Abstract
DNA vaccinations are able to induce strong cellular immune responses in mice and confer protection against infectious agents. However, DNA vaccination of large animals appears to be less effective and requires repeated injections of large amounts of plasmid DNA. Enhancement of the efficiency of DNA vaccines may be achieved by coapplication of cytokine-expressing plasmids. Here we investigated, with woodchucks, whether coadministration of an expression plasmid for woodchuck gamma interferon (IFN-γ), pWIFN-γ, can improve DNA vaccination with woodchuck hepatitis virus core antigen (WHcAg). Animals were immunized with pWHcIm (a plasmid expressing WHcAg) alone or with a combination of pWHcIm and pWIFN-γ using a gene gun. Six weeks postimmunization, all animals were challenged with 105 genome equivalents of woodchuck hepatitis virus (WHV). The antibody and lymphoproliferative immune responses to WHV proteins were determined after immunization and after challenge. Vaccination with pWHcIm and pWIFN-γ led to a pronounced lymphoproliferative response to WHcAg and protected woodchucks against subsequent virus challenge. Two of three animals vaccinated with pWHcIm alone did not show a detectable lymphoproliferative response to WHcAg. A low-level WHV infection occurred in these woodchucks after challenge, as WHV DNA was detectable in the serum by PCR. None of the pWHcIm-vaccinated animals showed an anti-WHcAg antibody response after DNA vaccination or an anamnestic response after virus challenge. Our results indicate that coadministration of the WIFN-γ gene with pWHcIm enhanced the specific cellular immune response and improved the protective efficacy of WHV-specific DNA vaccines.
Plasmid DNA vaccines are novel and powerful tools to induce humoral and cellular immune responses which are protective against bacterial and viral infections (25, 33; reviewed in reference 8). Altering the route of delivery and coapplication of stimulatory molecules can be used to improve DNA vaccines. DNA vaccines are commonly delivered by either intramuscular injection or intradermal application using a gene gun. The gene gun-mediated propulsion of DNA-coated gold particles into the dermis is an attractive mode of application, since only small amounts of plasmid DNA are needed for vaccination (13, 20, 26, 30, 31). The presence of large numbers of antigen-presenting Langerhans cells makes the skin a major immunological inductive site and may explain the high efficacy of gene gun vaccination (19, 29). The coapplication of plasmids expressing cytokines is an approach to modulate immune response to DNA vaccines (3, 4, 11, 14, 15). It has been demonstrated that gamma interferon (IFN-γ) plasmids support Th1 responses and suppress Th2 responses. Other biological effects of IFN-γ include the induction of major histocompatibility complex class I and II expression on cellular surfaces and hence an enhancement of antigen presentation. IFN-γ also converts various cell types into nonprofessional antigen-presenting cells and triggers the differentiation, maturation, and activation of resting macrophages (reviewed in reference 9). Furthermore, IFN-γ supports tumor necrosis factor alpha effects in a synergistic way. Consequently, it has been shown that the coinjection of IFN-γ and interleukin-12 expression vectors significantly enhances the cellular immune response in mice (15).
To evaluate DNA vaccines against hepatitis B, a number of immunogenicity studies and protection studies with different hepatitis B virus proteins have been carried out (1, 2, 7, 17, 18, 34, 35). Immunizations with plasmids expressing hepatitis B virus surface antigen (HBsAg) and hepatitis B virus core antigen (HBcAg) have been shown to induce high antibody titers and substantial T-cell responses in mice (1, 2). Protection from hepadnavirus infection by intramuscular DNA immunization has been demonstrated with ducks and woodchucks (22, 32). Antibody titers known to be protective in humans have also been induced by DNA vaccination of chimpanzees using a plasmid expressing HBsAg (6).
In previous studies, it has been demonstrated that an immune response against the woodchuck hepatitis virus (WHV) core antigen (WHcAg) primed by DNA vaccination effectively protected woodchucks against subsequent challenge with WHV (22). As the core protein inside the intact viral particle is covered by the surface antigen and therefore is not accessible to neutralizing antibodies, the cellular immune response may have played the major role in this protection. DNA vaccinations appeared to be less effective in large animals than in mice. Immunizations of woodchucks with a plasmid expressing WHcAg (pWHcIm) induced only a low level of WHcAg-specific lymphoproliferative and antibody responses. Therefore, we investigated whether coapplication of the recently characterized woodchuck IFN-γ (21) can improve the efficacy of pWHcIm-based DNA vaccination. We demonstrated that gene gun immunization using WHcAg in combination with woodchuck IFN-γ is sufficient to induce a lymphoproliferative immune response and to suppress viral replication after challenge with WHV.
