Abstract
Backgrounds & Aims
Bile acids (BAs) are core gastrointestinal metabolites with dual functions in lipid absorption and cell signaling. BAs circulate between the liver and distal small intestine (i.e., ileum), yet the dynamics through which complex BA pools are absorbed in the ileum and interact with host intestinal cells in vivo remain poorly understood. Because ileal absorption is rate-limiting in determining which BAs in the intestinal lumen gain access to host intestinal cells and receptors, and at what concentrations, we hypothesized that defining the rates and routes of ileal BA absorption in vivo would yield novel insights into the physiological forms and functions of mouse enterohepatic BA pools.
Methods
Using ex vivo mass spectrometry, we quantified 88 BA species and metabolites in the intestinal lumen and superior mesenteric vein of individual wild-type mice, and cage-mates lacking the ileal BA transporter, Asbt/Slc10a2.
Results
Using these data, we calculated that the pool of BAs circulating through ileal tissue (i.e., the ileal BA pool) in fasting C57BL/6J female mice is ∼0.3 μmol/g. Asbt-mediated transport accounted for ∼80% of this pool and amplified size. Passive permeability explained the remaining ∼20% and generated diversity. Compared with wild-type mice, the ileal BA pool in Asbt-deficient mice was ∼5-fold smaller, enriched in secondary BA species and metabolites normally found in the colon, and elicited unique transcriptional responses on addition to exvivo–cultured ileal explants.
Conclusions
This study defines quantitative traits of the mouse enterohepatic BA pool and reveals how aberrant BA metabolism can impinge directly on host intestinal physiology.
Keywords: Bile Acids, Enterohepatic Circulation, Intestinal Absorption, ASBT
Graphical abstract
Summary.
Knowledge of bile acid gene regulatory functions centers on individual species. However, physiological functions of bile acids reflect collective outputs of enterohepatic pools. This study identifies and functionally characterizes the pool of mouse bile acids that circulates through ileal tissue.
The mammalian gastrointestinal (GI) tract harbors more than 100 bile acid (BA) species and metabolites, each with unique physicochemical properties.1 As such, increasingly advanced analytical methods (e.g., metabolomics) are being developed to quantify large numbers of BAs within complex biospecimens (e.g., blood, stool).2,3 Although these studies have revolutionized understanding of BA biochemistry, even the most powerful instruments used for single-site measurements fail to inform broader regulatory dynamics that underlie BA circulation and signaling in vivo.
Hepatocytes synthesize a quantitatively large, but compositionally simple, pool of primary BAs (e.g., bile salts; cholic acid [CA] and chenodeoxycholic acid [CDCA] in humans; CA, CDCA, α-muricholic acid, and β-muricholic acid [βMCA] in mice) via cholesterol catabolism.4 These BAs are conjugated to amino acids glycine or taurine, transported into bile ducts, concentrated in the gallbladder, and secreted into the duodenum. BA n-acyl amidation limits membrane permeability and cytotoxicity and restricts BAs to the lumen of the proximal small intestine (SI) for efficient micelle formation.4,5
Because most BA-dependent lipid and vitamin absorption occurs in the proximal- and mid-SI (i.e., duodenum and jejunum, respectively), an increasing proportion of BAs reaching the distal SI (i.e., ileum) occur in free/nonmicellular forms. An estimated 95% of free BAs are reclaimed from the ileal lumen by specialized enterocytes expressing the apical sodium-dependent BA transporter (ASBT/SLC10A2).4,6 Intraepithelial BAs are bound by the ileal BA-binding protein (iBABP/FABP6), trafficked to the basolateral surface, and transported into underlying mucosal tissue (i.e., lamina propria) by the OSTα/β complex.7,8 Because of active absorption, ileal immune and epithelial cells are exposed to uniquely high BA concentrations.9,10 Ileal enterocytes manage BA toxicity via the nuclear receptor (NR), FXR/Nr1h4, which on activation by intracellular BAs promotes expression of several adaptive genes, including Fapb6 (to increase BA-binding capacity), OSTα/β (Slc51a/Slc51b; to increase basolateral efflux activity), and Fgf15 (FGF19 in humans; to restrict hepatic BA synthesis).11, 12, 13, 14, 15, 16, 17, 18 In parallel, immune cells in the ileal lamina propria leverage an orthogonal NR, CAR/Nr1i3, to limit BA-driven oxidative stress and inflammation.19,20 Ultimately, BAs in ileal tissue enter portal capillaries to be carried back to the liver for reuptake, potential enzymatic modification, and resecretion into bile, thus beginning another enterohepatic cycle.4,21
The fraction of primary BAs escaping ileal absorption (∼5% at homeostasis) enter the large intestine (LI) and become increasingly subject to metabolism by commensal microbiota.1 The gatekeeper reaction in bacterial BA metabolism is deconjugation (i.e., deamidation; (t)CA→CA) by bile salt hydrolase (bsh) enzymes.22 Following deconjugation, primary BAs can undergo diverse microbe-dependent reactions including dehydroxylation, epimerization of hydroxyl groups to form “iso” BAs, oxidation and reduction to form “allo” BAs, secondary side chain conjugation to other amino acids, and esterification at the 3-hydroxy position to fatty acids.1,23, 24, 25 7α-dehydroxylation is among the most common and important microbial BA biotransformation, producing major secondary BAs (e.g., deoxycholic acid [DCA], from CA; lithocholic acid [LCA], from CDCA), and using enzymes encoded by the BA-induced (bai) operon, a common genetic trait of several commensal genera (e.g., Clostridia, Bacillus).26 The LI BA pool is thus quantitatively smaller, but also more diverse and hydrophobic, than the SI BA pool. Colonic BAs are either excreted in feces or passively absorbed for portal recirculation to the liver. BAs diffusing across the colonic epithelium interface with mucosal immune cells, where LCA and several of its metabolites have been shown to interact with other NRs (VDR/Nr1i1, RORγt/Nr1f3) and enforce immune tolerance.27,28
In line with their diverse and critical functions, changes in BA circulation and/or metabolism have been linked to a growing number of human diseases, from metabolic dysfunction–associated steatotic liver disease and GI cancers, to inflammatory bowel disease (IBD) and cystic fibrosis (CF).29,30 Yet deciphering the causes and consequences of disease-associated BA dysmetabolism has proven more formidable. In IBD and CF, for example, levels of secondary BAs in stool are generally lower in patients vs. control subjects.31, 32, 33, 34 Although these results are widely interpreted as decreased bacterial BA metabolism caused by dysbiosis,1,35 alternative explanations cannot be excluded without augmented information about hepatic synthesis and/or intestinal absorption. Functionally, it is presumed that exposure of host intestinal cells to disease-associated BAs can precipitate immune and epithelial dysfunction, yet it remains unclear how BA concentrations in clinically accessible sites (e.g., stool, serum), or even in the intestinal lumen, correspond to local levels in intestinal mucosa, the latter being limited by variable and undefined rates of active and passive absorption in vivo. Finally, even if tissue-associated BA concentrations can be extrapolated or measured empirically, it is not known how BAs in complex enterohepatic pools compete for interactions with host cells and receptors.
Appreciating that BA homeostasis is an active process, governed by higher-order endocrine networks connecting the liver (where BAs are made), ileum (where most BAs are absorbed), and colonic microbiota (where most BAs are metabolized), we sought to develop an integrated methods set to quantify the form and functions of mouse enterohepatic BA pools that circulate through the ileum. The approach features parallel sampling of nearly 100 BA species in 3 GI sites of individual mice and yields quantitative insights into ileal BA absorption. Combining metabolomics with metagenomics informs aspects of BA dysmetabolism that involve altered microbial function. Incorporating host gene expression analyses, particularly in the liver and ileum, reveals whether, where, and how BA-dependent signaling is affected. Finally, molecular functions of enterohepatic BA pools can be examined independent of confounding in vivo variables (e.g., on microbiota, host metabolism) using cultured intestinal explants. We validate these approaches using mice lacking the ileal BA reuptake transporter, Asbt, and that harbor a markedly distinct enterohepatic BA pool. In the process, we provide working answers to long-standing questions about the form and functions of the ileal BA pool. Together, this work reshapes extant views of the gut-liver axis, moves BA understanding from individual species toward complex enterohepatic pools, and forges new paradigms in the regulation and function of BAs in human health and disease.
Results
System-Level Perspectives of In Vivo BA Circulation and Metabolism
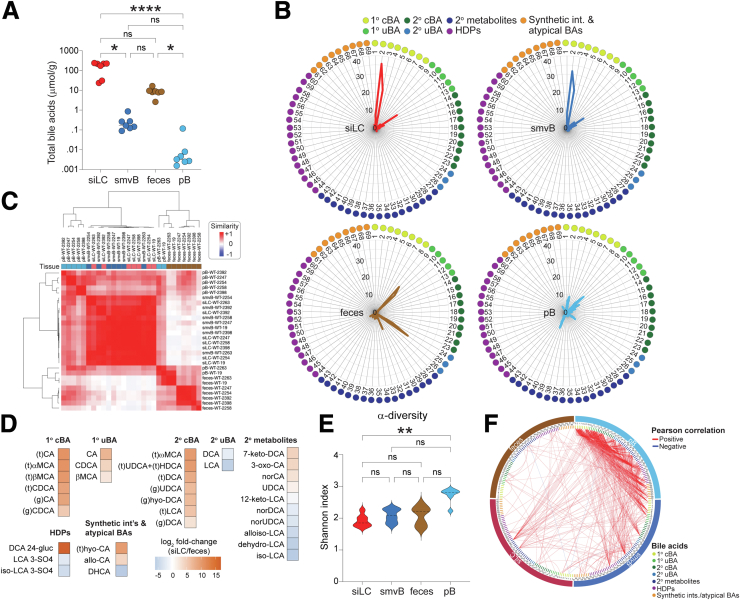
To leverage the strengths of mass spectrometry, while also appreciating its limitations (e.g., lack of spatial resolution in intestinal tissues), we surveyed 88 BA species and metabolites in 3 GI sites of 7 independently (specific pathogen free) housed female C57BL/6J (B6) wild-type mice: (1) SI luminal content (siLC), to define the pool of BAs made by the liver and secreted into the intestine; (2) superior mesenteric vein blood (smvB), to resolve the pool of luminal BAs reabsorbed primarily in the ileum; and (3) feces, to elaborate the fraction of BAs escaping ileal absorption and becoming metabolized in the LI (Figure 1A). We used fecal BAs as a surrogate for those in the colon lumen because an initial pilot study revealed that these pools were highly similar (Figure 1B). A fourth site, peripheral blood (pB), was included to formally establish whether or how BAs in peripheral circulation (an increasingly common clinical measurement) reflect those in local intestinal pools (Figure 1A). To avoid interanimal variation in postprandial BA secretion and ileal delivery,36 mice were fasted for 4 hours before sample collection.
Figure 1.
Preprocessing, filtering, and clustering of ex vivo BA metabolomics data from mice. (A) A schematic diagram of BA enterohepatic circulation adapted from.37 88 BA species and metabolites were analyzed by LC-MS/MS in the sites indicated by 1–4 numbering. (B) Concentrations (μmol/g) of individual BA species, determined by LC-MS/MS, in feces or cLC of separate 30-week-old wild-type C57BL/6J (B6) female mice (n = 1 each per sample type). Pearson correlation shows significant compositional similarity between the pools, despite being from different animals. (C) Median concentrations (μmol/g; n = 7) of individual BA species in the indicated sites of 30-week-old wild-type female B6 mice. 88 total BA species were analyzed in each site. Numbers of BA species with median values of >0 in each site are indicated on x-axis; white boxes (i, ii, iii) highlight BA species whose median values were 0 in all 3 GI sites and were removed from downstream analyses. No pB BA species with median values of 0 were excluded, because each of these were reliably detected in at least 1 of other 3 sites (siLC, smvB, feces). (D) Grouping of the 88 individual BA species analyzed based on: (1) reliability of detection by LC-MS/MS (all species where median concentrations i + ii + iii > 0 in B); and (2) likely biosynthetic origin. Poorly detected species (median concentrations i + ii + iii = 0 in B) are not strictly grouped. cLC, colon luminal content; GB, gallbladder; HDPs, hepatic phase 2 detoxification products; HPV, hepatic portal vein; imv, inferior mesenteric vein; IVC, inferior vena cava; LC-MS/MS, liquid chromatography coupled with tandem mass spectrometry; pB, peripheral blood; siLC, small intestine luminal contents; smvB, superior mesenteric vein blood; 1o cBAs, primary conjugated BAs; 1o uBAs, primary unconjugated BAs; 2o cBAs, secondary conjugated BAs; 2o uBAs, secondary unconjugated BAs; 2o metabolites, secondary metabolites.
