Abstract
This experiment aimed to reveal the dynamic changes of protein post-translational lactylation modifications and their correlations with the glycolytic process in broiler breast muscle within 48 h of postmortem acidification. The experiment involved 12 male AA broilers, 42 days old, with similar body weights (2.8 ± 0.05 kg). The breast fillets (Pectoralis major) were collected after slaughter, and samples were taken at various time points: 0, 15 min, 30 min, 45 min, 60 min, 2 h, 4 h, 6 h, 8 h, 12 h, 18 h, 24 h, 36 h, and 48 h postmortem. The results showed that the rate of glycogen decline in the muscle was highest at 45 min postmortem, and glycogen levels tended to stabilize at 8 h postmortem. The lactate content in the breast reached its highest level at 4 h postmortem and began to decrease, stabilizing at 24 h postmortem. Additionally, the glycolytic potential increased gradually in the first 4 h postmortem, decreased rapidly from 4 to 8 h. Similarly, lactylation modification levels were highest at 8 h postmortem, but stabilized at 12 h postmortem. During this process, the protein expression of the enzymatic lactylation modifier p300 showed no significant difference, while the content of the nonenzymatic lactylation substrate lactoylglutathione significantly decreased at 8 h and 24 h postmortem. Correlation analysis found that lactylation levels were negatively correlated with glycogen content, glucose content, glycolytic potential, and pH value, while positively correlated with lactate content. Besides, there was a positive correlation between lactylation levels and the protein expression of hexokinase, phosphoglycerate kinase 2, phosphoglucomutase 1, and triosephosphate isomerase. Additionally, lactylation levels were positively correlated with the activities of lactate dehydrogenase and phosphofructokinase. In summary, our experiment elucidated the dynamic changes in the entire glycolytic pathway in broiler pectoral muscle during acidification. During this process, lactylation modifications may participate in the glycolysis process by regulating the protein expression and activity of glycolytic enzymes.
Key words: broiler breast, postmortem, glycolytic, lactylation
INTRODUCTION
The process of postmortem muscle acidification in broiler breasts, also known as rigor mortis, is a critical determinant of meat quality (Cavitt et al., 2003). This process begins immediately after slaughter when the cessation of blood circulation leads to a halt in oxygen supply, forcing the muscles to switch from aerobic to anaerobic respiration (Zhao et al., 2023). This leads to glycolysis becoming the primary metabolic pathway, converting muscle glycogen into lactic acid and causing a decrease in pH (Alvarado et al., 2000). Typically, the muscle pH in a live broiler is around 7.2, which can decrease to about 5.5 postmortem (Chauhan et al., 2018). This decrease in pH affects many important meat attributes including texture, tenderness, juiciness, and color (Beauclercq et al., 2022). According to the Food and Agriculture Organization of the United Nations, the global broiler industry processed approximately 75 billion birds in 2022, underscoring the economic importance of broiler meat. Controlled postmortem processes are crucial for the poultry industry to ensure optimal meat quality. Therefore, studying these biochemical changes is both scientifically relevant and economically vital. The significance of the postmortem muscle acidification process directly influences consumer perceptions of meat quality. While the stages and impacts of glycolysis in postmortem muscles such as pH changes and energy depletion are well documented (Chauhan et al., 2018), the detailed transformations of specific enzymes involved during the postmortem period remain poorly documented.
The glycolytic pathway is essential for the postmortem conversion of muscle to meat, primarily because it leads to the production of lactic acid, a key player in muscle pH reduction (Chauhan et al., 2019). The rate and efficiency of glycolysis directly determine the extent of pH drop across the muscle tissues, which in turn influences enzymatic activities and the rate of rigor development (Wojtysiak et al., 2008). The breakdown of glycogen and the resultant lactic acid production are closely linked to several quality parameters of meat. For instance, an optimal rate of pH decline can prevent the denaturation of muscle proteins (England et al., 2016), which is crucial for maintaining the meat's water-holding capacity and texture (Apaoblaza et al., 2015). Conversely, a rapid or uneven pH decline can lead to the PSE-like meat, characterized by a pale color and a watery, soft texture that is generally rejected by consumers (Zhao et al., 2017). Current literature often describes the initial stages of the glycolytic pathway in postmortem muscle, however, detailed studies on the fate of glycolytic enzymes such as hexokinase, phosphofructokinase, pyruvate kinase, and lactate dehydrogenase during the progression of rigor mortis are sparse. Understanding these transformations is crucial, as they could reveal novel targets for enhancing meat quality through biochemical interventions.
Additionally, the modification of proteins through lactylation, a process where lactate is enzymatically or nonenzymatically added to lysine residues on proteins, is a relatively new area of study that has not been extensively explored in the context of meat science (Gaffney et al., 2020; Zhang et al., 2019a). This modification could play a crucial role in altering protein expression and metabolic reactions during the postmortem period, potentially affecting the quality of the meat. Briefly, the acetyltransferase p300 and lactyl-CoA enzymatically induce lactylation modifications at lysine sites (Zhang et al., 2019a), while lactoyl-glutathione promotes lactylation of lysine nonenzymatically (Gaffney et al., 2020), both modifications result in a 72 Dalton shift in the modified proteins, and alter binding capacity by affecting the secondary structure of the protein. By summarizing the sequencing results of lactylation in animals (Hong et al., 2023), plants (Meng et al., 2021), and microorganisms (Zhao et al., 2022) from previous studies, it has been found that lactylation primarily occurs in the cytoplasm, nucleus, and mitochondria. This suggests that lactylation modification is an important mechanism for regulating energy metabolism. For example, elevated lactylation at the K62 site of pyruvate kinase increases its activity, contributing to the suppression of inflammation by regulating the glycolytic pathway in macrophages (Wang et al., 2022b). The elevated lactylation at the K673 site of fatty acid synthase decreases its activity, thereby reducing the incidence of nonalcoholic fatty liver disease (Gao et al., 2023). However, the specific sources and mechanisms of lactylation in postmortem muscles are still not clear. Lactylation could potentially regulate the activity of enzymes through post-translational modifications, thus impacting the rate of glycolysis, acidification, and the ultimate pH of the meat. Despite the decline in muscle oxidative potential due to the cessation of oxygen supply and blood circulation after animal slaughter, the levels of lactic acid and enzyme activity in the muscle continue to undergo dynamic changes for an extended period as muscle glycogen shifts towards glycolysis (Wojtysiak et al., 2008). Therefore, investigating the dynamic changes in lactylation during postmortem muscle acidification and comprehensively understanding how lactylation interacts with muscle metabolism postmortem can provide important insights for new strategies to improve meat quality.