MATERIALS AND METHODS
Woodchucks.
Adult WHV-negative woodchucks trapped in the state of New York were purchased from North Eastern Wildlife (Ithaca, N.Y.). Previous exposure to WHV of these woodchucks was excluded by testing for anti-WHc, anti-WHs, and WHsAg. At the beginning of the study, the woodchucks were between 12 and 18 months old.
Construction, purification, and expression of plasmids pWHcIm and pWIFNγ.
Plasmids pWHcIm and pWIFNγ were constructed as described earlier (21, 22). Briefly, the core gene of WHV8 was amplified by PCR, and the PCR products were cloned into pCRII (Invitrogen, San Diego, Calif.) according to the manufacturer's instructions. The sequenced PCR fragment containing the WHV core gene was isolated by digestion with EcoRI and inserted into the EcoRI site of pcDNA3 (Invitrogen). The integrity of the clones was verified by sequencing.
Plasmids pWHcIm and pWIFNγ were prepared with a Giga plasmid purification kit (Qiagen, Hilden, Germany). Plasmids were dissolved in phosphate-buffered saline at a concentration of 1 mg/ml. The amount of bacterial protein contaminants in these preparations ranged from 0 to 10 ng/ml, as determined by using micro-BCA protein assay reagent (Pierce; Oud Beijerland, The Netherlands).
Expression was demonstrated as described earlier (22). Briefly, a BHK cell line and a woodchuck liver cell line, WH12/6, were used for transfection experiments. Transfection of cells was performed using lipofectamine (Gibco BRL, Eggenstein-Leopoldshafen, Germany). Four micrograms of plasmid was incubated with 10 μg of lipofectamine in 100 μl of media for 45 min and was incubated with cells in 1 ml of Opti-Media (Gibco BRL) for 6 h at 37°C, 5% CO2. pWHcIm-transfected cells were maintained for 48 h at 37°C in 5% CO2 and fixed with acetone and methanol (1:1). The expressed WHcAg was detected by indirect immunofluorescence staining using rabbit antisera. The expression of woodchuck IFN-γ was shown in a bioassay.
Virus protection assay.
A virus protection assay was carried out to measure the amounts of IFN-γ. Briefly, mouse L929 or woodchuck WH12/6 cells were seeded into 96-well microtiter plates and cultured in 100 μl of F12 medium supplemented with 10% fetal calf serum at 37°C in 5% CO2 until 100% confluent. After the culture medium was discarded, 100 μl of F12 medium containing appropriate dilutions of samples was added to cells for an additional incubation of 24 h. Afterwards, mouse encephalomyocarditis virus was added to cells and incubated an additional 24 h. Cells were stained and fixed with 0.1% crystal violet in 20% ethanol. One unit of IFN-γ was defined by its ability to protect 50% of cells per well.
Immunization of woodchucks by gene gun.
Gene gun immunizations were delivered to the shaved groin regions of ketamine-xylazine hydrochloride (Rompun)-anesthetized woodchucks using a Helios Gene Gun (Bio-Rad, Hercules, Calif.). Immunizations were performed as recommended by the manufacturer. DNA was precipitated onto 1-μm gold beads (Bio-Rad) at room temperature. Twenty-five milligrams of gold microcarriers was measured into a 1.5-ml microcentrifuge tube. One hundred microliters of 0.05 M spermidine (Sigma, St. Louis, Mo.) was added, and the mixture was vortexed for 20 s and sonicated for 5 s. Fifty micrograms of pWHcIm (or 50 μg of pWHcIm and 50 μg of pWIFN-γ) was added in a maximum volume of 100 μl. The mixture was again vortexed for 20 s. While the mixture was being vortexed at a moderate rate on a variable-speed vortexer, 100 μl of 1 M CaCl2 was added drop by drop. The DNA was allowed to precipitate at room temperature for 10 min. The supernatant was removed, and the gold microcarriers were washed three times with fresh 100% ethanol. After the last wash, 3 ml of ethanol containing 0.05 mg of polyvinylpyrrolidone (Bio-Rad) was added to the mixture. The gold microcarriers were then used to coat the inner wall of Tefzel tubing (Bio-Rad) according to the manufacturer's protocol. Cartridges were loaded into the gene gun. Ten cartridges were discharged per animal (five shots with 300 lb/in2 and five shots with 400 lb/in2), delivering a total of 10 μg of pWHcIm (or 10 μg of pWHcIm and 10 μg of pWIFN-γ).