69 of 88 BA species and metabolites measured displayed median values >0 in at least 1 of the 3 GI sites and were considered reliably detectable (Figure 1C). These became the focus of downstream analyses and clustered into 7 general classes based on likely biosynthetic origin (Figure 1D)26,38,39: (1) primary conjugated BAs (1o cBAs), most BAs synthesized and secreted by the liver at homeostasis; (2) primary unconjugated BAs (1o uBAs), products of bacterial 1o cBA deconjugation before further microbial or host metabolism; (3) secondary conjugated BAs (2o cBAs), secondary unconjugated BAs that get reconjugated in hepatocytes following recirculation from the intestines; (4) secondary unconjugated BAs (2o uBAs), canonical 7α-dehydroxylation products generated from 1o uBAs via LI bacterial metabolism; (5) secondary (2o) metabolites, more extensively modified 2o uBAs (e.g., iso-LCA, 3-oxo-CA) produced via gut bacterial metabolism; (6) hepatic phase 2 detoxification products, including sulfate and glucuronide conjugates produced principally in hepatocytes to limit BA toxicity and promote clearance; and (7) synthetic intermediates and atypical BAs, species secreted in trace amounts by healthy livers that tend to increase in settings of liver disease (e.g., hepatobiliary disease, cancer).40, 41, 42, 43
On aggregate, BAs were enriched in enterohepatic tissues (i.e., siLC, smvB), as expected, reaching fasting levels of ∼160 μmol/g in siLC and ∼0.16 μmol/g (∼200 μM) in smvB (Figure 2A). siLC and smvB BA pools were compositionally akin to each other, consistent with efficient ileocecal absorption in the mouse, whereas both were clearly distinct from matched fecal pools (Figure 2B and C). As expected, 1o cBAs and 1o uBAs, and 2o cBAs, were enriched in the enterohepatically cycling pool, whereas 2o uBAs and 2o metabolites (e.g., iso-LCA, alloiso-LCA) were elevated in feces (Figure 2D). Notably, the pool of pB BAs was most variable between animals, displayed the highest α-diversity (i.e., intrasample complexity), and presented mixed features of enterohepatic and fecal pools (Figure 2C and E). This result is consistent with the anatomic source, because metabolites in peripheral circulation include those that evade hepatic first-pass clearance following portal recirculation from both the SI (via the smv) and LI (via the inferior mesenteric vein [imv]) (Figure 1A). Correlation matrices of all 69 BAs in each of the 4 sites revealed that fecal BAs share the fewest relationships with BAs in other sites (Figure 2F), further highlighting strict compartmentalization of SI (enterohepatically cycling) and LI BA pools.
Figure 2.
Multisite analysis of in vivo BA metabolomes. (A) Total BA concentrations (μmol/g of luminal content or plasma; n = 7), determined by LC-MS/MS, in the 4 sites numbered and color-coded in Figure 1A from C57BL/6J (B6) wild-type female mice. Data are presented as the sum of 69 reliably detectable species described in Figure 1C and D. Each symbol represents data from 1 mouse; horizontal lines indicate means. ∗P < .05, ∗∗∗∗P < .0001, Friedman test with Dunn correction for multiple comparisons. (B) Percentages of each BA species within the total pool from the 4 sites numbered and color-coded in Figure 1A of B6 wild-type female mice. The 69 species shown here are listed, grouped and color-coded as in Figure 1D. Mean values are from 7 mice. (C) Similarity matrix of endogenous BA pools in the 4 sites numbered in Figure 1A from B6 wild-type female mice. Calculated based on levels of the 69 reliably detected BA species described in Figure 1C and D. Sample IDs list tissue type, genotype, and cage number; tissues are color-coded at top. (D) Heat maps of the relative abundance (Log2 fold-change) of individual BA species, determined as above by LC-MS/MS, that are significantly different (Padj < .05, Wilcoxon rank sum test) between siLC and feces. (E) α-diversity (Shannon index; n = 7) within indicated BA pools of B6 wild-type female mice analyzed by LC-MS/MS as above. ∗∗P < .01, Friedman test with Dunn correction for multiple comparisons. (F) Circos plot of Pearson correlations among the 69 reliably detected BA species in all 4 sites of B6 wild-type female mice. Line colors represent positive (red) and negative (blue) correlations; only correlation values of < -0.99 or > 0.99 are shown. 1o cBAs, primary conjugated BAs; 1o uBAs, primary unconjugated BAs; 2o cBAs, secondary conjugated BAs; 2o uBAs, secondary unconjugated BAs; 2o metabolites, secondary metabolites; HDPs, hepatic phase 2 detoxification products; LC-MS/MS, liquid chromatography coupled with tandem mass spectrometry; ns, not significant.
Interconnected Response Networks Underlying BA Homeostasis
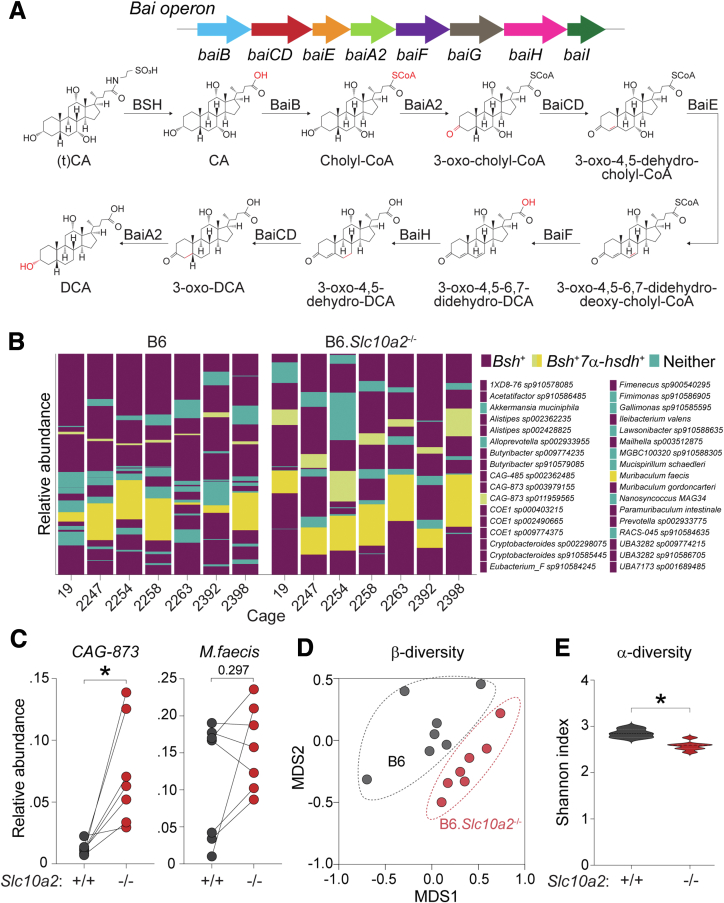
To explore the validity and utility of this approach further, we introduced a genetic perturbation (Asbt ablation) that drives BA dysmetabolism in robust and predictable ways.44 The same analyses performed on female Asbt-deficient (Slc10a2-/-) mice reared together with wild-type mice until the day of analysis reproduced previous findings that loss of Asbt-mediated ileal absorption leads to ∼5-fold fewer BAs in enterohepatic circulation (siLC, smvB), and commensurate ∼5-fold increases in fecal BA excretion (Figure 3A). More interestingly, the remnant pool of enterohepatically cycling BAs in Asbt-deficient mice displayed increased α-diversity, compared with wild-type control animals, and was compositionally similar to fecal BA pools from either wild-type or Asbt-deficient mice (Figure 3A–C). At the species level, reshaping of the Asbt-deficient enterohepatic BA pool involved depletion of virtually all 1o cBAs and 1o uBAs, consistent with previous notions that 1o cBAs are preferred Asbt transport substrates in the ileum,45 and concomitant increases in 2o uBAs and metabolites normally restricted to the LI (Figure 3D). These results suggest that Asbt-mediated ileal BA absorption sustains the unique size and composition of the enterohepatically circulating BA pool.
Figure 3.
Asbt is required for in vivo compartmentalization of enterohepatic and fecal BA pools. (A, Left) Total BA concentrations (μmol/g of luminal content or plasma; n = 7), determined by LC-MS/MS, in the 4 sites numbered and color-coded in Figure 1A from C57BL/6J wild-type (B6, open circles) or Asbt-deficient (B6.Slc10a2-/-, filled circles) female mice. Data shown are sums of 69 reliably detectable BA species described in Figure 1C and D. Each symbol represents data from 1 mouse; horizontal lines indicate means. (A, Right) α-diversity (Shannon index; n = 7) within indicated BA pools of wild-type or Asbt-deficient female mice analyzed as above. Data from wild-type are the same as in Figure 2A (for total BA concentrations) and Figure 2E (for α-diversity). ∗P < .05, paired 2-tailed Student t test. (B) Percentages of each BA species within the total pool from the indicated sites of wild-type (B6, grey/dashed lines) or Asbt-deficient (B6.Slc10a2-/-, solid/colored lines) female mice. The 69 reliably detectable species presented here are listed, grouped and color-coded as in Figure 1D. Mean values are from 7 mice per genotype. (C) Similarity matrix of endogenous BA pools in the 4 sites numbered in Figure 1A from wild-type or Asbt-deficient female mice. Calculated based on levels of the 69 reliably detected BA species described in Figure 1D. Sample IDs list tissue type, genotype and cage number; genotypes and tissues are color-coded at top. (D) Heat maps of the relative abundance (Log2 fold-change) of individual BA species, determined as above by LC-MS/MS, in the indicated tissues of Asbt-deficient vs. wild-type female mice. Separate heatmaps show BA species found in Figure 2D to be significantly enriched in either wild-type siLC (top) or feces (bottom). ∗Padj < .05, Wilcoxon rank sum test. (E) Mean expression (± SEM; n = 7) of BA metabolism-related genes, determined by Nanostring, in whole ileal tissue (top) or livers (bottom) of wild-type (B6) or Asbt-deficient (B6.Slc10a2-/-) female mice. ∗P < .05, unpaired 2-tailed Student t test; 1o cBAs, primary conjugated BAs; 1o uBAs, primary unconjugated BAs; 2o cBAs, secondary conjugated BAs; 2o uBAs, secondary unconjugated BAs; 2o metabolites, secondary metabolites; HDPs, hepatic phase 2 detoxification products; ns, not significant; pB, peripheral blood; siLC, small intestine luminal contents; smvB, superior mesenteric vein blood.
Three responses observed in Asbt-deficient mice were consistent with coordinated efforts to restore BA homeostasis. First, livers of Asbt-deficient mice increased hepatic BA biosynthesis, presumably to replace species lost to excretion. It is well-established that ileal absorption of 1o cBAs suppresses hepatic BA biosynthesis via 2 synergistic mechanisms. In one, BAs circulating through ileal enterocytes activate FXR-dependent expression of Fgf15 (FGF19 in humans), an enterokine hormone secreted into portal blood that signals through Fgfr4 in hepatocytes to suppress expression of rate-liming enzymes in BA biosynthesis (i.e., Cyp7a1, Cyp8b1).11,17 In another, BAs returning to the liver via portal blood activate hepatocyte FXR to promote expression of small heterodimer partner (SHP/Nr0b2), and in turn, repress Cyp7a1/Cyp8b1 transcription.15,46,47 In line with reduced enterohepatic circulation, ileal tissue from Asbt-deficient mice showed lower Fgf15 expression despite equivalent Fxr expression, whereas livers of these same animals showed depressed Shp and elevated Cyp7a1/Cyp8b1 expression (Figure 3E, data not shown).
Second, fecal microbiota from Asbt-deficient mice showed evidence of adapting to increased colonic BA flux by increasing the prevalence of organisms carrying BA-metabolizing (BSH, 7α-HSDH) enzymes (Figure 4A and B). Increased abundance of BA-metabolizing genes within fecal microbial communities of Asbt-deficient mice was explained mostly by a ∼7-fold enrichment of a single unannotated species (CAG-873 sp011959565) carrying both Bsh (deconjugation) and 7α-HSDH (dehydroxylation) enzymes; a second Bsh- and 7α-HSDH-positive organism, Muribaculum faecis, also trended higher in Asbt-deficient communities, although this was not statistically significant (Figure 4C). These results were notable because wild-type and Asbt-deficient mice in these experiments were intentionally cohoused for months after weaning, to normalize microflora and facilitate mixing of enteric microbiomes. Despite this, gut microbial communities from Asbt-deficient mice were clearly distinct from those of wild-type cage-mates, as judged by β-diversity, and showed reduced α-diversity, consistent with enrichment of select taxa (Figure 4D and E).
Figure 4.
Enrichment of bile acid-metabolizing taxa in Asbt-deficient mouse gut microbiota. (A) Schematic diagram of genes and chemical reactions involved in bacterial BA metabolism, adapted from.48 (B) Relative abundance of 34 metagenome-assembled genomes (MAGs) observed in feces from 7 pairs of C57BL/6J wild-type (B6) or Asbt-deficient (B6.Slc10a2-/-) female mice. Mice were cohoused at weaning (3–4 weeks of age) and reared together until 30 week of age. Fresh fecal pellets were collected from individual mice after separation and fasting for 4 hours. Individual taxa are colored and annotated based on presence of Bsh and/or 7α- hydroxysteroid dehydrogenase (7α-hsdh) genes; Bsh alone (purple), Bsh and 7α-hsdh (yellow), or neither (teal). (C) Relative abundance of Bsh- and 7α-hsdh-positive taxa (CAG-873 sp011959565, CAG-873, left; Muribaculum faecis, right), as determined in (B), within fecal microbiota of wild-type or Asbt-deficient mice (n = 7). ∗P < .05, Wilcoxon signed rank test. (D) β-diversity (Bray-Curtis dissimilarity) analysis of fecal microbiomes from wild-type or Asbt-deficient female mice (n = 7). (E) α-diversity (Shannon index) of fecal microbiomes from wild-type or Asbt-deficient female mice (n = 7). ∗P < .05, Wilcoxon signed rank test. Bai operon, bile acid-induced operon; BSH, bile salt hydrolase; (t)CA, tauro-cholic acid; DCA, deoxycholic acid.
