG-71320 to HZ); Alzheimer Drug Discovery Foundation, USA (#201809-2016862 to HZ); AD Strategic Fund and the Alzheimer's Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C to HZ); Bluefield Project, Olav Thon Foundation, Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270 to HZ); European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement (860197 MIRIADE to HZ); European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694 to HZ); National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre; UK Dementia Research Institute at UCL (UKDRI-1003 to HZ); Ray and the Dagmar Dolby Family Fund (to KA); Simon Family Trust (to KA); and NIH/NIA RF1 AG064926 and NIH/NINDS R35 NS097976 (to KA); NT is supported by 2R01AG057525-06A1; National Institutes of Health (R01 AG063849-01 to RDS); Swedish Research Council (#2017-00915 and #2022-00732 to KB); Swedish Alzheimer Foundation (#AF-930351, #AF-939721, and #AF-968270 to KB); Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006 to KB); Swedish state under the agreement between the Swedish government and the County Councils; ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240 to KB); Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495 to KB) and 2022–2025 grant (SG-23-1038904 QC to KB).
Data availability statement
The original data are available from the authors upon reasonable request.
Declarations of interests
HZ is a Wallenberg Scholar and has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). KA is the scientific founder, advisor, and shareholder of Therini Bio, Inc. Her interests are managed by Gladstone Institutes according to its conflict of interest policy. AMF is also a co-founder of Therini Bio, Inc. KB has served as a consultant for Acumen, AriBio, ALZpath, BioArctic, Biogen, Eisai, Lilly, Ono Pharma, Roche Pharma, Roche Diagnostics, and Siemens Healthineers. RDS is an editor at the British Journal of Anaesthesia.
Handling editor: Susan M. Goobie
Footnotes
Some aspects of this work were previously presented in an oral presentation at the Australasian Delirium Association ‘DECLARED’ conference in November 2023.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bjao.2024.100349.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
References
- 1.Jin Z., Hu J., Ma D. Postoperative delirium: perioperative assessment, risk reduction, and management. Br J Anaesth. 2020;125:492–504. doi: 10.1016/j.bja.2020.06.063. [DOI] [PubMed] [Google Scholar]
- 2.Boone M.D., Sites B., von Recklinghausen F.M., Mueller A., Taenzer A.H., Shaefi S. Economic burden of postoperative neurocognitive disorders among US Medicare patients. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson J.E., Mart M.F., Cunningham C., et al. Delirium. Nat Rev Dis Primers. 2020;6:90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders R.D. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. 2011;77:140–143. doi: 10.1016/j.mehy.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado J.R. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018;33:1428–1457. doi: 10.1002/gps.4823. [DOI] [PubMed] [Google Scholar]
- 6.Tanabe S., Mohanty R., Lindroth H., et al. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth. 2020;125:55–66. doi: 10.1016/j.bja.2020.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey C.P., Lindroth H., Mohanty R., et al. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2019;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen M.A., Ryu J.K., Akassoglou K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. 2018;19:283–301. doi: 10.1038/nrn.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiala M., Liu Q.N., Sayre J., et al. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood–brain barrier. Eur J Clin Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 10.Davalos D., Mahajan K.R., Trapp B.D. Brain fibrinogen deposition plays a key role in MS pathophysiology - yes. Mult Scler. 2019;25:1434–1435. doi: 10.1177/1352458519852723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenkins D.R., Craner M.J., Esiri M.M., DeLuca G.C. Contribution of fibrinogen to inflammation and neuronal density in human traumatic brain injury. J Neurotrauma. 2018;35:2259–2271. doi: 10.1089/neu.2017.5291. [DOI] [PubMed] [Google Scholar]