WHV challenge and statistical analysis.
Six weeks after vaccination, woodchucks were challenged intravenously with an inoculum containing 105 WHV genome equivalents. Serum derived from a chronic WHV carrier was used as a source of WHV. This serum contained 107 WHV genome equivalents per ml. The serum was sterile filtered, and aliquots were stored at −80°C. In previous experiments, similar doses of this stock have been used to challenge 14 woodchucks (22; our unpublished observations). All animals became viremic after challenge. These animals were included in the statistical analysis. The significance for the 2 × 2 table was calculated using a formula for small sample sizes: 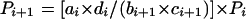 (10).
(10).
Serology and detection of WHV DNA.
Anti-WHc, anti-WHs, and WHsAg were detected by enzyme-linked immunosorbent assay (ELISA) as described previously (27, 28) The sensitivity of ELISA was determined in tests of serially diluted positive sera of woodchucks experimentally infected with WHV. The ELISA for anti-WHc was able to detect anti-WHc in woodchuck sera at dilutions of 10−3 to 10−6. The dot blot technique was routinely performed to detect WHV DNA in woodchuck sera. For PCR detection of WHV DNA in woodchuck sera, nucleic acids were isolated from sera by proteinase K digestion and phenol extraction. PCR for amplification of the WHV core gene was performed with primers wc1 (nucleotides nt 2015 to 2038, 5′-TGGGGCCATGGACATAGATCCTTA-3′) and fwc3a (nt 2537 to 2557, 5′-TCTGCGACGCGGTGATTGAGA-3′). By testing serial dilutions of a cloned WHV core fragment, 10 copies of specific templates were sufficient to give a positive result in the PCR. A virus DNA titer of 500 copies per ml of serum could be detected.
Measurement of WHV antigen-specific proliferation of woodchuck PBMCs.
Antigen-specific proliferation of woodchuck peripheral blood mononuclear cells (PBMCs) was determined by [2-3H]adenine assay as described previously (16). Briefly, woodchuck PBMCs were separated by Ficoll-Paque (Pharmacia, Freiburg, Germany) density gradient centrifugation and suspended in 0.9% NaCl. Triplicates of 5 × 104 PBMCs were cultured in flat-bottom 96-well microtiter plates (Falcon; Becton Dickinson, Paramus, N.J.) at 37°C in a humidified atmosphere containing 5% CO2. Two hundred microliters of AIM-V medium (Gibco BRL) supplemented with 2% 0.2 M l-glutamine (Sigma), 1% 0.125 M gentamicin sulfate (Sigma), and 10% fetal calf serum (Gibco BRL) was added to each well. PBMC proliferation in response to WHcAg or peptides was measured at an antigen concentration of 1 μg/ml. After a 5-day incubation, cells were labeled with 1 μCi of [2-3H]adenine (Amersham, Braunschweig, Germany) for 20 h and collected by a cell harvester (Skatron). Two panels, A and B, of WHcAg-derived peptides were used (see Table 2). Panel A consists of 16 overlapping 20-mer peptides and covers the complete WHcAg. Panel B, consisting of 6 additional peptides, covers the immunodominant region of the WHcAg, amino acids (aa) 97 to 140.
TABLE 2.