This study aimed to investigate the dynamic changes in the glycolytic pathway and lactylation modifications during the postmortem acidification process in broiler chicken breast, as well as their correlation. The experimental results could provide new insights into the post-translational modifications of proteins related to meat quality.
MATERIALS AND METHODS
The conduct of this experiment was in line with the regulations of the Institutional Animal Care and Use Committee of Nanjing Agricultural University (Nanjing, China).
Sample Collection
Twelve 42d male AA broiler chickens with similar body weights (2.8±0.05kg) were randomly selected. All broilers had been fed a corn-soybean meal diet formulated according to National Research Council (NRC) standards for each growth stage prior to sample collection, with free access to water and feed, and were exposed to 23 h of light per day. The ingredient composition and nutrient contents of diets are displayed in Table 1. The chickens were stunned using a closed CO2 stream and then exsanguinated via the jugular vein. The Pectoralis major samples were taken from the center of the right breast, with the epidermis and fascia removed, and immediately placed in a 4°C acid drainage room. Samples of approximately 2 grams with 3 times were collected at postmortem intervals of 0, 15 min, 30 min, 45 min, 60 min, 2 h, 4 h, 6 h, 8 h, 12 h, 18 h, 24 h, 36 h, and 48 h, then, these samples were rapidly frozen in liquid nitrogen and stored at -80°C for subsequent experiments.
Table 1.
Ingredient composition and nutrient contents of experimental diets.
| Ingredients (%) | 1–21 d | 22–42 d |
|---|---|---|
| Corn | 55.24 | 59.37 |
| Soybean meal | 36.92 | 31.9 |
| Soybean oil | 3.5 | 5 |
| Limestone | 1.12 | 1.23 |
| Dicalcium phosphate | 2.1 | 1.5 |
| L-lysine | 0.22 | 0.11 |
| DL-methionine | 0.28 | 0.27 |
| Salt | 0.3 | 0.3 |
| Vitamin premix1 | 0.03 | 0.03 |
| Mineral premix2 | 0.2 | 0.2 |
| 70%Choline chloride | 0.09 | 0.09 |
| Calculated nutrients | ||
| Metabolizable energy (MJ/kg) | 12.41 | 12.99 |
| Crude protein | 21 | 19 |
| Calcium | 1 | 0.9 |
| Total phosphorus | 0.67 | 0.56 |
| Available phosphorus | 0.45 | 0.35 |
| Lysine | 1.2 | 1 |
| Methionine + cystine | 0.85 | 0.8 |
| Threonine | 0.66 | 0.6 |
| Tryptophan | 0.22 | 0.2 |
Vitamin premix provided per kilogram of diet: Vitamin A, 12000 IU; Vitamin D3, 2500 IU; Vitamin E, 20 IU; menadione, 1.3 mg; thiamin, 2.21 mg; riboflavin, 7.8mg; nicotinamide, 40mg; calcium pantothenate, 16.5 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1.2 mg; Vitamin B12, 0.015 mg.
Mineral premix provided per kilogram of diet: iron, 80 mg; copper, 8.0 mg; manganese, 110 mg, zinc 65 mg; iodine, 1.1 mg; selenium, 0.3 mg.
The pH Measurement of Breast Muscle
One g of the sample was accurately weighed and added to 9 parts of precooled buffer (150 mM KCl, 5 mM sodium iodoacetate, pH=7.0) at 4°C as previously reported method (Xing et al., 2016a). The mixture was homogenized with a handheld high-speed homogenizer (PT 1300 D, Kinematica, Switzerland) (6000 rpm, 2 × 15 s), with a 10-second interval between homogenizations to allow the machine to cooling. The pH of the homogenate was then measured by a portable pH meter (Hanna Instrument Company, Porto, Portugal) and recorded the values. The measurement was repeated 3 times for each sample.
The Glycolytic Potential Measurement of Breast Muscle
The glucose (F006-1-1), glycogen (A043-1-1), and lactic acid (A019-2-1) contents in the muscle at different time points of aging were measured by the commercial kits. Considering the extremely low and almost undetectable levels of glucose-6-phosphate in broiler breast muscle, the glycolytic potential was calculated according to previous methods (Zhang et al., 2019b) as follows: Glycolytic Potential = 2 glycogen + lactic acid. All reagent kits were sourced from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
The Western Blot Analysis of Breast Muscle
The 50 mg of postmortem samples were accurately weighed at 0, 45 min, 8h, and 24h, and add ceramic zirconia beads along with 9 times the volume of precooled radioimmunoprecipitation assay buffer (RIPA) and 10% protease inhibitor. The samples were homogenized twice at 60 Hz, each for 60 seconds. After homogenization, the mixture was stood at 4°C for 30 min, then centrifuged at 12,000 rpm for 10 min at 4°C to collects the supernatant. The protein concentration of the supernatant was determined by a commercial bicinchoninic acid (BCA) kit (#C503061, Sangon Biotech, Shanghai, China). The protein concentration was diluted and standardized using the above RIPA. An appropriate amount of supernatant was mixed with 5 × loading buffer at a 1:4 ratio, then heated at 95°C for 5 min for western blot analysis. The appropriate stacking gel and resolving gel were selected based on the molecular weight of the target protein, and the appropriate transfer time was determined. The primary antibody was incubated at 4°C overnight. The corresponding secondary antibody was chosen based on the species of the primary antibody. An enhanced chemiluminescence (ECL) kit was used for detection the visualize protein expression bands on the ChemiDoc imaging system (Tanon Science & Technology, Shanghai, China). Additionally, the determination of lactylation modification levels was referenced form previous research methods, the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel bands were used as an internal control (Zhang et al., 2021). The specific method was as follows: after electrophoresis, the entire SDS-PAGE gel was placed in Coomassie Brilliant Blue R250 dye and shaken for 8 h. The gel was then shaken in a decolorizing solution containing methanol and acetic acid for 8 h. Then, the protein bands were imaged using the ChemiDoc imaging system. Finally, the grayscale values of the protein bands were quantified using ImageJ. The relative intensities of target proteins were normalized by tubulin, and their relative expression levels were calculated.