Finally, livers of Asbt-deficient mice upregulated BA detoxification pathways, likely in response to elevated portal recirculation of microbially derived 2o uBAs (e.g., DCA, LCA). 2o uBAs are more hydrophobic, and thus more cytotoxic at low concentrations, compared with 1o cBAs.4,49,50 As such, hepatocytes deploy multiple mechanisms to prevent BA-driven hepatotoxicity, including sulfation and glucuronidation (Figure 5A).51,52 Sulfation is particularly effective at rendering toxic BA species and other xenobiotics more hydrophilic, and at tagging BAs for urinary or fecal clearance.38,39,53 Both DCA and LCA, and their corresponding 3-sulfates (but not 24-glucuronides), were increased in Asbt-deficient vs. wild-type mice (Figure 5B and C). BA sulfates were almost entirely restricted to the SI lumen and feces, in line with the notion that these modified species are poor substrates for intestinal absorption (Figure 5C).54,55
Figure 5.
Increased hepatic BA sulfation in Asbt-deficient mice. (A) Schematic diagram of enterohepatic BA circulation adapted from.37 BAs recirculating from the small or large intestines can be modified in hepatocytes via sulfation or glucuronidation. (B) Concentrations (μmol/g; n = 7) of secondary BAs, DCA (top) or LCA (bottom), in smvB of C57BL/6J wild-type (B6) or Asbt-deficient (B6.Slc10a2-/-) female mice. Horizontal lines indicate means; ∗P < .05, paired 2-tailed Student t test. (C) Concentrations (μmol/g; n = 7) of secondary BA phase 2 detoxification products, DCA (top) or LCA (bottom) 3-sulfates (3-SO4) or 24-glucuronides (24-gluc), in the indicated sites of wild-type or Asbt-deficient female mice. Horizontal lines indicate means; ∗P < .05, paired 2-tailed Student t test (between the same sites of wild-type or Asbt-deficient mice). (D) Schematic diagram of the BA 3-sulfation pathway. BA sulfotransferase enzymes, encoded by the Sult2a gene cluster in mice, transfer a sulfonate (sulfate) group (-O3SO, highlighted red) from the universal sulfonate donor, PAPS to C-7 of the BA steroid nucleus. This produces PAPS as a byproduct, which is reconverted to PAPS by the PAPS-synthase enzyme, Papss2. (E) Mean expression (± SEM; n = 7) of Sult2a and Papss2 mRNA, determined by Nanostring, in livers of the same cohoused wild-type (B6) or Asbt-deficient (B6.Slc10a2-/-) female mice analyzed above. ∗P < .05, unpaired 2-tailed Student t test; DCA, deoxycholic acid; LCA, lithocholic acid; GB, gallbladder; HPV, hepatic portal vein; imv, inferior mesenteric vein; IVC, inferior vena cava; ns, not significant; PAPS, phosphoadenosine phosphosulfate; pB, peripheral blood; siLC, small intestine luminal contents; smvB, superior mesenteric vein blood.
Mechanistically, sulfation is evoked by BA-binding to xenobiotic-sensing NRs (e.g., VDR/Nr1i1, PXR/Nr1i2) in hepatocytes, which in turn promote expression of BA sulfotransferase enzymes encoded by the mouse Sult2a gene cluster (Figure 5D).50,56,57 Once expressed, Sult2a enzymes transfer a sulfonate group to C-3 or C-7 on the BA steroid nucleus from a universal donor, termed phosphoadenosine phosphosulfate (or PAPS), which itself is regenerated by a phosphoadenosine phosphosulfate synthase enzyme encoded by the Papss2 locus (Figure 5D).53 Consistent with enhanced hepatic BA sulfation, livers from Asbt-deficient mice displayed a collective increase in Sult2a, but not Papss2, gene expression, compared with wild-type control animals (Figure 5E). Thus, multisite and multiomics analyses unmask intricate response networks that follow loss of Asbt-dependent ileal BA reabsorption and that underpin BA homeostasis in vivo.
Mathematical Modeling of In Vivo Bile Acid Metabolism
Endocrine networks connecting the liver, ileum, and LI microbiota that coordinate BA homeostasis are tedious and expensive to study through experimentation alone. Thus, we sought to develop mathematical models capable of analyzing BA circulation and metabolism in more integrated and quantitative fashions (Figure 6A). A previous model by Sips et al reported that BA transit speed decreases from proximal to distal along the intestinal tract,37,58, 59, 60 causing BAs to accumulate in the ileum and colon in the absence of intestinal absorption (Figure 6B). Yet most BAs are reabsorbed either actively in the ileum or passively throughout the SI and LI, which drives their eventual reintroduction into the duodenum, together with the pool of newly synthesized BAs from the liver. To monitor each of these features, we incorporated independent rates and locations for active and passive intestinal BA absorption, and for hepatic BA synthesis, microbial 2o BA metabolism, and BA reintroduction to the duodenum after enterohepatic cycling. Once parameters were established and optimized that best fit our experimental data, we analyzed model outputs to determine how each step in BA circulation and metabolism influences the size and complexity of the enterohepatic BA pool.
Figure 6.
Mathematical modeling of BA enterohepatic circulation. (A) Schematic diagram of BA enterohepatic circulation. Two inputs for the circulating BA pool were considered: (1) 1o BAs synthesized de novo by the liver, to replace those not absorbed in the intestines and excrete in feces; and (2) BAs reintroduced into the small intestine after absorption in the small or large intestines, portal recirculation to the liver, and resecretion into bile. This includes 1o BAs that become metabolized into 2o BAs by colonic microbiota and reintroduced by hepatic resecretion after portal recirculation. (B) Changes of BA synthetic rates in the liver were considered in the model. Total amount of BAs along the intestinal tract in the absence of reabsorption is shown. Two different rates of hepatic BA synthesis (synthesis = 1, lower; synthesis = 2, higher) were used. BAs accumulate in the ileum and colon because of slower transit. The higher BA synthetic rate scales up BA amounts along the intestinal tract. (C) Effect of intestinal absorption on enterohepatic BA pool size and composition. BA levels are scaled by the amount of total BAs in the SI absent intestinal absorption. Combining active absorption in the ileum (90% efficiency) and passive absorption in the colon (50% efficiency) for a total of 95% intestinal absorption increases the small intestinal pool 20-fold relative to no intestinal absorption. Loss of Asbt-mediated active absorption, with only passive absorption remaining, explains the decrease in pool size in B6.Slc10a2-/- mice, but not changes in BA pool composition. (D) Passive BA reabsorption in the colon was considered. In this simulation, a fraction of the BA was recycled, increasing the pool size, and introducing 2o BA into the circulating pool in the SI. A 50% reabsorption efficiency doubles the size of the BA pool in the SI. (E) Active BA reabsorption in the ileum was considered. This model (assuming 90% ileal absorption efficiency) increased the size of BA pool 20-fold, compared with the absence of ileal absorption; note the size of the colonic BA pool does not change. Without passive absorption in the colon, there are no secondary BA introduced in the circulating pool. (F and G) Optimization of SI absorption and large intestinal (LI) microbial BA metabolism rates to fit experimental data for C57BL/6J wild-type (B6, C) and Asbt-deficient (B6.Slc10a2-/-, D) female mice. Differences in absorption rates between 1o BA and 2o BA were not considered. Increased conversion of 1o BA into 2o BA by the microbiota in B6.Slc10a2-/- mice explains increased BA pool diversity. IMV, inferior mesenteric vein; SMV, superior mesenteric vein.
At steady state (i.e., when enterohepatic circulation is stable), the amount of de novo–synthesized hepatic BAs introduced into the duodenum matched that escaping SI absorption and getting excreted in feces. When BAs were actively absorbed in the ileum, mimicking wild-type mice, this increased the size of the BA pool in enterohepatic circulation ∼20-fold, and BAs synthesized by the liver to replace those lost to excretion represented only a small fraction (∼5%) of the total pool (Figure 6C–E). Because most intestinal BA absorption in our model occurred via active transport in the ileum, only a small fraction of BAs reached the LI, became metabolized to 2o species, and were reintroduced to the SI pool (Figure 6F). This simulation suggested our model was appropriately calibrated to experimental data, and reinforced established concepts that Asbt-mediated ileal BA uptake sustains pool size, whereas bacterial BA metabolism in the LI generates pool diversity (Figures 2A, 3A–D, and 6F).61,62
Abolishing active BA absorption in our model to mimic that in Asbt-deficient mice reproduced the ∼5-fold reduction in SI BA pool size observed in our experiments but was not sufficient to explain increased fecal BA excretion (Figures 3A, 6C, and 6E). In this case, although the size of the SI BA pool was reduced markedly, a far higher fraction of this smaller pool escaped ileal absorption and was excreted. Importantly, increased fecal BA excretion in the absence of active ileal absorption, which are physiologically linked in Asbt-deficient mice (Figure 3A and E), only occurred in our model when hepatic synthesis rates were increased (Figure 6B). These simulations support and extend our experimental observations in Asbt-deficient mice, where reduced ileal absorption and increased hepatic synthesis may contribute independently to depletion of SI BAs and bloom of fecal BAs, respectively.
Finally, we used our model to interrogate determinants of enterohepatic BA pool complexity. Neither increasing hepatic synthesis nor decreasing active ileal absorption substantially altered representation of bacterially derived 2o BAs within the SI pool (Figure 6B and C). In these simulations, increases in hepatic resecretion of LI-derived 2o BAs were negated by proportionate increases in 1o BA biosynthesis by the liver (Figure 6B and C). In fact, we tested a range of ileal (and colonic) absorption rates in our simulations and found no conditions that overtly modified composition of the SI BA pool (Figure 6C). Thus, we considered that the increased proportion of 2o BAs in the SI pool of Asbt-deficient mice might require parallel increases in 2o BA metabolism by LI microbiota (Figure 6A). In testing this, we varied bacterial BA metabolism rates in our model and searched for those that best fit our experimental data. These simulations suggested a 9-fold increase in LI bacterial BA metabolism rates would be necessary to explain the increased 2o BA representation seen in the SI of Asbt-deficient animals (Figure 6F and G), a number surprisingly close to the ∼7-fold enrichment of Bsh+Bai+ taxa observed experimentally in Asbt-deficient vs. wild-type fecal microbiota (Figure 4B and C). We attempted to explore this predicted increase in microbial BA metabolism more directly by assessing metabolism of an isotopically labelled (t)CA tracer by cultured fecal microbiota from wild-type or Asbt-deficient mice, but these communities, and bai+ taxa in particular, were poorly maintained in our culture conditions (see methods). Nonetheless, these results suggest that mathematical models can augment experimental investigation of in vivo BA metabolism; they also suggest that hepatic synthesis, ileal absorption, and bacterial metabolism each contribute nonredundant inputs to establish and maintain key features of the enterohepatic BA pool in mice.
Quantifying Ileal BA Absorption In Vivo
The estimate that ∼95% of all BAs are absorbed in the ileum stems from classical studies in which SIs of experimental animals were perfused, or human volunteers were dosed orally, with individual BAs and serum levels were followed over time to estimate absorption.45,63, 64, 65, 66, 67, 68, 69, 70, 71 However, because each BA species possesses unique pharmacokinetic properties, including rates and routes of intestinal absorption,72,73 this general estimate cannot be uniformly applied to all BA species within complex enterohepatic pools. Furthermore, neither active nor passive intestinal BA absorption are biologic constants; BA malabsorption is an underdiagnosed manifestation of myriad intestinal disorders because of reduced ASBT expression or function, whereas changes in epithelial barrier function caused by disease, diet, or dysbiosis may affect passive BA absorption.74 Because intestinal absorption is a key and rate-limiting determinant of which luminal BAs ultimately seed mucosal tissues, and at what concentrations, we asked if our metabolomics datasets (Figure 1, Figure 2, Figure 3) could be used to empirically infer rates and routes of ileal BA reabsorption in vivo.