WHcAg-derived peptides for proliferation assay
| Peptide no. | Amino acid no. | Sequence |
|---|---|---|
| Panel A | ||
| 1 | 1–20 | MDIDPYKEFGSSYQLLNFLP |
| 2 | 15–34 | LLNFLPLDFFPDLNALVDTA |
| 3 | 28–47 | NALVDTATALYEEELTGREH |
| 4 | 38–57 | YEEELTGREHCSPHHTAIRQ |
| 5 | 50–69 | PHHTAIRQALVCWDELTKLI |
| 6 | 61–80 | CWDELTKLIAWMSSNITSEQ |
| 7 | 70–89 | AWMSSNITSEQVRTIIVNHV |
| 8 | 82–101 | RTIIVNHVNDTWGLKVRQSL |
| 9 | 90–109 | NDTWGLKVRQSLWFHLSCLT |
| 10 | 100–119 | SLWFHLSCLTFGQHTVQEFL |
| 11 | 112–131 | QHTVQEFLVSFGVWIRTPAP |
| 12 | 120–139 | VSFGVWIRTPAPYRPPNAPI |
| 13 | 136–155 | NAPILSTLPEHTVIRRRGGA |
| 14 | 146–165 | HTVIRRRGGARASRSPRRRT |
| 15 | 156–175 | RASRSPRRRTPSPRRRRSQS |
| 16 | 169–188 | RRRRSQSPRRRRSQSPSANC |
| Panel B | ||
| 17 | 97–110 | VRQSLWFHLSCLTF |
| 18 | 100–113 | SLWFHLSCLTFGQH |
| 19 | 111–124 | GQHTVQEFLVSFGV |
| 20 | 120–131 | VSFGVWIRTPAP |
| 21 | 124–137 | VWIRTPAPYRPPNA |
| 22 | 129–140 | PAPYRPPNAPIL |
Results for triplicate cultures are presented as a mean stimulation index (SI, mean total absorption for stimulated PBMCs divided by the mean total absorption for control). The standard deviation of the means was less than 30% of the mean (range, 15 to 50%). An SI of ≥2 was considered significant, to distinguish the specific stimulation and possible variation within an assay, as described previously (24).
RESULTS
Immune response succeeding a single-shot DNA vaccination.
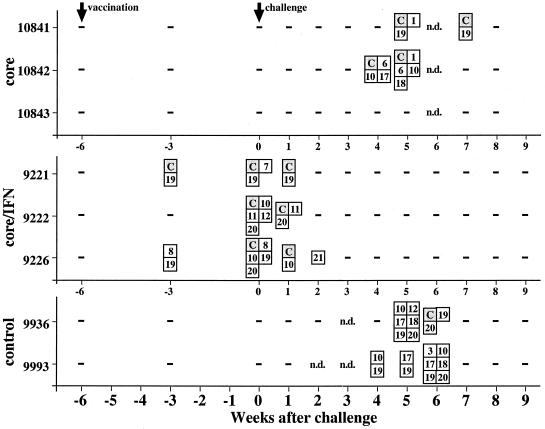
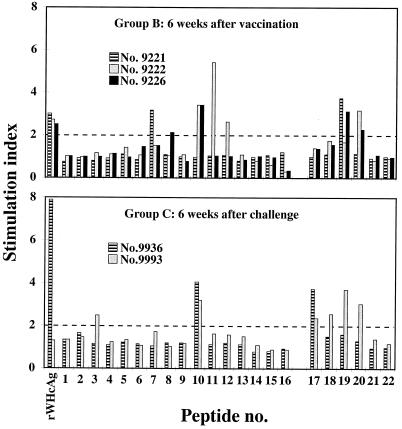
To test whether a single-shot DNA vaccination induced an efficient immune response, three woodchucks (group A) were immunized with 10 μg of an expression vector for WHcAg (pWHcIm) using the gene gun (Table 1). To evaluate the augmentation of immune response by coadministration of an expression vector for IFN-γ, three woodchucks (group B) were immunized with 10 μg of pWHcIm and 10 μg of pWIFN-γ. Sera were tested for anti-WHcAg and for proliferative responses of PBMCs to WHcAg and WHcAg-derived peptides (Table 2) after vaccination. Woodchucks of group A immunized with pWHcIm developed neither anti-WHcAg (Fig. 1A) nor a proliferative response to WHcAg or core peptides (Fig. 2). Likewise, no antibody response to WHcAg was induced by immunization of animals in group B with pWHcIm in combination with pWIFN-γ (Fig. 1B). However, for all animals in group B, a proliferative response to core and core peptides was detected at week 3 after immunization (Fig. 2). At this time point, PBMCs derived from animals 9221 and 9226 proliferated in response to peptide 19 (aa 111 to 124), located in the middle of WHcAg. Additionally, animal 9226 showed a proliferation in response to peptide 8, and animal 9221 responded to WHcAg. Six weeks after immunization, a multispecific response against WHcAg and WHcAg-derived peptides was detected in all animals in group B. PBMCs obtained from the control animals vaccinated with the empty plasmid pcDNA3 did not show a proliferative response to WHcAg or core peptides after immunization.