The suppliers and dilution ratios for the primary and secondary antibodies were listed in Table 2.
Table 2.
The suppliers and dilution ratios for the primary and secondary antibodies.
| Name1 | Supplier | Dilution ratios | Cat no. |
|---|---|---|---|
| HK | Proteintech | 1:5000 | 22029-1-AP |
| PGM1 | Abclonal | 1:1000 | A21121 |
| PGI | Abclonal | 1:1000 | A4401 |
| PFKM | Proteintech | 1:2000 | 55028-1-AP |
| ALDOC | Proteintech | 1:2000 | 14884-1-AP |
| TPI1 | Proteintech | 1:2000 | 10713-1-AP |
| GAPDH | Proteintech | 1:10000 | 60004-1-Ig |
| PGK2 | Abclonal | 1:1000 | A12952 |
| PGAM1 | Abclonal | 1:1000 | A4170 |
| ENO3 | Abclonal | 1:2000 | A3852 |
| PKM | Proteintech | 1:1000 | 15822-1-AP |
| LDHA | Abclonal | 1:2000 | A1146 |
| GLO1 | Abclonal | 1:2000 | A4329 |
| Tubulin | Proteintech | 1:3000 | 11224-1-AP |
| Lactylation | PTM BIO | 1:1000 | PTM-1401 |
| p300 | Cell Signaling Technology | 1:1000 | 86377T |
| Anti-rabbit IgG | Cell Signaling Technology | 1:3000 | 7074S |
| Anti-mouse IgG | Cell Signaling Technology | 1:3000 | 7076S |
HK, hexokinase; PGM1, phosphoglucomutase 1; PGI, phosphoglucose isomerase; PFKM, phosphofructokinase muscle type; ALDOC, aldolase C; TPI1, triosephosphate isomerase 1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PGAM1, phosphoglycerate msutase 1; PGK2, phosphoglycerate kinase 2; ENO3, β-enolase; PKM, pyruvate kinase muscle type; LDHA, lactate dehydrogenase A type; GLO1, glyoxalase I.
The Enzyme Activity Assay of Breast Muscle
The enzyme activities of Hexokinase (A077-3-1), Phosphofructokinase (A129-1-1) (Muscle Type), Pyruvate Kinase (A076-1-1) (Muscle Type), and Lactate Dehydrogenase A (A020-1-2) in broiler breast muscle at 0, 45 min, 8 h, and 24 h of postmortem was measured by commercial kits. All reagent kits were provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
The Lactylation Substrate Content Measurement of Breast Muscle
The contents of methylglyoxal at different time points in breast muscle were measured by 2,4-dinitrophenylhydrazine (DNPH) derivatization and high-performance liquid chromatography (HPLC) analysis (Zhang et al., 2019b). The specific steps are as follows: Approximate 500 mg of samples from different time points were weighed, and added 9 times the volume of precooled PBS buffer (pH=7.0), and homogenized at 4°C, 6000 rpm for 2 × 15 s. The homogenates were centrifuged at 12,000 rpm for 10 min at 4°C to collect the supernatant. The protein concentration of the supernatant was measured with a BCA kit, and the protein concentration was standardized with the mentioned PBS buffer. Approximate 500 µL of the supernatant with 500 µL of DNPH buffer (0.1% DNPH, 2 M HCl) were incubated at 37°C for 1 h. After incubation, added 1 mL of acetonitrile to the mixture, vortex, and centrifuge at 12,000 rpm for 5 min at 4°C to collect the supernatant. A 20 µL volume of the extracted supernatant was injected into the HPLC system, with a C18 column and gradient elution (water with 0.1% formic acid and acetonitrile with 0.1% formic acid) being used. Finally, detection was carried out at 360 nm, and the contents of acetaldehyde was calculated based on the peak area of the acetaldehyde-DNPH standard. The contents of glutathione in breast muscle at different acidification time points were measured by a commercial kit (A006-2-1) from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), while the contents of lactoylglutathione (LGSH) were determined by an enzyme-linked immunosorbent assay (ELISA) kits (AF0016-ChA) from AiFang Biotechnology Co., Ltd (Wuhan, China).
Statistical Analysis
The experimental data were analyzed using repeated measures ANOVA in the SPSS (22.0) system, and Duncan's method was employed for multiple comparisons. The results were expressed as mean ± standard error (n = 12). The spearman correlation analysis was performed in heatmap using the pheatmap package of R software (Version 4.3.2). It is important to note that in the western blot experiment, to represent all samples from each time point in a single band, we mixed 4 samples from each time point into one, so n=3. P < 0.05 was considered statistically significant.
RESULTS
pH Changes During the Postmortem Acidification Process
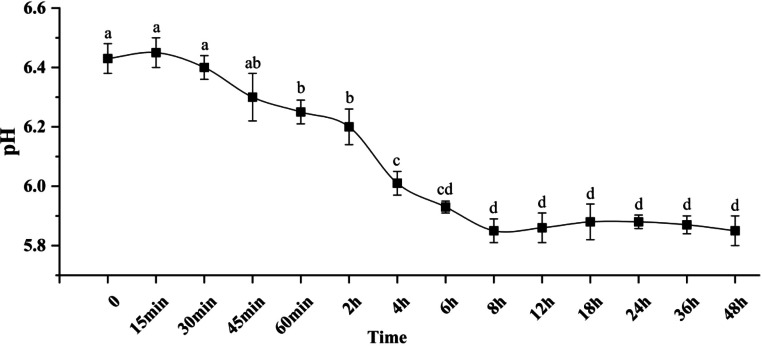
As shown in Figure 1, The time points at which pH significantly decreased were 60 min, 4 h, and 8 h during the acidification process (P < 0.05). After 8 h, the pH stabilized (P > 0.05).