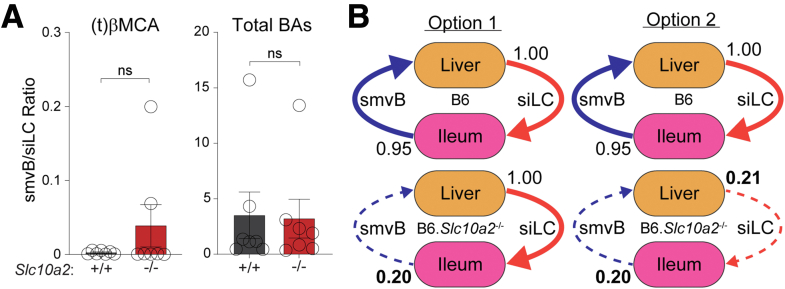
We first considered that smvB/siLC BA ratios alone might reflect ileal absorption rates. However, these were similar between wild-type and Asbt-deficient mice, both for individual BA species (e.g., (t)βMCA) and for the total pool (Figure 7A). In considering this result further, we noted that this ratio assumes siLC and smvB BA levels are maintained in a constant state of disequilibrium absent Asbt (i.e., that only smvB, not siLC, BAs decrease in Asbt-deficient mice; Figure 7B). Conversely, both our experimental data and computational modeling better supported a model in which siLC and smvB BAs are depleted together in Asbt-deficient mice, with the remaining BAs circulating enterohepatically at a new equilibrium limited by passive absorption rates. (Figures 3A, 7B, and 8A). In exploring siLC-smvB BA relationships further, we found that the natural variation in siLC and smvB BA levels across cohorts of wild-type or Asbt-deficient mice generated line slopes (m), which were generally lower in Asbt-deficient animals and better intimated intestinal absorption rates. By defining these slopes (rates) for each BA species, and in both wild-type and Asbt-deficient animals, we were able to use standard line equations (y = mx + b) to estimate amounts of active and passive absorption, for each individual BA species, and the pool as a whole (Figure 8B and C and Table 1).
Figure 7.
Inferring ileal BA absorption from BA levels in the small intestinal lumen and superior mesenteric vein. (A) Ratios (± SEM; n = 7) of superior mesenteric vein blood (smvB)/small intestine luminal content (siLC) BA concentrations, determined by LC-MS/MS, in C57BL/6J wild-type (B6) or Asbt-deficient (B6.Slc10a2-/-) female mice. Ratios are shown for both the primary conjugated mouse BA, tauro-beta-muricholic acid, (t)βMCA; left) and for the sum of all BAs (total BAs, right); Wilcoxon signed rank test. (B) Schematic diagrams of 2 distinct models for how loss of Asbt-mediated ileal BA transport could affect enterohepatic BA circulation. Option 1 (left) assumes disequilibrium between siLC and smvB BA levels in the absence of Asbt. Here, 95% of all BAs in siLC of wild-type mice (in B6 mice, top) are reabsorbed in the ileum and return to the liver via smvB. Loss of Asbt (in B6.Slc10a2-/- mice, bottom) reduces the amount of smvB BAs (in this example, from 95% of siLC BAs in wild-type mice to 20% of siLC BAs in Asbt-deficient mice), but siLC BA levels remain unchanged. Option 2 (right) assumes that both siLC and smvB BAs are depleted in the absence of Asbt. The latter is more consistent with both our experimental observations in mice (Figure 3A) and our mathematical modeling of BA enterohepatic circulation (Figure 6). LC-MS/MS, liquid chromatography coupled with tandem mass spectrometry; ns, not significant,
Figure 8.
Estimating active and passive ileal BA reabsorption from ex vivo metabolomics data. (A) Relationship between total BA concentrations in small intestine luminal content (siLC, x axis) and superior mesenteric vein blood (smvB, y axis) of the same C57BL/6J wild-type (B6, grey) or Asbt-deficient (B6.Slc10a2-/-, red) female mice (n = 7). Sum totals of 69 reliably detectable BA species (described in Figure 1C and D) for individual mice are shown in small, clear circles; averages values per genotype are indicated by large, filled circles. Dashed diagonal line shows the y=x axis. (B) Example of how active and passive ileal reabsorption is calculated for individual BA species. Individual (small, clear circles) and average (large, filled circles) siLC and smvB concentrations of (t)βMCA in wild-type (grey) or Asbt-deficient (red) female mice are shown (n = 7). Linear regression analyses, per genotype, produced line slopes (m) indicative of ileal absorption rates. Line equations (y = mx+b) were used to determine the amount of each BA, if present in the Asbt-deficient siLC at the same concentration as in the wild-type siLC (x), would be absorbed and reach smvB (y). Because the amount of each BA in wild-type smvB (large, filled grey circle) is known, and represents total ileal absorption when both active and passive ileal absorption are intact, and we can solve for amount of passive BA absorption in Asbt-deficient mice (large, clear red circle), the difference between wild-type and Asbt-deficient y-axis points equals the amount of active (Asbt-mediated) ileal absorption. (C, Top) Amount of total, active and passive ileal absorption (in μmol/g) inferred for 57 individual BAs, as in B. Amounts for (t)βMCA and (t)CA are shown as examples. (C, Bottom) Pie chart showing the percentage of total ileal BA absorption, calculated as in B (Table 1), attributed to active (Asbt-mediated, grey) or passive (red) absorption. (D) Pie charts showing percentages of total active (left) or passive (right) ileal BA absorption, calculated as in B and C, for each BA species. Total percentages of the top 5 BAs vs. all others are listed at bottom. (E) Rank-orders of all 57 BAs from lowest to highest active (left) or passive (right) ileal absorption rates (m), calculated as in B and color-coded based on putative biosynthetic origin as in Figure 1D. (F) Heatmap of Pearson correlation (r) values between rates (m) of active or passive ileal absorption rates, inferred here (as in B and E), with previously measured physicochemical properties for 10 major primary and secondary BAs.72 Jmax, maximal amount of Asbt-dependent transport (both theoretically related to active, Asbt-mediated BA transport); Kt, Asbt transport constant; Pp, passive permeability (theoretically related to passive BA absorption).
Table 1.
Absorption Amounts for All 57 BA Species Shown in Figure 8B and C
| µmol/g | Total | Active | Passive |
|---|---|---|---|
| (t)βMCA | 0.09802 | 0.08872 | 0.00930 |
| (t)CA | 0.04895 | 0.03969 | 0.00926 |
| CA | 0.02853 | 0.02109 | 0.00744 |
| (t)αMCA | 0.02473 | 0.02312 | 0.00162 |
| βMCA | 0.02175 | 0.01910 | 0.00264 |
| (t)ωMCA | 0.01350 | 0.01350 | 0a |
| αMCA | 0.00573 | 0.00387 | 0.00186 |
| (t)UDCA+(t)HDCA | 0.00483 | 0.00392 | 0.00091 |
| 7-keto-DCA | 0.00429 | 0.00357 | 0.00072 |
| ωMCA | 0.00386 | 0.00316 | 0.00070 |
| (t)allo-CA | 0.00378 | 0.00346 | 0.00032 |
| DCA | 0.00371 | 0a | 0.00371 |
| (t)CDCA | 0.00293 | 0.00114 | 0.00179 |
| UDCA | 0.00202 | 0.00124 | 0.00079 |
| (t)DCA | 0.00200 | 0a | 0.00200 |
| 12-keto-CDCA | 0.00141 | 6.819e-5 | 0.00134 |
| hyo-DCA | 0.00103 | 0a | 0.00103 |
| allo-CA | 0.00090 | 0.00069 | 0.00021 |
| CDCA | 0.00071 | 0a | 0.00071 |
| UCA | 0.00040 | 0a | 0.00040 |
| MCA | 0.00032 | 0a | 0.00032 |
| norCA | 0.00029 | 0a | 0.00029 |
| apo-CA | 0.00027 | 0a | 0.00027 |
| 3-oxo-CA | 0.00026 | 0.00017 | 9.162e-5 |
| norDCA | 0.00025 | 0.00023 | 1.826e-5 |
| (g)CA | 0.00020 | 0.00019 | 5.687e-6 |
| 7-keto-LCA | 0.00015 | 0a | 0.00015 |
| LCA | 0.00012 | 0a | 0.00012 |
| DHCA | 0.00012 | 0a | 0.00012 |
| (t)LCA | 0.00011 | 0a | 0.00011 |
| allo-CA 3-SO4 | 6.497e-5 | 0a | 6.497e-5 |
| 7αOH-3-oxo-4-chol. | 6.152e-5 | 0a | 6.152e-5 |
| (t)hyo-CA | 4.841e-5 | 4.739e-5 | 1.019e-6 |
| 12-keto-LCA | 1.978e-5 | 0a | 1.978e-5 |
| hyo-CA | 1.972e-5 | 8.83a 8e-6 | 1.089e-5 |
| hyo-DCA 3-SO4 | 1.529e-5 | 7.388e-6 | 7.905e-6 |
| DCA 24-gluc | 1.483e-5 | 0a | 1.483e-5 |
| CA 3-SO4 | 1.455e-5 | 1.359e-5 | 9.649e-7 |
| 3β-OH-5-chol. | 1.348e-5 | 1.726e-6 | 1.175e-5 |
| THCA | 1.248e-5 | 0a | 1.248e-5 |
| 6,7-diketo-LCA | 1.087e-5 | 9.366e-6 | 1.500e-6 |
| (t)DCA 3-SO4 | 9.514e-6 | 7.687e-6 | 1.827e-6 |
| (g)UDCA | 6.235e-6 | 5.222e-6 | 1.013e-6 |
| dehydro-LCA | 5.535e-6 | 0a | 5.535e-6 |
| (g)CDCA | 5.204e-6 | 3.549e-6 | 1.655e-6 |
| alloiso-LCA | 4.391e-6 | 1.450e-6 | 2.940e-6 |
| iso-LCA | 3.225e-6 | 0a | 3.225e-6 |
| dioxo-LCA | 3.170e-6 | 0a | 3.170e-6 |
| (g)DCA | 2.562e-6 | 1.594e-6 | 9.680e-7 |
| CDCA 24-gluc | 2.526e-6 | 1.003e-6 | 1.522e-6 |
| (g)hyo-DCA | 1.967e-6 | 1.919e-6 | 4.844e-8 |
| LCA 24-gluc | 1.870e-6 | 0a | 1.870e-6 |
| UDCA 3-SO4 | 1.852e-6 | 1.366e-6 | 4.861e-7 |
| DCA 3-SO4 | 7.695e-7 | 0a | 7.695e-7 |
| LCA 3-SO4 | 4.541e-7 | 0a | 4.541e-7 |
| iso-LCA 3-SO4 | 1.174e-7 | 0a | 1.174e-7 |
| ωMCA 3-SO4 | 6.512e-8 | 6.512e-8 | 0a |
| sum | 0.27551 | 0.22705 | 0.04846 |
BA, bile acid.
The value is less than 1e-8 or undetectable.
After removing 12 additional BAs whose median concentration in wild-type siLC was 0, we calculated the size of the ileal BA pool in fasted female wild-type mice to be ∼0.2755 μmol/g (Figure 8C and Table 1). More than 80% of this pool could be attributed to Asbt-dependent transport, and ∼85% of actively transported BAs comprised only 5 abundant 1o BA species, including conjugated and unconjugated CA, and the rodent-biased muricholates (e.g., (t)α/βMCA, βMCA) (Figure 8C and D and Table 1). 1o cBAs, which are thought to strictly require active absorption for their enterohepatic circulation,72 displayed surprisingly high levels of passive absorption, likely caused by sheer abundance, whereas passive absorption also drove ileal entry of many less abundant, but also more hydrophobic, secondary BAs (e.g., DCA), thus increasing pool diversity (Figure 8D). Intriguingly, these in vivo active ileal absorption rates (mB6 - mKO) did not correlate with Asbt transport kinetic parameters (Jmax, KT) previously defined for 10 individual BAs using standard in vitro MDCK cell-based transport assays (Figure 8E and F).72 Similarly, in vivo passive absorption rates (mKO) did not align with passive permeability rates estimated in the same in vitro study (Figure 8E and F).72 It is not clear if these discrepancies speak more to divergent behaviors of individual BA species in vitro vs. within complex enterohepatic pools, or to other complex in vivo variables (e.g., BA retention within mucosal tissue). Nevertheless, these results provide a framework for empirically measuring ileal BA absorption in mice, which is an important step toward relating putative BA functions to in situ concentrations.
Physiological Functions of the Enterohepatic BA Pool
Much of contemporary BA research (i.e., since the discovery of FXR as an endogenous BA receptor 25 years ago12, 13, 14,75) has centered on defining interactions with host receptors and elucidating their impacts on GI physiology. Yet most BA functional studies focus on individual species, not complex endogenous pools, leaving sizeable knowledge gaps about how BAs influence host intestinal responses in vivo. Thus, we finally sought to leverage our new insights into the size and complexity of mouse ileal BA pools (Figure 8) to examine their cellular and molecular functions.