- 12.Lee M.-H., Perl D.P., Nair G., et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2020;384:481–483. doi: 10.1056/NEJMc2033369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montagne A., Nation D.A., Sagare A.P., et al. APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline. Nature. 2020;581:71–76. doi: 10.1038/s41586-020-2247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Oijen M., Witteman J.C., Hofman A., Koudstaal P.J., Breteler M.M.B. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36:2637–2641. doi: 10.1161/01.STR.0000189721.31432.26. [DOI] [PubMed] [Google Scholar]
- 15.Fan D.Y., Sun H.L., Sun P.Y., et al. The correlations between plasma fibrinogen with amyloid-beta and tau levels in patients with Alzheimer's disease. Front Neurosci. 2020;14 doi: 10.3389/fnins.2020.625844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig-Schapiro R., Kuhn M., Xiong C., et al. Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer's disease diagnosis and prognosis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.W., Namkoong H., Kim H.K., et al. Fibrinogen gamma-A chain precursor in CSF: a candidate biomarker for Alzheimer's disease. BMC Neurol. 2007;7:14. doi: 10.1186/1471-2377-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devinney M.J., Wong M.K., Wright M.C., et al. Role of blood–brain barrier dysfunction in delirium following non-cardiac surgery in older adults. Annal Neurol. 2023;94:1024–1035. doi: 10.1002/ana.26771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hov K.R., Berg J.P., Frihagen F., et al. Blood-cerebrospinal fluid barrier integrity in delirium determined by Q-albumin. Dement Geriatr Cogn Disord. 2016;41:192–198. doi: 10.1159/000443789. [DOI] [PubMed] [Google Scholar]
- 20.Taylor J., Parker M., Casey C.P., et al. Postoperative delirium and changes in the blood-brain barrier, neuroinflammation, and cerebrospinal fluid lactate: a prospective cohort study. Br J Anaesth. 2022;129:219–230. doi: 10.1016/j.bja.2022.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terrando N., Akassoglou K. Breaking barriers in postoperative delirium. Br J Anaesth. 2022;129:147–150. doi: 10.1016/j.bja.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Payne T., Taylor J., Casey C., et al. Prospective analysis of plasma amyloid beta and postoperative delirium in the Interventions for Postoperative Delirium: biomarker-3 study. Br J Anaesth. 2023;130:546–556. doi: 10.1016/j.bja.2023.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor J., Payne T., Casey C., et al. Sevoflurane dose and postoperative delirium: a prospective cohort analysis. Br J Anaesth. 2023;130:e289–e297. doi: 10.1016/j.bja.2022.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 25.Parker M., White M., Casey C., et al. Cohort analysis of the association of delirium severity with cerebrospinal fluid amyloid-tau-neurodegeneration pathologies. J Gerontol A Biol Sci Med Sci. 2022;77:494–501. doi: 10.1093/gerona/glab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcantonio E.R., Ngo L.H., O'Connor M., et al. 3D-CAM: derivation and validation of a 3-minute diagnostic interview for CAM-defined delirium: a cross-sectional diagnostic test study. Ann Intern Med. 2014;161:554–561. doi: 10.7326/M14-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ely E.W., Margolin R., Francis J., et al. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive Care unit (CAM-ICU) Crit Care Med. 2001;29:1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Boly M., Moran R., Murphy M., et al. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gjini K., Casey C., Kunkel D., et al. Delirium is associated with loss of feedback cortical connectivity. Alzheimers Dement. 2024;20:511–524. doi: 10.1002/alz.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelman A., Hill J. Cambridge University Press; Cambridge: 2006. Data analysis using regression and multilevel/hierarchical models. [Google Scholar]
- 31.Dunn P.K., Smyth G.K. Randomized quantile residuals. J Comput Graph Stat. 1996;5:236–244. [Google Scholar]
- 32.Arel-Bundock V. 2024. How to interpret statistical models using marginaleffects in R and Python.https://marginaleffects.com Available from: [Google Scholar]