Figure 1.
Dynamic changes in the pH of broiler breast at different acidification time points. The data are presented as Mean ± SE (n = 12), a-dRepresents significant differences (P < 0.05).
Glycolytic Potential Changes During the Postmortem Acidification Process
As shown in Figure 2, the progress of acidification in broiler chicken breast muscle resulted in a rapid decrease in glucose content within the first 15 min (P < 0.05), reaching its lowest value at 48 h (Figure 2A) (P < 0.05). The time points at which glycogen significantly decreased were at 60 min, 2 h, 4 h, and 8 during the acidification process (P < 0.05), after which it stabilized (P > 0.05) (Figure 2B). The first time point at which a significant increase in lactate content in the breast muscle occurred during the acidification process was at 60 min (P < 0.05), reaching its highest level at 4 h (P < 0.05) (Figure 2C). The glycolytic potential significantly increased at the time of 30min in acidification (P < 0.05), reaching its peak at 4 h (P < 0.05), and significantly decreased at 6 h, 24 h, and 48 h during the acidification process (P < 0.05) (Figure 2D).
Figure 2.
Dynamic changes in the glycolysis of broiler breast at different acidification time points. (A) Contents of glucose. (B) Contents of glycogen. (C) Contents of lactic acid. (D) Glycolytic Potential. The data are presented as Mean ± SE (n = 12), a-f Represents significant differences (P < 0.05).
Lactylation Level Changes During the Postmortem Acidification Process
Figure 3 shows that during the postmortem acidification process of broiler breast, the lactylation level significantly increased in the first 2h (P < 0.05). At the time of 8 h of acidification, the lactylation level reached its highest level (P < 0.05). After 18 h of the acidification, the level of lactylation was significantly decreased (P < 0.05), and then remained stable (P > 0.05).
Figure 3.
Dynamic changes in the lactylation levels of broiler breast at different acidification time points. The data are presented as Mean ± SE (n = 3), a-e Represents significant differences (P < 0.05).
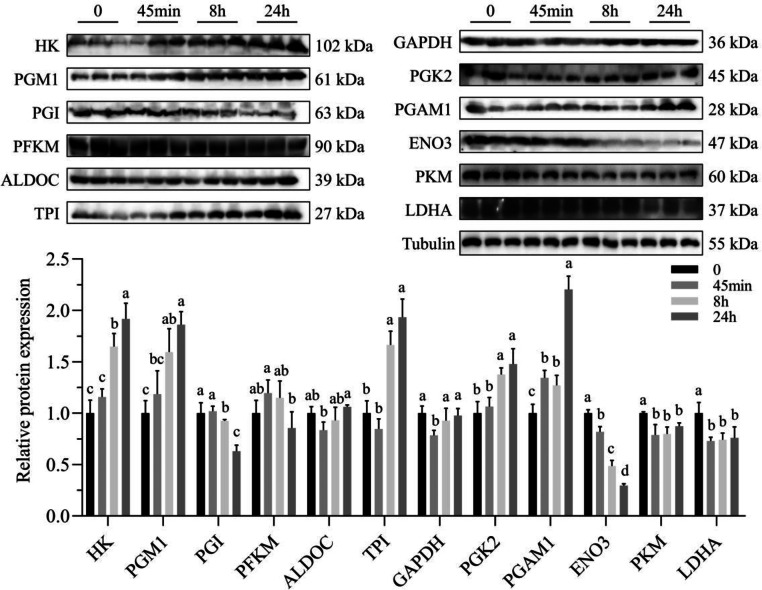
Protein Expression of Enzymes Involved in the Glycolytic Pathway
As shown in Figure 4, compared to immediately postslaughter, the protein expression of GAPDH, ENO3, PKM, and LDHA was significantly down regulated (P < 0.05)at 45 min of postmortem, while the protein expression of PGAM1 was significantly upregulated (P < 0.05). At 8 h of postmortem, the protein expression levels of HK, PGM1, TPI, and PGK2 were significantly upregulated (P < 0.05), whereas the protein expression levels of PGI, ENO3, PKM, and LDHA were significantly downregulated (P < 0.05). At 24 h of postmortem, the protein expression levels of HK, PGM1, TPI, PGK2, and PGAM1 were significantly upregulated (P < 0.05), while the protein expression levels of PGI, ENO3, and LDHA were significantly downregulated (P < 0.05).
Figure 4.
Dynamic changes in the glycolytic enzyme protein expression of broiler breast at different acidification time points. The data are presented as Mean ± SE (n=3). a-d Represents significant differences (P < 0.05).
The Enzymatic Activity of Glycolysis
As shown in Figure 5, compared to immediately postslaughter, the enzymatic activity of HK significantly decreased (P < 0.05) at 45 min of postmortem, while it significantly increased (P < 0.05) at 8 h and 24 h postmortem (Figure 5A). As the acidification time increased, the PFKM activity gradually increased (P < 0.05) (Figure 5B). However, the PKM activity significantly decreased (P < 0.05) at 45 min of postmortem and showed no differences in subsequent acidification stages (P > 0.05) (Figure 5C). The enzymatic activity of LDHA significantly increased (P < 0.05) only at 8 h of acidification, with no significant differences detected at the other time points (P > 0.05) (Figure 5D).
Figure 5.
Dynamic changes in the glycolytic enzyme activities of broiler breast at different acidification time points. (A) The activity of HK in breast. (B) The activity of PFKM in breast. (C) The activity of PKM in breast. (D) The activity of LDHA in breast. The data are presented as Mean ± SE (n=12). a-b Represents significant differences (P < 0.05).