After extracting bulk metabolites from siLC of fasted and cohoused wild-type or Asbt-deficient mice, which included but were not limited to BAs, contents from each animal were divided and either left intact or depleted of BAs using the BA-binding resin, cholestyramine (CME), (Figure 9A). Total BA concentrations were normalized between intact extracts to focus downstream analyses on compositional differences between pools, and individual species were quantified by LC-MS/MS. After accounting for dilution factors used in functional studies (see later), which we intentionally designed to approximate the size of the ileal BA pool in fasted wild-type mice (0.2755 μmol/g; ∼ 291 μM) (Figure 8C and Table 1), we confirmed that the mean total BA concentration in intact wild-type siLC extracts was ∼286 μM, and that CME treatment depleted ∼97% of these BAs (Figure 9A). Intact extracts from Asbt-deficient mice contained ∼2-fold fewer total BAs (mean total concentration ∼112 μM), and CME again depleted ∼93% of these (Figure 9A). At the species level, CME treatment depleted 55 of 69 reliably detected BAs by at least 95% in both wild-type and Asbt-deficient siLC extracts; only 2 minor BA metabolites (7-keto-DCA, UCA) were depleted with <75% efficiency (Figure 9B). Most importantly, intact siLC extracts produced here displayed the same Asbt-dependent hallmarks as before (Figure 3): fewer 1o cBAs and more 1o uBAs, 2o uBAs, and BA 3-sulfates in Asbt-deficient vs. wild-type siLC extracts (Figure 9A).
Figure 9.
Transcriptional responses of cultured ileal explants to synthetic and endogenous BAs. (A, Top) Schematic diagram of the experimental workflow for interrogating direct, ex vivo functions of endogenous, enterohepatic BA pools in mice. Organic extracts from small intestine luminal content (siLC) of C57BL/6J wild-type (B6, black) or Asbt-deficient (B6.Slc10a2-/-, red) male and female mice were divided, and either treated with CME to deplete BAs (BA-depleted) or left intact (BA-replete). (A, Bottom) Mean total BA concentrations (n = 3), determined by LC-MS/MS, in siLC extracts indicated above (BA-replete or BA-depleted from wild-type or Asbt-deficient mice). Proportions of discrete BAs in defined categories are color-coded as in Figures 1D, 2B, 3B. (B) Efficiency of CME-mediated BA-depletion in siLC extracts from C57BL/6J wild-type (B6) or Asbt-deficient (B6.Slc10a2-/-) mice. Each symbol represents an individual BA species; values were calculated based on mean BA concentrations (μmol/g) in CME-treated vs. untreated siLC extracts [100 – (+CME concentration/-CME concentration)] from 3 mice (2 females, 1 male) per genotype. Pearson correlation (r) value, in red, demonstrates nearly identical BA depletion efficiencies by CME pretreatment in both wild-type and Asbt-deficient siLC extracts. ∗∗∗∗P < .0001, Pearson correlation test. (C, Left) Kinetics of Fgf15 mRNA upregulation in cultured ileal explants after treatment with 200 μM tauro-chenodeoxycholic acid [(t)CDCA] or vehicle (DMSO) alone. (C, Right) Dose response of (t)CDCA on Fgf15 mRNA expression in cultured ileal explants after 8 hours. Data are presented as mean RQ values (± SEM), determined by qPCR, in 3-independent experiments using ileal explants from separate C57BL/6J wild-type (B6) male mice; average values from 3 technical replicates (i.e., 3 pieces of ileal tissue) were calculated in each independent experiment. ∗∗P < .01, 2-way analysis of variance with Sidak correction for multiple comparisons. (D) Mean Fgf15 mRNA expression (± SEM; n = 9) in cultured ileal (left) or proximal colon (prox. colon, right) explants from separate wild-type male mice, determined by qPCR as in A, after treatment with or without 200 μM (t)CDCA or 200 μM GW4064 (FXR agonist) for 8 hours. ∗P < .05, ∗∗∗∗P < .0001, 1-way analysis of variance with Dunnett correction for multiple comparisons. (E) Mean Fgf15 mRNA expression (± SEM; n = 8–9) in cultured ileal explants from separate wild-type male mice, determined by qPCR as in C and D, after treatment with 200 μM (t)CDCA or 200 μM GW4064 (FXR agonist) in the presence or absence of the Asbt inhibitor, odevixibat (10 μM) for 8 hours. ∗P < .05, unpaired 2-tailed Student t test. (F, Left) Total BA concentrations (± SEM; n = 7), presented as μmol/g and determined by colorimetric kit, in male C57BL/6J wild-type (B6) siLC extracts with or without CME-mediated BA depletion. (F, Right) Mean Fgf15 mRNA expression (± SEM; n = 7–9) in cultured ileal explants from separate wild-type male mice, determined by qPCR as in (C–E), after 8-hour treatment with or without 200 μM (t)CDCA, or equivalent dilutions of intact or BA-depleted siLC extracts normalized to add 200 μM total BAs in intact extracts. ∗∗P < .01, ∗∗∗P < .001, Kruskal-Wallis test with Dunn correction for multiple comparisons or Wilcoxon signed rank test. ns, not significant. qPCR, quantitative polymerase chain reaction; SEM, standard error of the mean
To assess functions of these distinct enterohepatic BA pools, we used cultured intestinal explants.76 In optimizing culture conditions, we confirmed that ileal, but not colonic, explants isolated from wild-type mice responded rapidly (within 4–8 hours) to low micromolar concentrations of (t)CDCA by upregulating the canonical FXR target gene, Fgf15; similar results were seen with the synthetic FXR agonist, GW4064 (Figure 9C and D).77 Blocking Asbt transport activity with the FDA-approved small molecule, odevixibat,78 blunted (t)CDCA-induced Fgf15 expression in ileal explants (Figure 9E), confirming that FXR activation by conjugated BAs in ileal explants, as in vivo, requires Asbt-mediated uptake by enterocytes. Intact siLC extracts from wild-type mice also evoked Fgf15 upregulation, akin to (t)CDCA alone, whereas this response was muted on CME-mediated BA depletion (Figure 9F).
Having established the system, we went on to use RNA-seq to compare genome-wide transcriptional responses of wild-type ileal explants to (t)CDCA alone, or the 4 siLC extracts generated previously (i.e., from wild-type or Asbt-deficient mice with or without BA depletion). To quantify wild-type and Asbt-deficient BA pool activities, we enumerated genes whose expression was significantly altered (i.e., induced, repressed) by each BA extract vs. vehicle (dimethyl sulfoxide [DMSO]) alone (Figure 10A). Fewer than 150 genes showed differential expression in response to BA-replete, but not BA-depleted, siLC extracts from wild-type mice, Asbt-deficient mice, or both. By contrast, more than 900 genes were induced or repressed by a standard (supraphysiologic) concentration of (t)CDCA alone (200 μM) (Figure 10A-C). By itself, this result highlights the unique information that comes from examining host tissue responses to physiologically defined BA pools.
Figure 10.
Compositionally distinct small intestinal BA pools induce divergent transcriptional responses in cultured ileal explants. (A) Differential gene expression (Padj < .05), determined by DESeq2 analysis of RNA-seq data (n = 3–4), in cultured ileal explants (isolated from WT male mice) after treatment for 8 hours with 200 μM (t)CDCA, or equal dilutions of intact (CME-) or BA-depleted (CME+) siLC extracts from WT (i.e., B6) or Asbt-deficient (KO; i.e., B6.Slc10a2-/-) male and female mice. Intact extracts were normalized to add 200–300 μM total BAs of each WT or KO extract, based on colorimetric kit analysis, as in Figure 9F; identical dilutions of parallel BA-depleted extracts were used. Numbers of differentially expressed genes (red, upregulated; blue, downregulated), for each condition vs. vehicle (DMSO), are indicated by color-matched text. Data were pooled from 2-independent experiments (1 or 3 biologic replicates each) using siLC extracts from separate WT or Asbt-deficient male or female mice. (B) Mean normalized Fgf15 mRNA expression (± SEM; n = 3–4; expressed as transcripts per million [TPM]) in cultured ileal explants, determined by RNA-seq as in A. ∗P < .05, ∗∗P < .01, 1-way analysis of variance with Holm-Sidak correction for multiple comparisons or unpaired 2-tailed Student t test. Venn diagrams of gene numbers (C) and heatmap of gene expression (D) for genes significantly altered (Padj < .05, DESeq2; as in A and B, n = 3–4) in cultured ileal explants 8 hours after treatment with 200 μM (t)CDCA, or equal dilutions of intact (CME-) or BA-depleted (CME+) siLC extracts from WT (B6) or Asbt-deficient (B6.Slc10a2-/-) male or female mice. KO, knock-out; WT, wild-type.
Of the ∼150 genes regulated by either or both BA-replete, but neither BA-depleted, siLC extracts, most (n = 130; 87%) reflected unique responses to only wild-type or Asbt-deficient BA pools (Figure 10C and D). Asbt-deficient extracts elicited more unique transcriptional responses in ileal explants (n = 106) than those evoked by wild-type extracts (n = 24) (Figure 10A and C). Unique responses of ileal explants to the 2 SI BA pools spanned multiple key areas of intestinal physiology, from prostaglandin (Ptgr2) and nitric oxide (Nos2) metabolism to antimicrobial activity (Defa5, Defa30) and xenobiotic metabolism (Slco2a1, Sult2b1) (Figure 10D). Just more than half (∼52%) of each uniquely responsive gene set (i.e., to wild-type or Asbt-deficient BA-replete extracts) were predicted by the explant response to (t)CDCA alone, whereas nearly all (18/19; 95%) of the genes commonly influenced by both wild-type and Asbt-deficient BA pools, including FXR gene targets Fgf15, Slc51b, and Slc10a2, were similarly affected by (t)CDCA (Figure 10B–D). These results establish new experimental paradigms for examining how altered BA metabolism can impinge directly on host intestinal physiology.
Discussion
Appreciation is growing for complex endocrine connections among the liver, intestines, and gut microbial communities that underlie most aspects of GI physiology, including the circulation and metabolism of BAs. Still, contemporary studies of human or mouse BAs rely on single-site measurements, most commonly in plasma (to catalog BAs as biomarkers), liver/gallbladder (to explore BA biosynthetic mechanisms), ileum (to decipher BA absorption or signaling), or stool (to study microbial metabolic pathways). The results presented here suggest that BAs, gut microbial communities, and host enterohepatic tissues should be considered integrated functional units, wherein perturbation of any one component necessarily affects the others and confounds experimental readouts if all nodes are not monitored and controlled for. The approaches, analyses, and mathematical models presented here were developed as an integrated methods set to enable microbiome scientists to monitor effects of dysbiosis on hepatic metabolism or intestinal absorption, and vice versa.
Our multisite analyses emphasize strict compartmentalization between SI and LI BA pools. Although this was anticipated, based on historical knowledge and more recent metabolomics studies,79 the comprehensive and quantitative comparisons shown here have important implications for multiple areas of biology. Compartmentalization of SI and LI BA pools likely influences everything from the behavior of local immune and epithelial subsets (discussed later) to that of small vs. large bowel microbes and cancers. Compartmentalization also suggests that analysis of fecal BAs alone, an increasingly common measurement, has limited relevance to BAs in enterohepatic circulation. BAs in peripheral circulation are even more diverse and variable than those in feces, displaying mixed features of both SI and LI BA pools. Thus, great care must be taken when using only fecal or serum BA data to infer features in enterohepatic circulation.
Our results offer working answers to long-standing questions in BA research. For example, by sampling BA pools in siLC and smv of individual mice in parallel, we were able to estimate the size, complexity, and routes of absorption of the ileal BA pool in mice. Our results suggest that of the ∼95% of BAs absorbed in the wild-type mouse ileum at steady-state, more than 80% of this pool can be attributed directly to Asbt-dependent transport, whereas less than 20% owes to passive (Asbt-independent) diffusion. As expected, most (∼70%) of ileal BAs in fasted wild-type mice were comprised of a select set of the most abundant primary conjugated and unconjugated BAs in mice, namely CA and α/βMCA. At the same time, we observed unexpectedly high passive absorption of taurine-conjugated primary BAs, which are generally viewed as requiring Asbt-mediated transport for ileal entry.72 Intriguingly, neither active nor passive in vivo ileal absorption rates inferred here were predicted by prior in vitro studies where major primary or secondary BAs were added individually to control or Asbt-expressing MDCK cells.41 One explanation for this discrepancy is in vivo competition between myriad BA species within intestinal pools for finite numbers of (Asbt) transporters. If true, in vitro Asbt transport kinetics of individual BAs will be influenced by the presence of other (competing) species. It also seems plausible that more complex in vivo variables, such as retention of BAs in intestinal tissues/egress from tissues into portal blood, contribute to the limited resolution with which one can reasonably approximate Asbt transport kinetics in vivo. It should also be noted that we used linear regression models to estimate ileal absorption rates in vivo (Figure 8A and B), whereas Asbt transport kinetics may be better approached using semilogarithmic curve-fitting to follow Michaelis–Menten enzyme principles. Whatever the dynamics, features of the mouse ileal BA pool proposed here can be refined and expanded in future studies, such as to estimate whether or how ileal BA absorption varies throughout the postprandial period, in male vs. female mice, in response to different diets or disease states, or across discrete inbred mouse strains (e.g., C57BL/6, BALB/C, FVB/N).1,80, 81, 82, 83
Through combined in vivo experimentation and mathematical modeling, we show that Asbt-mediated ileal BA absorption not only maintains the size of the SI BA pool; it also conserves pool composition, particularly enrichment of 1o cBAs. At steady-state, the SI BA pool is enriched in 1o cBAs and specialized for lipid and fat-soluble vitamin absorption. Conversely, loss of Asbt-mediated absorption not only reduced the size of the enterohepatic BA pool; it also becomes infiltrated with hydrophobic 2o BAs generated by bacterial metabolism in the LI. Mechanistically, encroachment of 2o BAs into the SI pool is likely to involve increased rates of 2o BA metabolism by colonic bacteria. However, we cannot exclude additional contributions, such as preferential Asbt-mediated transport of 1o BAs and/or biased hepatic synthesis of CA vs. MCAs in Asbt-deficient mice, which were not accounted for in our mathematical models. Compositional shifts in the enterohepatic BA pool of Asbt-deficient mice also raise intriguing clinical questions that to our knowledge have not been addressed. For example, our results suggest that composition of enterohepatic BA pools may be overtly altered in individuals with intestinal diseases, surgical procedures, or pharmacologic interventions that impair ASBT expression or function. If so, do these pools display disproportionately high levels of 2o BAs? If this is the case, does enrichment of 2° BAs affect other properties of the enterohepatic pool, such as lipid or fat-soluble vitamin absorption? These and other questions are important for understanding broader impacts of BA metabolism on human health and disease.