TABLE 1.
DNA vaccination of woodchucks with plasmids pWHcIm and pWIFN-γ and control plasmid pcDNA3
| Group | Vaccine construct(s) | Dose(s)a (μg) | No. of animals |
|---|---|---|---|
| A | pWHcIm | 10 | 3 |
| B | pWHcIm and pWIFN-γ | 10, 10b | 3 |
| C | pcDNA3 | 10 | 2 |
Animals were vaccinated once using the gene gun.
Animals received 10 μg of each plasmid.
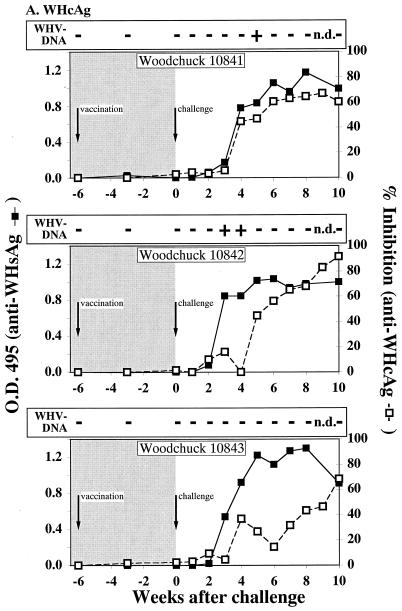
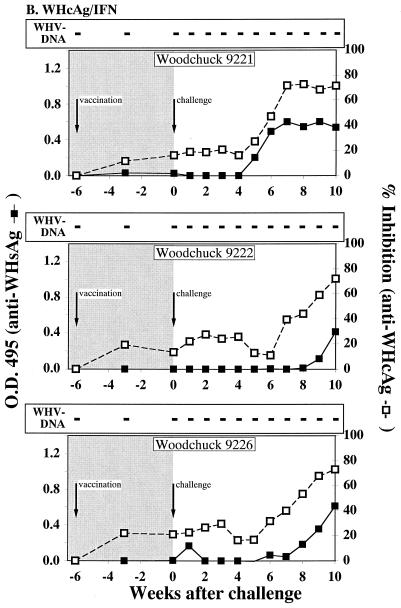
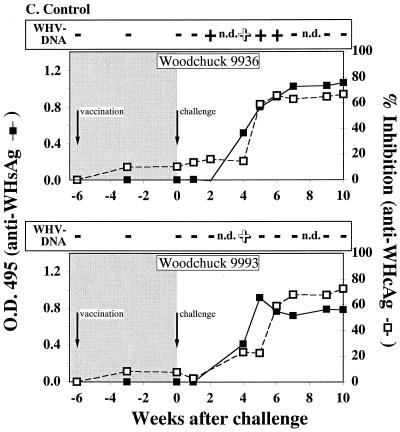
FIG. 1.
Serological profiles of woodchucks after immunization and WHV challenge. (A) Woodchucks immunized with pWHcIm. (B) Woodchucks immunized with pWHcIm and pWIFN-γ. (C) Woodchucks immunized with the control plasmid pcDNA3. The woodchucks were immunized at week −6 and challenged at week 0, as indicated. Antibody reactivity of woodchuck sera to WHsAg (open square) or WHcAg (black square) is shown. The presence of WHV DNA is indicated by a black plus sign for PCR positive and by a white plus sign for PCR and dot blot hybridization positive. Dot blot hybridization was carried out using the titration of a standard (1010 to 106 copies/ml) and comparing the signals to the experimental signals. All detected signals were in the 106 range except for those of animal 9936 in week 4 (107 copies/ml) and animal 9993 in week 4 (107 copies/ml).
FIG. 2.
Lymphoproliferative responses to WHcAg and WHcAg-derived peptides during the immunization study. Positive results (SI > 3) are indicated by boxes. Numbers stand for peptides as shown in Table 2. C, WHcAg.
Magnitude of the proliferative immune response after immunization.