Protein Expression of Lactylation-Related Substrates and Enzymes
As shown in Figure 6, compared to immediately postslaughter, the methylglyoxal content in the tissue significantly decreased (P < 0.05) at 8 and 24 h of acidification (Figure 6A), and the glutathione content significantly decreased (P < 0.05) at 45 min of acidification (Figure 6B). The nonenzymatic lactylation substrate, LGSH, showed no significant changes (P > 0.05) at 45 min of acidification, but its content significantly decreased (P < 0.05) at 8 h and 24 h of acidification (Figure 6C). The protein expression of GLO-1 was significantly downregulated (P < 0.05) at 45 min of acidification but significantly upregulated (P < 0.05) at 8 h and 24 h of acidification. There were no significant differences (P > 0.05) in the expression of p300 protein at any time point (Figure 6D).
Figure 6.
Dynamic changes in the lactylation substrate and enzyme protein expression of broiler breast at different acidification time points. (A) The contents of methylglyoxal in breast. (B) The contents of GSH in breast. (C) The contents of LGSH in breast. (D) Relative expression of lactylation enzymes. The data are presented as Mean ± SE (n = 12). a-b Represents significant differences (P < 0.05).
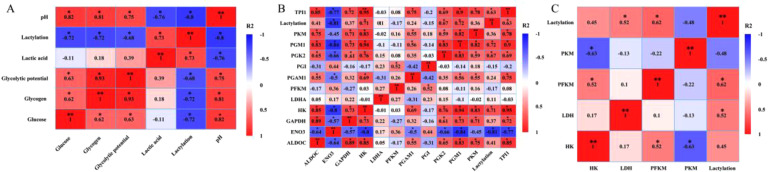
Correlation Analysis
Spearman correlation analysis revealed that postmortem lactylation modification levels were positively correlated with lsactic acid content (R2 > 0.5, P < 0.05) and negatively correlated with glucose content, glycogen content, glycolytic potential, and pH value (R2 < -0.5, P < 0.05) (Figure 7A). Additionally, lactylation modification levels were positively correlated with the protein expression of HK, PGK2, PGM1, and TPI (R2 > 0.5, P < 0.05), and negatively correlated with the protein expression of ENO3 (R2 < -0.5, P < 0.05) (Figure 7B). Furthermore, lactylation modification levels were positively correlated with LDH and PFKM activities (R2 > 0.5, P < 0.05) (Figure 7C).
Figure 7.
Correlation analysis between lactylation levels and glycolytic metabolic pathway during postmortem acidification. Each correlation coefficient is calculated by the spearman method, with positive and negative correlations shaded in red and blue, respectively. The correlation strength is expressed as the color shade. The symbol * represents the difference with a significance level of P < 0.05. (A) Correlation between lactylation and glycolytic substrates. (B) Correlation between lactylation and glycolysis pathway enzyme protein expression. (C) Correlation between lactylation and glycolytic pathway rate-limiting enzyme activity.
DISCUSSION
Acidification is an important process in the transformation of muscle into meat. Although this part of the process is not usually a major focus for chicken, the time from slaughter/soaking, precooling/subdividing, packaging, and transportation to the final sale to consumers typically exceeds 2 d, and in remote areas, it can even exceed a week (Raj et al., 2001). The biochemical reactions during this process still hold significant research importance. According to the National Chicken Council, with improvements in breeding technology and optimized feeding management, the market weight of broiler chickens has increased from an average of 2.5 pounds to 6.54 pounds from 1925 to 2023, while the market age was reached to 47 d in the United States. During these changes, researchers have focused more on the effects of nutrition and genetic selection on growth performance, with fewer reports on the biochemical dynamic changes during the postmortem acidification process of broiler chicken muscle. Notably, under this breeding model, the physiological indicators of broiler chickens at slaughter have continuously changed, and the occurrence of some myopathies has severely impacted the economic benefits of current broiler farming. Research has found that the incidence rate of PSE-like meat at commercial slaughterhouses is 20 to 40% (Xing et al., 2016b), the incidence rate of spaghetti meat is similarly up to 20% (Wu et al., 2024), and the incidence rate of woody breast meat is astonishingly up to 60% in China (Xing et al., 2020). The occurrence of these abnormal meats suggests that there are significant differences in the energy substance content of broilers at market age, especially in glucose, glycogen, and lactic acid, which are crucial energy substrates affecting postmortem acidification and muscle maturation.
Glycogen and glucose are important energy storage and supply substances in muscles (Liu et al., 2021). After the slaughter of broiler chickens, the cessation of blood circulation and the interruption of oxygen supply necessitate energy provision through glycolysis to meet energy demands (England et al., 2016). The pH change caused by the end products of glycolysis, lactic acid, is a crucial factor in the conversion of muscle to meat (Beauclercq et al., 2022). In recent years, although there have been reports on the dynamic changes of glycogen and glucose postmortem in broiler chickens, these reports are relatively dated, and the time points for sample collection were limited (Chauhan et al., 2019). Additionally, with the current frequent occurrence of low-quality meat in slaughterhouses, it is urgently necessary to understand the dynamic metabolic patterns of these energy substrates in postmortem. Glycogen breakdown is generally considered the hallmark of the onset of postmortem glycolysis and is the primary factor influencing the ultimate pH during acidification (England et al., 2016). This experiment found that within approximately the first 45 min postmortem, the rate of glycogen decline in the muscle was relatively slow. During this period, there is typically a substantial amount of ATP and phosphocreatine in the muscle to meet energy demands (Wang et al., 2022a). The period from 45 min to 4 h postmortem marked a rapid phase of glycogen breakdown, indicating that glycolysis is at a high level during this stage. After 8 h of acidification, glycogen levels essentially remained unchanged, and pH measurements also indicated that the ultimate pH was reached at 8 h postmortem. In a previous study, it was reported that complete glycolysis of glycogen in the breast muscle of broilers and the ultimate pH both occurred at 4 h postmortem (Chauhan et al., 2019). This discrepancy to some extent supports our hypothesis that genetic selection and nutritional regulation have altered the levels of energy substrates in the breast muscle of broilers. In fact, the ultimate pH value typically affects the formation of meat quality. For example, the ultimate pH of woody breast is significantly higher than the normal level (Zhang et al., 2020), while PSE-like breast shows the opposite (Xing et al., 2020). Therefore, understanding the current acidification patterns of breast meat has important reference value for regulating meat quality formation.