Our modeling results suggest that ileal absorption and hepatic synthesis act independently to dictate the size of the SI BA pool and the amount of fecal BA excretion, respectively. This principle could provide a mechanistic explanation for the uncoupling of ileal BA absorption and fecal BA excretion in mice or humans lacking OSTα/β-mediated basolateral BA efflux in ileal enterocytes. Despite a block in ileal BA absorption, fecal BA excretion is not elevated in OSTα/β-deficient preclinical or clinical settings because of increased gut-liver signaling via Fgf-15 (in Slc51a-/- mice) or FGF-19 (in children born with homozygous loss of SLC51A function), which act dominantly to limit increased hepatic BA biosynthesis despite reduced enterohepatic BA circulation.84,85
Finally, our study establishes new paradigms in how complex enterohepatic BA pools influence host intestinal cell function. Numerous studies to-date have explored gene regulatory functions of individual BAs, either in vitro or in vivo. Yet in vivo studies where mice are fed BA-supplemented diets19,28 are confounded by effects on host (e.g., hepatic) and microbial metabolism (Figure 3, Figure 4, Figure 5, Figure 6). Similarly, in vitro studies are limited by routine use of arbitrary and supraphysiologic BA concentrations that also fail to account for in vivo competition between neighboring BA species. The notion of BA competition becomes particularly important when considering that different BA species can bind competitively to the same NR, but elicit opposite functions (CDCA activates FXR, whereas (t)βMCA antagonizes it).75,86, 87, 88 The extent to which these or other competitive interactions shape host intestinal responses to BAs have yet to be rigorously examined.
Using cultured ileal explants, we show that direct ex vivo functions of 2 SI BA pools with markedly different compositions (in this case from wild-type or Asbt-deficient mice) are more different than they are similar. Despite possessing ∼2-fold fewer total BAs after normalization attempts, SI BA extracts from Asbt-deficient mice elicited more differential gene expression that those evoked by wild-type BA pools. Further study is needed to elucidate underlying mechanisms, but one possibility is differing ratios of FXR agonist (e.g., CDCA) vs. antagonist (e.g., (t)βMCA) BAs within the pools. Although both CDCA and (t)βMCA were depleted in Asbt-deficient vs. wild-type SI extracts (Figure 3D), loss of Asbt disproportionately depleted muricholates, resulting in a ∼20-fold relative increase in the CDCA/(t)βMCA ratio. However, several known FXR target genes (Fgf15, Slc51b, and Slc10a2) were similarly influenced by both wild-type and Asbt-deficient SI BA pools, suggesting that differential FXR activation is not the only contributing factor underlying unique transcriptional responses of ileal explants to wild-type or Asbt-deficient BA pools. Another, perhaps additive, function of the enterohepatic BA pool in Asbt-deficient mice is the increase in multiple 2o BA species and metabolites, including DCA, LCA, and their corresponding metabolites, known for activating orthogonal NR pathways (e.g., VDR, PXR).50,89
In conclusion, substantial work remains to appreciate the full impacts of BA metabolism on host physiology, and in turn, to leverage these insights for therapeutic benefit in human patients. Our study takes key next steps by empirically defining the form and functions of ileal BA pools in mice. These approaches should be broadly useful for interrogating functions of BA pools related to age, sex, disease, or treatment differences. In addition, the paradigms established here are forward-looking and useful for informing the synthesis, intestinal absorption, and functions of recently, or still-to-be identified, BAs.
Methods
Mice
C57BL/6J (B6) mice (stock no: 000664) were purchased from the Jackson Laboratory. B6-derived Slc10a2-/- (B6.Slc10a2-/-) mice were obtained from Dr. Paul Dawson (Emory University, Atlanta, GA).44 Most mice used in these experiments were female, to avoid fighting by males of different litters; animals were weaned at 4–5 weeks old, cohoused at 8–10 weeks old, and sacrificed at 29–32 weeks old unless otherwise stated. Mice were maintained under specific pathogen-free conditions, fed irradiated 2920 Teklad Global Soy Protein-Free Extruded Rodent Diet (Inotiv), and maintained on chlorinated water. Mice were housed in individually ventilated microisolator cages placed on Allentown ventilated racks, and maintained under 7am/7pm light on/off cycles. Cages, bedding, and all equipment were autoclaved before use. All breeding and experimental use of animals was conducted in accordance with protocols approved by IACUC committees at Scripps Florida and Dartmouth College.
Bile Acid Metabolomics
Mice were separated into individual cages and fasted for 4 hours before sample collection. Samples (siLC, smvB, feces, pB) were collected, weighed, and stored at -80°C until sent for BA quantification. siLC was harvested from the whole SI. pB from submandibular cheek bleeds with 5 mm Goldenrod animal lancet (Medipoint) were collected into MiniCollect 1 mL lithium heparin coated tubes (Greiner Bio-One). smvB was subsequently collected from surgically open live mice under 3% isoflurane nose cone administered anesthesia using 5-mL syringe attached to a 30G mouse PE CSF collection lines (SAI) coated in lithium heparin. siLC was harvested from the whole SI following EA-34000 SMARTBOX CO2 euthanasia (E-Z System Inc) immediately after smvB collection. BA quantification analyses were performed by Creative Proteomics. Briefly, for blood, 20 μL samples were mixed with 80 μL of the internal standard (IS) solution (50% methanol/50% water containing 0.01% formic acid, deuterated BAs) and 900 μL water. The deuterated BAs were d4-CA, 2,2,4,4-d4-UDCA, d4-CDCA, d4-DCA, d4-LCA, d4-(t)UDCA, d4-(t)CA, 2,2,4,4-d4-(g)UDCA, d4-(g)CA, d4-(t)CDCA, d6-(t)DCA, d4-(g)CDCA, 2,2,4,4-d4-(g)DCA, 2,2,4,4-d4-(g)LCA. After samples were sonicated on ice for 10 minutes, the mixture was loaded onto a polymeric reversed-phase SPE cartridge (60 mg/mL). After washing with water, BAs were eluted with 1 mL methanol. The flow-through fraction was collected under a 2-psi positive pressure, dried under a nitrogen gas flow, and reconstituted in 80 mL of 50% acetonitrile. For siLC and feces, samples were homogenized in 20 μL of 70% acetonitrile per 1 mg tissue using a Mixer mill MM 400 (Retsch, Haan, Germany) at 30 Hz for 3 minutes, sonicated in a water bath for 3 minutes, then centrifugation. The supernatant was diluted with IS solution containing a mixture of standards containing all the target BAs was dissolved in an IS solution containing 14 D-labeled BAs and used for calibration. An Agilent 1290 UHPLC system (Agilent) coupled to a Sciex 4000 QTRAP mass spectrometer (Sciex) was used. The MS instrument was operated in the multiple reaction monitoring mode with negative-ion detection. A Waters BEH C18 column (2.1∗150 mm, 1.7 mm) was used, and the mobile phase was (A) 0.01% formic acid in water and (B) 0.01% formic acid in acetonitrile for binary-solvent gradient elution. For further analyses with similar matrix algorithm and Pearson correlation, Morpheus (https://software.broadinstitute.org/morpheus) was used.
Intestinal Explant Cultures
The terminal 6 cm of ileum, or 3 cm of the proximal colon, was harvested from 8–18 weeks old male B6 mice after fasting for 4 hours. Fat was removed and tissues were opened longitudinally. Luminal contents were gently flushed with ice-cold phosphate-buffered saline (PBS), and fragments were first cut into 0.5-cm pieces along the vertical axis; these pieces were subsequently cut into halves along the horizontal axis and placed in ice-cold PBS before culture. Fragments containing Peyer's patches were not used. Square sponges of 1 cm × 1 cm (Surgifoam; Ethicon) were soaked in 1 mL of serum-free Advanced DMEM/F12 (Gibco) containing 100 U/mL penicillin, 100 μg/mL streptomycin, 10 mM HEPES, 2 mM GlutaMAX (Gibco) in 12-well plate wells. Sponges of 0.5 cm × 1 cm and 0.5 mL of medium were used for cultures in 24-well plates. Air in sponges was released by applying manual pressure. Intestinal fragments were then dried gently on kimwipes, opened apical side up, and placed on media-soaked sponge. Plates were incubated at 37°C with 5% CO2. In some experiments, 0.2%–0.6% DMSO, 50-400 μM (t)CDCA (Sigma-Aldrich), 200 μM GW4064 (MedChemExpress), and/or 10 μM odevixibat (MedChemExpress) were used.
For experiments where cultured explants were treated with endogenous siLC extracts, siLC was collected from whole SI of 8- to 9-week-old B6 or B6.Slc10a2-/- mouse cohoused for at least 4 weeks, weighed and stored at -20°C. Samples were homogenized in 1 mL of a mixture of 75% acetonitrile, 10% methanol, 15% water with 0.5 g of 2.7 mm dia glass beads (BioSpec Products), and SPEX SamplePrep 1600 miniG (Cole-Parmer) at 1500 strokes/minute for 1 minute with 3 sets. After incubated on a nutator (60 minutes, room temperature), samples were centrifuged (16,000 g, 10 minutes, 4°C) and the supernatants were harvested. These steps were repeated using the pellets from the first extraction. The pooled supernatants were dried up by Savant SpeedVac SPD120 (Thermo Fisher Scientific) and redissolved in DMSO. To deplete BAs, the samples were incubated with 19-fold volume of 50 mM cholestyramine (Sigma-Aldrich)-containing PBS for 60 minutes on a shaker (200 rpm, room temperature). After centrifugation (16,000 g, 10 minutes, 4°C), the supernatants were harvested. As cholestyramine-untreated groups, the samples were diluted in a 19-fold volume of PBS. Total BA assay (Diazyme Laboratories) and BioTek Epoch Microplate Spectrophotometer (Agilent Technologies) were used to measure total BA concentrations to determine the volume of cholestyramine-untreated extract needed to add for a final concentration of ∼300 μM BAs in the ileal explant culture media. The same volume of cholestyramine-treated extract was used for the BA-depleted media.
Gene Expression Assays
Quantitative Polymerase Chain Reaction
Total RNA was isolated from cultured or ex vivo tissues using TRIzol (Invitrogen) or RNeasy Mini Kit (Qiagen; with on-column DNase I treatment) after mechanical disruption with 0.5 g of 2.7 mm dia glass beads and SPEX SamplePrep 1600 miniG (1500 strokes/minute, 1 minute, 2 sets). RNA was quantified on a NanoDrop 2000 (Thermo Scientific). Quantitative polymerase chain reaction was performed with SuperScript III Platinum One-Step qRT-PCR Kit (Invitrogen) and commercial Taqman primer/probe sets (Applied Biosystems) on a QuantStudio 6 Pro (Applied Biosystems). Probes included: Actb (Mm00607939_s1) and Fgf15 (Mm00433278_m1). Data were analyzed with Design & Analysis (Applied Biosystems).
Nanostring
Tissues (spleen, liver, colon, terminal ileum) were harvested postmortem from mice after a 4-hour fasting period and blood (peripheral, portal) collection, as mentioned previously. Total RNA was isolated using RNeasy Mini kits with on-column DNase I treatment. Gene expression was analyzed using a custom codeset run on an nCounter Pro system (Nanostring) as per manufacturer’s instructions. Data were analyzed with nSolver software (Nanostring).
Anaerobic Bacterial Culture
The preserved fecal pellets were thawed, weighed, and resuspended in sterile PBS supplemented with 10 mM L-cysteine in a ratio of 1 to 10 wt/vol. Following homogenization, the samples were inoculated into MiPro medium90 in a sterile 12-well plate to a final vol/vol ratio of 2%. Each culture was passaged anaerobically for 24 hours at 37°C in a Whitley A55 anaerobic chamber (Don Whitley Scientific, Victoria 412 Works) with gas composition of 10% CO2, 10% H2, and 80% N2. The culture was analyzed as follows: (1) CFU/mL were calculated by plating aliquot of the planktonic culture on sheep blood agar that was then incubated at 0% or 21% oxygen; (2) the cell pellets were collected and stored in RNAprotect (Qiagen) at -80°C for DNA extraction, 16S rRNA gene amplicon library sequencing and metagenomic sequencing; and (3) culture supernatants were filter-sterilized and stored at -80°C for subsequent analysis of BA compositions.