The protective efficacy of DNA vaccination may be correlated to the intensity of the lymphoproliferative response. Therefore, the magnitude of the proliferative immune response was analyzed after vaccination. The proliferative immune response induced by coimmunization with core and IFN-γ 6 weeks after vaccination was compared to the proliferative response detected in the control animals 6 weeks after WHV infection (Fig. 3). The SI of PBMCs derived from animals of group B ranged from 3 to 5. The strongest proliferation (SI > 5) was detected in PBMCs of animal 9222 in response to peptide 11. A proliferative response of the same magnitude was demonstrated with PBMCs derived from the control animals 6 weeks after WHV inoculation.
FIG. 3.
Magnitude of T-cell proliferation induced by the combination vaccination. Lymphocytes derived from vaccinated and challenged woodchucks were stimulated with WHcAg and WHcAg-derived peptides. Results for PBMCs (triplicates) are presented as mean SI.
Challenge of vaccinated animals with WHV.
Woodchucks of all groups were challenged with 105 genome equivalents of WHV 6 weeks after the single-shot immunization, and sera were analyzed for WHV DNA by dot blot hybridization as well as by PCR and for antibodies against WHcAg. WHV DNA was detected in the sera from two animals of group A (10841, 10842), while the third animal (10843) stayed negative for viral DNA throughout the follow-up (Fig. 1A). Animal 10841 was PCR positive at week 5 after challenge and animal 10842 was positive at weeks 3 and 4 after challenge. However, WHV DNA could not be detected by dot blot hybridization in the sera of these animals, indicating that the number of WHV genomes stayed below the detection limit of 106 genomes per ml. The observed protection of one of three animals without detectable WHV DNA was not significant (P = 0.2). In contrast to animals in group A, all animals in group B immunized with pWHcIm in combination with pWIFN-γ remained negative for WHV throughout the observation period (Fig. 1B). Neither by dot blot hybridization nor by PCR could WHV DNA be detected. Animals in this group were significantly protected (P = 0.0015) from viremia. As expected, the two control animals of group C, immunized with the empty pcDNA3 vector, were viremic after inoculation of infectious WHV (Fig. 1C). WHV DNA was repeatedly detected by PCR in the serum of animal 9936 between weeks 2 and 6 after infection, while animal 9993 was PCR positive only in week 4. In both animals, WHV DNA was detected by dot blot hybridization 4 weeks after challenge with a titer of 107 genomes per ml of serum.
Humoral immune response after challenge.
Antibodies against WHsAg and WHcAg were determined following the inoculation of WHV. All animals of group A developed antibodies against WHcAg after challenge (Fig. 1A). Additionally, antibodies against WHsAg were detected at weeks 3 and 4 after challenge in the sera of animals 10841 and 10842. Although no WHV DNA could be detected in the serum of woodchuck 10843 3 weeks after challenge, the animal seroconverted to anti-WHsAg. Even though a proliferative response to WHcAg and WHcAg-derived peptides was detected after immunization with pWHcIm and pWIFN-γ, no anamnestic humoral immune response to WHcAg was observed in animals of group B seroconverting (≥50% inhibition) between weeks 6 and 8 after challenge (Fig. 1B). Furthermore, all animals, including the WHV DNA-negative animals, seroconverted to anti-HBsAg, indicating a low-level infection of the liver. Woodchucks 9222 and 9226 of group B displayed a delayed seroconversion to anti-WHsAg (weeks 9 and 10 after challenge) and to anti-WHcAg (weeks 8 and 9 after challenge) when compared to control animals. The two control animals, immunized with the empty pcDNA3 vector, seroconverted to anti-WHcAg and anti-WHsAg between 4 and 6 weeks after challenge, respectively (Fig. 1C).
Lymphoproliferative response after challenge.
The cellular immune response to WHcAg and WHcAg-derived peptides was determined by proliferation assay after challenge. Two animals of group A immunized with pWHcIm developed a proliferative response to WHcAg and various core peptides after challenge (Fig. 2). Simultaneous to the detection of WHV DNA in the serum of woodchuck 10841 in week 4 after infection, PBMCs derived from this animal showed an initial proliferative response. The proliferative response was directed against WHcAg, peptide 19, located within the middle part of the core protein, and peptide 1, located at the amino terminus. PBMCs derived from woodchuck 10842 showed a multispecific proliferative response to WHcAg, to peptide 6, and to peptides covering aa 97 to 137 of the core protein in weeks 4 and 5 after challenge (Fig. 2). The third woodchuck of group A (10843) did not show a proliferative response to WHcAg or core peptides. The proliferative responses of animals 10841 and 10842 of group A did not show major differences in time of occurrence and peptides recognized from the proliferative responses of the control animals. The proliferative response to WHcAg or core peptides of PBMCs derived from animals of group B seen at week 3 after vaccination was present up to weeks 1 and 2 after challenge. However, they did not show a proliferative response 4 and 5 weeks after challenge, whereas at that time the two control animals developed a proliferative response.