Glycolysis is an enzyme-dependent metabolic pathway. Therefore, in addition to substrate levels, the activity of metabolic enzymes involved in glycolysis is also an important factor restricting the acidification process (Sun et al., 2021). However, there are few reports on the dynamic changes in glycolytic enzyme activity in the breast muscle of broilers during acidification. Thus, by measuring the expression of enzyme proteins throughout the entire glycolysis process at 4 key time points—immediately postslaughter (0), onset of acidification (45 min), onset of rigor mortis (8 h), and end of acidification (24 h)—could reflect their dynamic changes to a certain extent. This includes the activity determination of key rate-limiting enzymes such as hexokinase, phosphofructokinase, pyruvate kinase, and lactate dehydrogenase. Hexokinase is the first rate-limiting enzyme in glycolysis, primarily responsible for phosphorylating glucose to produce glucose-6-phosphate (Rabbani et al., 2022). Phosphofructokinase is the second rate-limiting enzyme, which phosphorylates fructose-6-phosphate to produce fructose-1,6-bisphosphate (Wegener et al., 2002). The third rate-limiting enzyme is pyruvate kinase, which phosphorylates phosphoenolpyruvate to produce pyruvate, which is finally converted to lactate under the catalysis of lactate dehydrogenase (Rajala 2020). Although previous studies have reported that phosphofructokinase activity initially increases and then decreases during postmortem acidification, peaking at 1 h postmortem (Sun et al., 2019), our experimental results indicated that phosphofructokinase activity significantly increased with the extension of acidification time, reaching its highest activity at 24 h postmortem. This discrepancy may be due to the complexity of the factors influencing phosphofructokinase activity, among which acid environment and energy levels are significant factors (England et al., 2014). Additionally, in our experiment, we found that at 45 min postmortem, the activity of PFKM and LDHA did not significantly differ, indicating that glycolysis was not fully activated in the early postmortem period, which is consistent with previous descriptions (Ruixia et al., 2022). At 8 h postmortem, the activity of HK, PFKM, and LDHA in the muscle significantly increased, indicating that the glycolysis pathway was active between 45 min and 8 h postmortem. However, at 24 h postmortem, the activity of PFKM and LDHA significantly decreased, explaining the reduced efficiency of lactate production during the rigor mortis period. Interestingly, the western blot results did not completely align with the activity levels. For example, the protein expression level of PFKM at 24 h postmortem was significantly downregulated, yet its enzyme activity was significantly increased. Similarly, the protein level of LDHA at 8 h postmortem was significantly downregulated, but its enzyme activity was also significantly increased. This suggests that these metabolic enzymes may undergo post-translational modifications during the postmortem acidification process, which affect their activity (Zhang et al., 2020). However, there are few reports on the dynamic changes in the activity of these rate-limiting enzymes during postmortem acidification currently. Therefore, our results filled this gap.
Lactylation is a newly discovered type of protein post-translational modification (Zhang et al., 2019a), widely present in the nuclei, cytoplasm, and mitochondria of animals, plants, and microorganisms (An et al., 2022; Hong et al., 2023; Meng et al., 2021), playing a crucial regulatory role in energy metabolism (Zhang et al., 2021). However, there are currently no reports on lactylation in broiler tissues. The fiber type in broiler breast muscle is typically glycolytic, primarily composed of type IIb fibers, which have a significantly higher lactate production capacity during stress and postmortem aging than other tissues (Hosotani et al., 2021). It can be hypothesized that lactylation plays an important regulatory role in the metabolism of broiler breast muscle, especially in the glycolysis process during postmortem acidification that produces lactate from glucose and glycogen. Firstly, our results showed that the level of lactylation in the muscle significantly increased with the extension of acidification time. Correlation analysis also revealed a negative correlation between lactylation levels and glycogen/glucose levels and a positive correlation with lactate levels, consistent with the well-accepted conclusion that lactate is the sole substrate for lactylation (Wang et al., 2024). Additionally, by measuring the levels of key enzymatic and nonenzymatic substrates and enzyme protein expression, we found that the protein expression level of the enzymatic lactylation modifier p300 did not significantly changed throughout the muscle aging phase. In contrast, the level of the nonenzymatic lactylation substrate LGSH significantly decreased at 8 h and 24 h postmortem, despite the significant upregulation of the GLO1 protein, which synthesizes LGSH (Gaffney et al., 2020), at these time points. Therefore, we speculated that the enzymatic lactylation pathway might dominate the increase in lactylation levels of postmortem. To further elucidated the relationship between lactylation and glycolysis during acidification, we first analyzed the correlation between lactylation levels and enzyme protein expression. Current research suggests that lactylation can regulate protein expression by affecting epigenetics (Wang et al., 2023). The results showed that the correlation coefficients between lactylation levels and the expression of HK, PGK2, PGM1, and TP1 all exceed 0.5. Rho et al. (2023) reported that high lactate levels could upregulate HK expression by increasing histone H3K18 lactylation levels, while Jiang et al. (2021) believed that the change in HK expression induced by a high lactate environment was due to increased lactylation levels in the HK promoter region. Furthermore, the correlation coefficients between lactylation levels and the enzyme activities of PFKM and LDH exceeded 0.5. Although there are no reports on lactylation affecting PFKM and LDH activities, recent tissue sequencing results indicate a significant increase in the number and frequency of lysine lactylation sites on these 2 proteins as lactate levels increase (Meng et al., 2021; Zhang et al., 2021), which suggests the potential for lactylation to regulate PFKM and LDH activities.
CONCLUSION
In summary, this study investigated the dynamic changes and correlations of glucose, glycogen, lactate, and lactylation modifications in broiler breast muscle within 48 h postmortem acidification. The study revealed that during the postmortem acidification phase, lactylation modifications might be involved in the glycolytic metabolic process by regulating the expression and activity of key glycolytic enzymes. Therefore, this experiment provided a theoretical basis for the potential pathways through which lactylation modifications influencing chicken meat quality formation and offering new insights into the post-translational modifications of meat quality proteins. However, the specific types of amino acids and modification sites involved in lactylation, as well as the functional effects of these modifications, require further investigation.