- 33.Miners J.S., Schulz I., Love S. Differing associations between Aβ accumulation, hypoperfusion, blood-brain barrier dysfunction and loss of PDGFRB pericyte marker in the precuneus and parietal white matter in Alzheimer's disease. J Cereb Blood Flow Metab. 2018;38:103–115. doi: 10.1177/0271678X17690761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendiola A.S., Yan Z., Dixit K., et al. Defining blood-induced microglia functions in neurodegeneration through multiomic profiling. Nat Immunol. 2023;24:1173–1187. doi: 10.1038/s41590-023-01522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merlini M., Rafalski V.A., Rios Coronado P.E., et al. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an Alzheimer's disease model. Neuron. 2019;101:1099–1108.e6. doi: 10.1016/j.neuron.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ballweg T., White M., Parker M., et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth. 2021;126:458–466. doi: 10.1016/j.bja.2020.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan B.A., Perkins A.J., Prasad N.K., et al. Biomarkers of delirium duration and delirium severity in the ICU. Crit Care Med. 2020;48:353–361. doi: 10.1097/CCM.0000000000004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedrini S., Gupta V.B., Hone E., et al. A blood-based biomarker panel indicates IL-10 and IL-12/23p40 are jointly associated as predictors of β-amyloid load in an AD cohort. Sci Rep. 2017;7 doi: 10.1038/s41598-017-14020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakrabarty P., Li A., Ceballos-Diaz C., et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85:519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guillot-Sestier M.V., Doty K.R., Gate D., et al. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85:534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall R.J., Watne L.O., Cunningham E., et al. CSF biomarkers in delirium: a systematic review. Int J Geriatr Psychiatry. 2018;33:1479–1500. doi: 10.1002/gps.4720. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt E.M., Marcantonio E.R., Alsop D.C., et al. Novel risk markers and long-term outcomes of delirium: the Successful Aging after Elective Surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13:10. doi: 10.1016/j.jamda.2012.08.004. 818.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thisayakorn P., Thipakorn Y., Tantavisut S., Sirivichayakul S., Maes M. Delirium due to hip fracture is associated with activated immune-inflammatory pathways and a reduction in negative immunoregulatory mechanisms. BMC Psychiatry. 2022;22:369. doi: 10.1186/s12888-022-04021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasunilashorn S.M., Ngo L., Inouye S.K., et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J Gerontol A Biol Sci Med Sci. 2015;70:1289–1295. doi: 10.1093/gerona/glv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cape E., Hall R.J., van Munster B.C., et al. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J Psychosom Res. 2014;77:219–225. doi: 10.1016/j.jpsychores.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kealy J., Murray C., Griffin E.W., et al. Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. J Neurosci. 2020;40:5681–5696. doi: 10.1523/JNEUROSCI.2876-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X., Nemeth D.P., McKim D.B., et al. Cell-type-specific interleukin 1 receptor 1 signaling in the brain regulates distinct neuroimmune activities. Immunity. 2019;50:317–333.e6. doi: 10.1016/j.immuni.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He J.T., Zhao X., Xu L., Mao C.Y. Vascular risk factors and Alzheimer's disease: blood-brain barrier disruption, metabolic syndromes, and molecular links. J Alzheimers Dis. 2020;73:39–58. doi: 10.3233/JAD-190764. [DOI] [PubMed] [Google Scholar]
- 49.Marrie R.A., Rudick R., Horwitz R., et al. Vascular comorbidity is associated with more rapid disability progression in multiple sclerosis. Neurology. 2010;74:1041–1047. doi: 10.1212/WNL.0b013e3181d6b125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westhoff D., Witlox J., Koenderman L., et al. Preoperative cerebrospinal fluid cytokine levels and the risk of postoperative delirium in elderly hip fracture patients. J Neuroinflammation. 2013;10:889. doi: 10.1186/1742-2094-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirsch J., Vacas S., Terrando N. Perioperative cerebrospinal fluid and plasma inflammatory markers after orthopedic surgery. J Neuroinflammation. 2016;13:211. doi: 10.1186/s12974-016-0681-9. Handling editor. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data are available from the authors upon reasonable request.