Bioinformatics
Metagenomics
Feces were collected from individually housed mice after a 4-hour fasting time and stored at -80°C before shipment to TransnetYX for library preparation and sequencing. DNA was extracted using Qiagen DNeasy 96 PowerSoil Pro QIAcube HT kits (Qiagen). Sequencing libraries were prepared with Watchmaker DNA Library Prep kits with Fragmentation (Watchmaker) and sequenced using the Illumina NovaSeq instrument (Illumina) with the shotgun sequencing method (a depth of 2 million 2 x 150 bp read pairs). Acquired data were analyzed with the One Codex database. Metagenomics reads were processed using atlas v.2.13.0.91 In short, tools from the STAR suite v.2.7.2b92 were used to filter out contaminations from the mouse host genome. Reads were error corrected and merged before assembly with metaSpades v.3.15.3.93 Contigs were binned using maxbin2 v.2.2.94 The predicted MAGs were clustered (95% average nucleotide identity) resulting in 34 representative genomes (referred later as genomes). Genes of each genome were predicted using eggNOG v.2.1.94 α- and β-diversity of metagenome communities were calculated using the relative abundance of MAGs from the atlas workflow with vegan R package v.2.6-4.95
RNA-seq
Bulk RNA-seq analysis was performed on wild-type (B6) ileal fragments cultured with DMSO, (t)CDCA, or endogenous BA extracts with or without CME treatment from B6 or B6.Slc10a2-/- mice, as described previously. Total RNA was isolated with TRIzol (Invitrogen) after mechanical disruption with 0.5 g of 2.7 mm dia glass beads and SPEX SamplePrep 1600 miniG (1500 strokes/minute, 1 minute, 2 sets), and quantified using a Qubit Fluorometer (Invitrogen). RNA quality was evaluated using a 5200 Fragment Analyzer System (Agilent Technologies). QuantSeq 3’ mRNA-Seq V2 Library Prep Kit with UDI (Lexogen, Vienna, Austria) was used for cDNA library preparation from 100 ng total RNA. Libraries were pooled and sequenced using 10 M, single-end, 100-bp forward strand reads per sample on NextSeq2000 (Illumina). DNA alignment, quantification, and annotation were performed using the Dartmouth Analytics Core RNAseq pipeline. Quality control was evaluated using fastQC. Reads trimmed using Cutadapt were aligned to the NCBI mm39 mouse reference genome using the HSIAT2 aligner. Sequencing metrics and ribosomal RNA identification were performed using the Picard Toolkit, and a report was created using MultiQC. Samples with low sequencing depths were removed. Differential gene expression analysis was performed with DESeq2 (Padj < .05) to enumerate genes differentially expressed in each condition, relative to DMSO. Gene symbols were annotated by Entrez gene ID using the org.Mm.eg.db database. ggplot2 was used to visualize data. Metagenomic and RNA-seq data are publicly available at the NIH BioProject (PRJNA1078280; https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1078280).
Mathematical Modeling of BA Circulation and Metabolism
BA flux through the intestine was modeled as a series of connected compartments.37 Additional compartments, such as liver and gallbladder, were not modeled to focus on the effects of reabsorption on the intestinal BA pool. Only 1o and 2o BA species were used as a measure of the BA pool diversification. BA concentrations were governed by differential equations accounting for synthesis, transit, reabsorption, and conversion reactions. Units were provided in molar amounts, and the fluxes between the compartments were balanced. Reaction rates that fit our experimental data qualitatively were numerically calculated through parameter optimization. The compartment volumes were required to quantitatively compare with the actual rates determined in the experiment to convert BA units to concentrations. Changes in BA amount in compartment were given by:
for 1o BAs and
for 2o BAs where was the input rate of BAs transiting from the previous compartment; was the removal rate of BAs from the compartment; and were the reabsorption rates of 1o- and 2o BAs, respectively; represented the metabolization rate of 1o BAs into 2o BAs. In the first compartment, the rate of 1o BA synthesis plus the sum of all reabsorbed BAs were used as the input, instead of BAs input from recirculation:
for 1o BAs and
for 2o BAs
The whole intestine was divided into 15 compartments, (10 in SI, 5 in colon). BA intestinal transit speed was set to rates in SI compartments 1–8, reduced to in SI compartments 9–10, and decreased further to in colon compartments 11–15 (Table 2). The colonic transiting speed was considered 10-fold slower than that in the SI, based on the transit of technetium-labeled activated charcoal DTPA in mice.96 Rates of passive absorption were considered different in the SI and colon, with rates and , respectively (Table 2). Active ileal reabsorption was considered in simulations of wild-type B6 mice (i.e., ). The reabsorption rates of 1o- and 2o BAs were considered same in the initial simulation. 1o → 2o BA metabolism was considered only in the colon (i.e., and ).
Table 2.
Experimental Data and Parameters Used for Figure 6
| Experimental data used in optimization | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mice | BA in SI (μmol) | BA in feces (μmol) | 1o BA in SI | 1o BA in feces | ||||||
| B6 | 215 | 9 | 88% | 32% | ||||||
| B6.Slc10a2-/- | 44 | 43 | 60% | 14% | ||||||
| Parameters used in simulations (rates given in min-1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.05 | 0.01 | 0.29 | 0.025 | 0.0012 | 0.0030 | 0.0010 | 0.0090 | 1.20 | 4.29 |
BA, bile acid; SI, small intestine.
MatLab solver ode113 was used to implement simulations of this system, and an optimization of absorption and conversion rates were performed to qualitatively fit our experimental data. For the optimization, a single set of parameters ( was considered and the system was simulated twice in the presence or absence of active reabsorption. fminsearch and the sum of squared errors were used as a cost function. Code is available at github.com/schultz-lab/Bile_Acids.
Because elimination of active reabsorption alone was not sufficient to explain the diversification of the BA pool in B6.Slc10a2-/- mice, an increase of bacterial BA metabolism was implemented as a possible mechanism to increase the amount of 2o BA in the absence of active ileal absorption. Different rates of bacterial BA metabolization were used, and for B6 and B6.Slc10a2-/- mice in our optimization (Table 2).
Statistical Analysis
Statistical analyses were performed with Prism (GraphPad Software). P values were determined as shown in the figure legends. Data are expressed as the mean ± standard error of the mean.
Acknowledgements
The authors thank Core Facility staff at UF Scripps, the Genomics and Molecular Biology Shared Resources at Dartmouth’s Norris Cotton Cancer Center with NCI Cancer Center Support Grant 5P30 CA023108-37, and Creative Proteomics for technical support. They further acknowledge Drs. Casey Weaver (University of Alabama-Birmingham) and Maria Abreu (University of Miami) for critical discussions.
CRediT Authorship Contributions
Koichi Sudo (Formal analysis: Lead; Investigation: Lead; Software: Supporting; Validation: Lead; Writing – original draft: Lead; Writing – review & editing: Supporting)
Amber Delmas-Eliason (Data curation: Supporting; Formal analysis: Supporting; Investigation: Supporting; Software: Supporting; Validation: Supporting; Writing – review & editing: Supporting)
Shannon Soucy (Data curation: Supporting; Formal analysis: Supporting; Investigation: Supporting; Software: Supporting; Validation: Supporting)
Kaitlyn E. Barrack (Formal analysis: Supporting; Funding acquisition: Supporting; Investigation: Supporting; Methodology: Supporting; Validation: Supporting)
Jiabao Liu (Software: Supporting; Writing – review & editing: Supporting)
Akshaya Balasubramanian (Formal analysis: Supporting; Investigation: Supporting; Software: Supporting; Validation: Supporting)
Chengyi Jenny Shu (Investigation: Supporting; Validation: Supporting)
Michael J. James (Investigation: Supporting; Validation: Supporting)
Courtney L. Hegner (Investigation: Supporting; Validation: Supporting)
Henry D. Dionne (Formal analysis: Supporting; Software: Supporting)
Alex Rodriguez-Palacios (Writing – review & editing: Supporting)
Henry M. Krause (Funding acquisition: Supporting; Writing – review & editing: Supporting)
George A. O'Toole Jr. (Funding acquisition: Supporting; Writing – review & editing: Supporting)
Saul J. Karpen (Writing – review & editing: Supporting)
Paul A. Dawson (Conceptualization: Supporting; Funding acquisition: Supporting; Writing – review & editing: Supporting)
Daniel Schultz (Conceptualization: Supporting; Formal analysis: Supporting; Investigation: Supporting; Validation: Supporting; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Mark S. Sundrud (Conceptualization: Lead; Formal analysis: Supporting; Funding acquisition: Lead; Writing – original draft: Supporting; Writing – review & editing: Supporting)
Footnotes
Conflicts of interest These authors disclose the following: Chengyi Jenny Shu and Michael J. James were employees of Synlogic Therapeutics during this study. Mark S. Sundrud was a consultant to Synlogic, Inc; and a member of the immunology advisory board at Sage Therapeutics. The remaining authors disclose no conflicts.
Funding Financial support was provided by National Institutes of Health grants ES033988-01A1 (to G.A.O.); T32HL134598 (to K.E.B.); DK047987 (to P.A.D.); and R01AI143821, R01AI164772, U01AI163063 and a subaward from the Dartmouth Cystic Fibrosis Research Center (P30DK117469; to M.S.S.). A Canadian Institutes of Health Research grant (PJT-186117; to H.M.K.) provided additional support.