DISCLOSURES
The authors declare that there are no conflicts of interest.
ACKNOWLEDGMENTS
This study was supported by the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS [2024]).
REFERENCES
- Alvarado C.Z., Sams A.R. The influence of postmortem electrical stimulation on rigor mortis development, calpastatin activity, and tenderness in broiler and duck pectoralis. Poult. Sci. 2000;79:1364–1368. doi: 10.1093/ps/79.9.1364. [DOI] [PubMed] [Google Scholar]
- An D., Song L., Li Y., Shen L., Miao P., Wang Y., Liu D., Jiang L., Wang F., Yang J. Comprehensive analysis of lysine lactylation in Frankliniella occidentalis. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.1014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apaoblaza A., Galaz A., Strobel P., Ramírez-Reveco A., Jeréz-Timaure N., Gallo C. Glycolytic potential and activity of adenosine monophosphate kinase (AMPK), glycogen phosphorylase (GP) and glycogen debranching enzyme (GDE) in steer carcasses with normal (<5.8) or high (>5.9) 24h pH determined in M. longissimus dorsi. Meat Sci. 2015;101:83–89. doi: 10.1016/j.meatsci.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Beauclercq S., Mignon-Grasteau S., Petit A., Berger Q., Lefèvre A., Métayer-Coustard S., Tesseraud S., Emond P., Berri C., Bihan-Duval E.L.e. A divergent selection on breast meat ultimate pH, a key factor for chicken meat quality, is associated with different circulating lipid profiles. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.935868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavitt L.C., Sams A.R. Evaluation of physical dimension changes as nondestructive measurements for monitoring rigor mortis development in broiler muscles. Poult. Sci. 2003;82:1198–1204. doi: 10.1093/ps/82.7.1198. [DOI] [PubMed] [Google Scholar]
- Chauhan S.S., England E.M. Postmortem glycolysis and glycogenolysis: insights from species comparisons. Meat Sci. 2018;144:118–126. doi: 10.1016/j.meatsci.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Chauhan S.S., LeMaster M.N., Clark D.L., Foster M.K., Miller C.E., England E.M. Glycolysis and pH decline terminate prematurely in oxidative muscles despite the presence of excess glycogen. Meat Muscle Biol. 2019;3:254–264. [Google Scholar]
- England E.M., Matarneh S.K., Scheffler T.L., Wachet C., Gerrard D.E. pH inactivation of phosphofructokinase arrests postmortem glycolysis. Meat Sci. 2014;98:850–857. doi: 10.1016/j.meatsci.2014.07.019. [DOI] [PubMed] [Google Scholar]
- England E.M., Matarneh S.K., Oliver E.M., Apaoblaza A., Scheffler T.L., Shi H., Gerrard D.E. Excess glycogen does not resolve high ultimate pH of oxidative muscle. Meat Sci. 2016;114:95–102. doi: 10.1016/j.meatsci.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Gaffney D.O., Jennings E.Q., Anderson C.C., Marentette J.O., Shi T., Schou Oxvig A.M., Streeter M.D., Johannsen M., Spiegel D.A., Chapman E., Roede J.R., Galligan J.J. Non-enzymatic lysine lactoylation of glycolytic enzymes. Cell. Chem. Biol. 2020;27:206–213.e206. doi: 10.1016/j.chembiol.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R., Li Y., Xu Z., Zhang F., Xu J., Hu Y., Yin J., Yang K., Sun L., Wang Q., He X., Huang K. Mitochondrial pyruvate carrier 1 regulates fatty acid synthase lactylation and mediates treatment of nonalcoholic fatty liver disease. Hepatology. 2023;78:1800–1815. doi: 10.1097/HEP.0000000000000279. [DOI] [PubMed] [Google Scholar]
- Hong H., Chen X., Wang H., Gu X., Yuan Y., Zhang Z. Global profiling of protein lysine lactylation and potential target modified protein analysis in hepatocellular carcinoma. Proteomics. 2023;23 doi: 10.1002/pmic.202200432. [DOI] [PubMed] [Google Scholar]
- Hosotani M., Kametani K., Ohno N., Hiramatsu K., Kawasaki T., Hasegawa Y., Iwasaki T., Watanabe T. The unique physiological features of the broiler pectoralis major muscle as suggested by the three-dimensional ultrastructural study of mitochondria in type IIb muscle fibers. J. Vet. Med. Sci. 2021;83:1764–1771. doi: 10.1292/jvms.21-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Huang D., Jiang Y., Hou J., Tian M., Li J., Sun L., Zhang Y., Zhang T., Li Z., Li Z., Tong S., Ma Y. Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.647559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu L., Wang J., Cui H., Zhao G., Wen J. FOSL2 is involved in the regulation of glycogen content in chicken breast muscle tissue. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.682441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Baine J.M., Yan T., Wang S. Comprehensive analysis of lysine lactylation in rice (Oryza sativa) grains. J. Agric. Food Chem. 2021;69:8287–8297. doi: 10.1021/acs.jafc.1c00760. [DOI] [PubMed] [Google Scholar]
- Rabbani N., Xue M., Thornalley P.J. Hexokinase-2-linked glycolytic overload and unscheduled glycolysis-driver of insulin resistance and development of vascular complications of diabetes. Int. J. Mol. Sci. 2022;23:2165. doi: 10.3390/ijms23042165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A.B., Wilkins L.J., O'Callaghan M., Phillips A.J. Effect of electrical stun/kill method, interval between killing and neck cutting and blood vessels cut on blood loss and meat quality in broilers. Br. Poult. Sci. 2001;42:51–56. [PubMed] [Google Scholar]
- Rajala R.V.S. Aerobic glycolysis in the retina: Functional roles of pyruvate kinase isoforms. Front. Cell. Dev. Biol. 2020;8:266. doi: 10.3389/fcell.2020.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H., Terry A.R., Chronis C., Hay N. Hexokinase 2-mediated gene expression via histone lactylation is required for hepatic stellate cell activation and liver fibrosis. Cell. Metab. 2023;35:1406–1423. doi: 10.1016/j.cmet.2023.06.013. e1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruixia L., Wei L., Wang Y., Wu F. AMP-activated protein kinase mediates glycolysis in post-mortem breast muscle of broilers. Ital. J. Anim. Sci. 2022;21:1067–1073. [Google Scholar]
- Sun X.B., Huang J.C., Li T.T., Ang Y., Xu X.L., Huang M. Effects of preslaughter shackling on postmortem glycolysis, meat quality, changes of water distribution, and protein structures of broiler breast meat. Poult. Sci. 2019;98:4212–4220. doi: 10.3382/ps/pez175. [DOI] [PubMed] [Google Scholar]
- Sun X., Peng Y., Zhao J., Xie Z., Lei X., Tang G. Discovery and development of tumor glycolysis rate-limiting enzyme inhibitors. Bioorg. Chem. 2021;112 doi: 10.1016/j.bioorg.2021.104891. [DOI] [PubMed] [Google Scholar]
- Wang C., Matarneh S.K., Gerrard D., Tan J. Contributions of energy pathways to ATP production and pH variations in postmortem muscles. Meat Sci. 2022;189 doi: 10.1016/j.meatsci.2022.108828. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang P., Yu T., Gao M., Liu D., Zhang J., Lu C., Chen X., Zhang X., Liu Y. Lactylation of PKM2 suppresses inflammatory metabolic adaptation in pro-inflammatory macrophages. Int. J. Biol. Sci. 2022;18:6210–6225. doi: 10.7150/ijbs.75434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Ye Z., Li Z., Jing D.S., Fan G.X., Liu M.Q., Zhuo Q.F., Ji S.R., Yu X.J., Xu X.W., Qin Y. Lactate-induced protein lactylation: A bridge between epigenetics and metabolic reprogramming in cancer. Cell. Prolif. 2023;56:e13478. doi: 10.1111/cpr.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Wang Z., Wang Q., Li X., Guo Y. Ubiquitous protein lactylation in health and diseases. Cell. Mol. Biol. Lett. 2024;29:23. doi: 10.1186/s11658-024-00541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener G., Krause U. Different modes of activating phosphofructokinase, a key regulatory enzyme of glycolysis, in working vertebrate muscle. Biochem. Soc. Trans. 2002;30:264–270. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Wojtysiak D., Połtowicz K., Karasiński J. Relationship between post mortem desmin degradation and meat quality of poultry breast muscle. Med. Weter. 2008;64:1003–1006. [Google Scholar]
- Wu T., Liu P., Wu J., Jiang Y., Zhou N., Zhang Y., Xu Q., Zhang Y. Broiler spaghetti meat abnormalities: Muscle characteristics and metabolomic profiles. Animals (Basel) 2024;14:1236. doi: 10.3390/ani14081236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T., Li Y.H., Li M., Jiang N.N., Xu X.L., Zhou G.H. Influence of transport conditions and pre-slaughter water shower spray during summer on protein characteristics and water distribution of broiler breast meat. Anim. Sci. J. 2016;87:1413–1420. doi: 10.1111/asj.12593. [DOI] [PubMed] [Google Scholar]
- Xing T., Xu X., Jiang N., Deng S. Effect of transportation and pre-slaughter water shower spray with resting on AMP-activated protein kinase, glycolysis and meat quality of broilers during summer. Anim. Sci. J. 2016;87:299–307. doi: 10.1111/asj.12426. [DOI] [PubMed] [Google Scholar]
- Xing T., Zhao X., Zhang L., Li J.L., Zhou G.H., Xu X.L., Gao F. Characteristics and incidence of broiler chicken wooden breast meat under commercial conditions in China. Poult Sci. 2020;99:620–628. doi: 10.3382/ps/pez560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Tang Z., Huang H., Zhou G., Cui C., Weng Y., Liu W., Kim S., Lee S., Perez-Neut M., Ding J., Czyz D., Hu R., Ye Z., He M., Zheng Y.G., Shuman H.A., Dai L., Ren B., Roeder R.G., Becker L., Zhao Y. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Li J.L., Wang X.F., Zhu X.D., Gao F., Zhou G.H. Attenuating effects of guanidinoacetic acid on preslaughter transport-induced muscle energy expenditure and rapid glycolysis of broilers. Poult. Sci. 2019;98:3223–3232. doi: 10.3382/ps/pez052. [DOI] [PubMed] [Google Scholar]
- Zhang N., Jiang N., Yu L., Guan T., Sang X., Feng Y., Chen R., Chen Q. Protein lactylation critically regulates energy metabolism in the protozoan parasite Trypanosoma brucei. Front. Cell. Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.719720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhai W., Li S., Suman S.P., Chen J., Zhu H., Antonelo D.S., Schilling M.W. Early postmortem proteome changes in normal and woody broiler breast muscles. J. Agric. Food Chem. 2020;68:11000–11010. doi: 10.1021/acs.jafc.0c03200. [DOI] [PubMed] [Google Scholar]
- Zhao X., Xing T., Chen X., Han M.Y., Li X., Xu X.L., Zhou G.H. Precipitation and ultimate pH effect on chemical and gelation properties of protein prepared by isoelectric solubilization/precipitation process from pale, soft, exudative (PSE)-like chicken breast meat1. Poult. Sci. 2017;96:1504–1512. doi: 10.3382/ps/pew412. [DOI] [PubMed] [Google Scholar]
- Zhao W., Yu H., Liu X., Wang T., Yao Y., Zhou Q., Zheng X., Tan F. Systematic identification of the lysine lactylation in the protozoan parasite Toxoplasma gondii. Parasit. Vect. 2022;15:180. doi: 10.1186/s13071-022-05315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Li J., Shi Q., Shan H., Liu L., Geng T., Yu L., Gong D. The effects of in ovo feeding of selenized glucose on selenium concentration and antioxidant capacity of breast muscle in neonatal broilers. Biol. Trace Elem. Res. 2023;201:5764–5773. doi: 10.1007/s12011-023-03611-5. [DOI] [PubMed] [Google Scholar]