References
- 1.Collins S., Stine J., Bisanz J., et al. Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol. 2023;21:236–247. doi: 10.1038/s41579-022-00805-x. [DOI] [PubMed] [Google Scholar]
- 2.Oduyebo I., Camilleri M. Bile acid disease: the emerging epidemic. Curr Opin Gastroenterol. 2017;33:189–195. doi: 10.1097/MOG.0000000000000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuei J., Chau T., Mills D., et al. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med (Maywood) 2014;239:1489–1504. doi: 10.1177/1535370214538743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Aguiar Vallim T., Tarling E., Edwards P. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013;17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Setchell K., Heubi J., Shah S., et al. Genetic defects in bile acid conjugation cause fat-soluble vitamin deficiency. Gastroenterology. 2013;144:945–955.e946. doi: 10.1053/j.gastro.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong M., Oelkers P., Craddock A., et al. Expression cloning and characterization of the hamster ileal sodium-dependent bile acid transporter. J Biol Chem. 1994;269:1340–1347. [PubMed] [Google Scholar]
- 7.Dawson P., Hubbert M., Haywood J., et al. The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Praslickova D., Torchia E., Sugiyama M., et al. The ileal lipid binding protein is required for efficient absorption and transport of bile acids in the distal portion of the murine small intestine. PloS One. 2012;7 doi: 10.1371/journal.pone.0050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Northfield T., McColl I. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut. 1973;14:513–518. doi: 10.1136/gut.14.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann A., Hagey L. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55:1553–1595. doi: 10.1194/jlr.R049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki T., Choi M., Moschetta A., et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Makishima M., Okamoto A., Repa J., et al. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 13.Parks D., Blanchard S., Bledsoe R., et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Chen J., Hollister K., et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 15.Lu T., Makishima M., Repa J., et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–515. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 16.Sinal C., Tohkin M., Miyata M., et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 17.Holt J., Luo G., Billin A., et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17:1581–1591. doi: 10.1101/gad.1083503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrebee C., Li J., Haywood J., et al. Organic solute transporter a-b protects ileal enterocytes from bile acid-induced injury. Cell Mol Gastroenterol Hepatol. 2018;5:499–522. doi: 10.1016/j.jcmgh.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M., Huang X., Wang H., et al. CAR directs T cell adaptation to bile acids in the small intestine. Nature. 2021;593:147–151. doi: 10.1038/s41586-021-03421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao W., Kayama H., Chen M., et al. The xenobiotic transporter Mdr1 enforces T cell homeostasis in the presence of intestinal bile acids. Immunity. 2017;47:1182–1196. doi: 10.1016/j.immuni.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell D. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 22.Ridlon J., Kang D., Hylemon P. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Sato Y., Atarashi K., Plichta D.R., et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature. 2021;599:458–464. doi: 10.1038/s41586-021-03832-5. [DOI] [PubMed] [Google Scholar]
- 24.Rimal B., Collins S.L., Tanes C.E., et al. Bile salt hydrolase catalyses formation of amine-conjugated bile acids. Nature. 2024;626:859–863. doi: 10.1038/s41586-023-06990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takei H., Narushima S., Suzuki M., et al. Characterization of long-chain fatty acid-linked bile acids: a major conjugation form of 3β-hydroxy bile acids in feces. J Lipid Res. 2022;63 doi: 10.1016/j.jlr.2022.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahlström A., Sayin S., Marschall H., et al. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Hang S., Paik D., Yao L., et al. Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 2019;576:143–148. doi: 10.1038/s41586-019-1785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X., Sun X., Oh S., et al. Microbial bile acid metabolites modulate gut RORg+ regulatory T cell homeostasis. Nature. 2020;577:410–415. doi: 10.1038/s41586-019-1865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao J., Ge T., Chen S., et al. Role of bile acids in liver diseases mediated by the gut microbiome. World J Gastroenterol. 2021;27:3010–3021. doi: 10.3748/wjg.v27.i22.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Režen T., Rozman D., Kovács T., et al. The role of bile acids in carcinogenesis. Cell Mol Life Sci. 2022;79:243. doi: 10.1007/s00018-022-04278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi L., Chen Y. Circulating bile acids as biomarkers for disease diagnosis and prevention. J Clin Endocrinol Metab. 2023;108:251–270. doi: 10.1210/clinem/dgac659. [DOI] [PubMed] [Google Scholar]
- 32.Bodewes F., van der Wulp M., Beharry S., et al. Altered intestinal bile salt biotransformation in a cystic fibrosis (Cftr-/-) mouse model with hepato-biliary pathology. J Cyst Fibros. 2015;14:440–446. doi: 10.1016/j.jcf.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Duboc H., Rajca S., Rainteau D., et al. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 34.Lloyd-Price J., Arze C., Ananthakrishnan A., et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569:655–662. doi: 10.1038/s41586-019-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefebvre D., Ratelle S., Chartrand L., et al. Reduced microbial transformation of bile acids in cystic fibrosis. Experientia. 1977;33:616–618. doi: 10.1007/BF01946533. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q., Lin H., Shen C., et al. Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling. Gut Microbes. 2023;15 doi: 10.1080/19490976.2023.2274124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sips F., Eggink H., Hilbers P., et al. In silico analysis identifies intestinal transit as a key determinant of systemic bile acid metabolism. Front Physiol. 2018;9:631. doi: 10.3389/fphys.2018.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trottier J., Milkiewicz P., Kaeding J., et al. Coordinate regulation of hepatic bile acid oxidation and conjugation by nuclear receptors. Mol Pharm. 2006;3:212–222. doi: 10.1021/mp060020t. [DOI] [PubMed] [Google Scholar]
- 39.Alnouti Y. Bile Acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108:225–246. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 40.Hanson R., Szczepanik-VanLeeuwen P., Williams G., et al. Defects of bile acid synthesis in Zellweger's syndrome. Science. 1979;203:1107–1108. doi: 10.1126/science.424737. [DOI] [PubMed] [Google Scholar]
- 41.Mouzaki M., Wang A., Bandsma R., et al. Bile acids and dysbiosis in non-alcoholic fatty liver disease. PloS One. 2016;11 doi: 10.1371/journal.pone.0151829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camilleri M., Nadeau A., Tremaine W., et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734.e743. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Axelson M., Aly A., Sjövall J. Levels of 7 alpha-hydroxy-4-cholesten-3-one in plasma reflect rates of bile acid synthesis in man. FEBS Lett. 1988;239:324–328. doi: 10.1016/0014-5793(88)80944-x. [DOI] [PubMed] [Google Scholar]
- 44.Dawson P., Haywood J., Craddock A., et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- 45.Marcus S.N., Schteingart C.D., Marquez M.L., et al. Active absorption of conjugated bile acids in vivo. Kinetic parameters and molecular specificity of the ileal transport system in the rat. Gastroenterology. 1991;100:212–221. doi: 10.1016/0016-5085(91)90603-i. [DOI] [PubMed] [Google Scholar]
- 46.Goodwin B., Jones S., Price R., et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 47.Boulias K., Katrakili N., Bamberg K., et al. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. EMBO J. 2005;24:2624–2633. doi: 10.1038/sj.emboj.7600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Funabashi M., Grove T., Wang M., et al. A metabolic pathway for bile acid dehydroxylation by the gut microbiome. Nature. 2020;582:566–570. doi: 10.1038/s41586-020-2396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heuman D., Mills A., McCall J., et al. Conjugates of ursodeoxycholate protect against cholestasis and hepatocellular necrosis caused by more hydrophobic bile salts. In vivo studies in the rat. Gastroenterology. 1991;100:203–211. doi: 10.1016/0016-5085(91)90602-h. [DOI] [PubMed] [Google Scholar]
- 50.Staudinger J., Goodwin B., Jones S., et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zollner G., Wagner M., Moustafa T., et al. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 52.Wagner M., Halilbasic E., Marschall H.U., et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 53.Strott C. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732. doi: 10.1210/er.2001-0040. [DOI] [PubMed] [Google Scholar]
- 54.De Witt E., Lack L. Effects of sulfation patterns on intestinal transport of bile salt sulfate esters. Am J Physiol. 1980;238:G34–G39. doi: 10.1152/ajpgi.1980.238.1.G34. [DOI] [PubMed] [Google Scholar]
- 55.Low-Beer T., Tyor M., Lack L. Effects of sulfation of taurolithocholic and glycolithocholic acids on their intestinal transport. Gastroenterology. 1969;56:721–726. [PubMed] [Google Scholar]
- 56.Sonoda J., Xie W., Rosenfeld J., et al. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Natl Acad Sci U S A. 2002;99:13801–13806. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miyata M., Watase H., Hori W., et al. Role for enhanced faecal excretion of bile acid in hydroxysteroid sulfotransferase-mediated protection against lithocholic acid-induced liver toxicity. Xenobiotica. 2006;36:631–644. doi: 10.1080/00498250600776827. [DOI] [PubMed] [Google Scholar]
- 58.Worsøe J., Fynne L., Gregersen T., et al. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterol. 2011;11:145. doi: 10.1186/1471-230X-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pišlar M., Brelih H., Mrhar A., et al. Analysis of small intestinal transit and colon arrival times of non-disintegrating tablets administered in the fasted state. Eur J Pharm Sci. 2015;75:131–141. doi: 10.1016/j.ejps.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Lee Y., Erdogan A., Rao S. How to assess regional and whole gut transit time with wireless motility capsule. J Neurogastroenterol Motil. 2014;20:265–270. doi: 10.5056/jnm.2014.20.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahlström A., Kovatcheva-Datchary P., Ståhlman M., et al. Induction of farnesoid X receptor signaling in germ-free mice colonized with a human microbiota. J Lipid Res. 2017;58:412–419. doi: 10.1194/jlr.M072819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li J., Dawson P. Animal models to study bile acid metabolism. Biochim Biophys Acta Mol. Basis Dis. 2019;1865:895–911. doi: 10.1016/j.bbadis.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dietschy J. The role of bile salts in controlling the rate of intestinal cholesterogenesis. J Clin Invest. 1968;47:286–300. doi: 10.1172/JCI105725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dietschy J. Mechanisms for the intestinal absorption of bile acids. J Lipid Res. 1968;9:297–309. [PubMed] [Google Scholar]
- 65.Tyor M., Garbutt J., Lack L. Metabolism and transport of bile salts in the intestine. Am J Med. 1971;51:614–626. doi: 10.1016/0002-9343(71)90285-3. [DOI] [PubMed] [Google Scholar]
- 66.Boyd G., Merrick M., Monks R., et al. Se-75-labeled bile acid analogs, new radiopharmaceuticals for investigating the enterohepatic circulation. J Nucl Med. 1981;22:720–725. [PubMed] [Google Scholar]
- 67.Aldini R., Ussia G., Roda A., et al. Evaluation of the ileal absorption capacity for bile acids in the rabbit. Eur Surg Res. 1990;22:93–100. doi: 10.1159/000129088. [DOI] [PubMed] [Google Scholar]
- 68.Aldini R., Roda A., Lenzi P.L., et al. Bile acid active and passive ileal transport in the rabbit: effect of luminal stirring. Eur J Clin Invest. 1992;22:744–750. doi: 10.1111/j.1365-2362.1992.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 69.Aldini R., Roda A., Montagnani M., et al. Hepatic uptake and intestinal absorption of bile acids in the rabbit. Eur J Clin Invest. 1994;24:691–697. doi: 10.1111/j.1365-2362.1994.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 70.Aldini R., Montagnani M., Roda A., et al. Intestinal absorption of bile acids in the rabbit: different transport rates in jejunum and ileum. Gastroenterology. 1996;110:459–468. doi: 10.1053/gast.1996.v110.pm8566593. [DOI] [PubMed] [Google Scholar]
- 71.Aldini R., Roda A., Montagnani M., et al. Relationship between structure and intestinal absorption of bile acids with a steroid or side-chain modification. Steroids. 1996;61:590–597. doi: 10.1016/s0039-128x(96)00119-5. [DOI] [PubMed] [Google Scholar]
- 72.Balakrishnan A., Wring S., Polli J. Interaction of native bile acids with human apical sodium-dependent bile acid transporter (hASBT): influence of steroidal hydroxylation pattern and C-24 conjugation. Pharm Res. 2006;23:1451–1459. doi: 10.1007/s11095-006-0219-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Krag E., Phillips S. Active and passive bile acid absorption in man. Perfusion studies of the ileum and jejunum. J Clin Invest. 1974;53:1686–1694. doi: 10.1172/JCI107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vítek L. Bile acid malabsorption in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:476–483. doi: 10.1097/MIB.0000000000000193. [DOI] [PubMed] [Google Scholar]
- 75.Chiang J.Y.L., Ferrell J.M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol Cell Endocrinol. 2022;548 doi: 10.1016/j.mce.2022.111618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russo I., Zeppa P., Iovino P., et al. The culture of gut explants: a model to study the mucosal response. J Immunol Methods. 2016;438:1–10. doi: 10.1016/j.jim.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 77.Maloney P.R., Parks D.J., Haffner C.D., et al. Identification of a chemical tool for the orphan nuclear receptor FXR. J Med Chem. 2000;43:2971–2974. doi: 10.1021/jm0002127. [DOI] [PubMed] [Google Scholar]
- 78.Graffner H., Gillberg P.G., Rikner L., et al. The ileal bile acid transporter inhibitor A4250 decreases serum bile acids by interrupting the enterohepatic circulation. Aliment Pharmacol Ther. 2016;43:303–310. doi: 10.1111/apt.13457. [DOI] [PubMed] [Google Scholar]
- 79.Shalon D., Culver R., Grembi J., et al. Profiling the human intestinal environment under physiological conditions. Nature. 2023;617:581–591. doi: 10.1038/s41586-023-05989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trottier J., Perreault M., Rudkowska I., et al. Profiling serum bile acid glucuronides in humans: gender divergences, genetic determinants, and response to fenofibrate. Clin Pharmacol Ther. 2013;94:533–543. doi: 10.1038/clpt.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phelps T., Snyder E., Rodriguez E., et al. The influence of biological sex and sex hormones on bile acid synthesis and cholesterol homeostasis. Biol Sex Differ. 2019;10:52. doi: 10.1186/s13293-019-0265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma C., Guo Y., Klaassen C.D. Effect of gender and various diets on bile acid profile and related genes in mice. Drug Metab Dispos. 2021;49:62–71. doi: 10.1124/dmd.120.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evangelakos I., Heeren J., Verkade E., et al. Role of bile acids in inflammatory liver diseases. Semin Immunopathol. 2021;43:577–590. doi: 10.1007/s00281-021-00869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rao A., Haywood J., Craddock A.L., et al. The organic solute transporter alpha-beta, Ostalpha-Ostbeta, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–3896. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tronstad R.R., Berland S., Tjora E., et al. Fat malabsorption and ursodeoxycholic acid treatment in children with reduced organic solute transporter-α (SLC51A) expression. JPGN Rep. 2022;3 doi: 10.1097/PG9.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sayin S., Wahlström A., Felin J., et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Li F., Jiang C., Krausz K.W., et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jiang C., Xie C., Lv Y., et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6 doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makishima M., Lu T.T., Xie W., et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 90.Li L., Abou-Samra E., Ning Z., et al. An in vitro model maintaining taxon-specific functional activities of the gut microbiome. Nat Commun. 2019;10:4146. doi: 10.1038/s41467-019-12087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kieser S., Brown J., Zdobnov E., et al. ATLAS: a Snakemake workflow for assembly, annotation, and genomic binning of metagenome sequence data. BMC Bioinformatics. 2020;21:257. doi: 10.1186/s12859-020-03585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dobin A., Davis C., Schlesinger F., et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bankevich A., Nurk S., Antipov D., et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cantalapiedra C., Hernández-Plaza A., Letunic I., et al. eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol Biol Evol. 2021;38:5825–5829. doi: 10.1093/molbev/msab293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oksanen J., Simpson G., Blanchet F., et al. vegan: Community Ecology Package. 2.6–4. CRAN. 2022 doi: 10.32614/CRAN.package.vegan. [DOI] [Google Scholar]
- 96.Padmanabhan P., Grosse J., Asad A., et al. Gastrointestinal transit measurements in mice with 99mTc-DTPA-labeled activated charcoal using NanoSPECT-CT. EJNMMI Res. 2013;3:60. doi: 10.1186/2191-219X-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]