DISCUSSION
In this study, we have demonstrated that a combined DNA vaccination of plasmids expressing WHcAg and IFN-γ using the gene gun is sufficient to induce an immune response and to control WHV replication in woodchucks after challenge.
The lymphoproliferative responses to WHcAg in vaccinated woodchucks are closely associated with effective control of WHV infection. Menne et al. demonstrated that priming T-cell response to a single epitope within WHcAg by peptide immunization was sufficient to control WHV infection (24). However, the protective efficacy of DNA vaccination may be linked to the potency of the lymphoproliferative response. Therefore, the magnitude of the proliferative response was analyzed after vaccination. The lymphoproliferative response after combined DNA vaccination with core antigen and IFN-γ was as strong as the proliferative response induced by viral replication following experimental WHV infection. These results are in accordance with previously published experiments showing that the coadministration of IFN-γ leads to an enhanced proliferative response and a reduced antibody response to HBsAg in mice (3). In this study, PBMCs derived from three animals immunized with core antigen and IFN-γ showed strong proliferation in response to WHcAg. All three animals were protected from viremia. These results corroborate the importance of WHcAg-specific lymphoproliferative responses in the control of WHV infection. In woodchucks immunized with pWHcIm alone, viremia occurred but remained low. In woodchuck 10843, WHV DNA was below the PCR detection limit. Thus, vaccination with pWHcIm alone may induce a weak immune response, which was generally insufficient to control WHV infection in woodchucks. We have demonstrated previously that the viremic phase in woodchucks acutely infected with WHV is accompanied by a proliferative response to WHcAg and core-derived peptides (23). Accordingly, all viremic animals of groups A and C showed a proliferative response to WHcAg and different core-derived peptides 4 to 7 weeks after challenge. A proliferative response was also demonstrated in all animals of group B 3 weeks prior to challenge and between 1 and 2 weeks after challenge. This response can be attributed to the previous vaccination. However, animal 10843 was also protected but did not show any measurable lymphoproliferative responses to WHcAg; although it applies to only one animal, this observation may suggest that other cellular immune responses such as cytotoxic T lymphocyte may be involved in the protective mechanism. Nevertheless, these results provide additional evidence for the importance of WHcAg-specific lymphoproliferative responses in WHV infection.
Woodchucks develop high antibody titers against WHcAg during a natural WHV infection, although WHcAg is primarily localized intracellularly (5, 12). However, no anti-WHcAg was detected after DNA vaccination with 10 μg of plasmid, even by using core antigen in combination with IFN-γ. Previously, we had vaccinated woodchucks intramuscularly with different plasmid doses (22). Immunization with 100 μg of plasmid DNA did not induce measurable anti-WHcAg antibody titers. Only after three injections using 1-mg DNA doses was a low, transient antibody titer to WHcAg detected. It appears that DNA vaccination was unable to induce an antibody response to WHcAg in woodchucks. An interesting observation in our experiments is the lack of anamnestic antibody responses against WHcAg after challenge in animals of groups A and B immunized with pWHcIm. We have demonstrated previously that intramuscular vaccination with pWHcIm induces low titers of anti-WHcAg and a slight increase in anti-WHcAg titers after challenge with WHV (22). However, this increase was probably due to the carryover from the high anti-WHcAg antibody titer in the challenge virus stock. In contrast, a strong anamnestic antibody response to WHsAg after challenge was observed with woodchucks previously immunized with a vector expressing WHsAg. A strong anamnestic humoral immune response was also observed after boosting chimpanzees immunized with a single injection of 400 μg of plasmid DNA expressing HBsAg (6). These results indicate that the development of antibodies against WHcAg and WHsAg is controlled by distinct mechanisms. This may be explained by different cellular locations of these antigens, which influence their exposure to B cells.
WHcAg is a nucleoprotein and does not induce neutralizing antibodies against WHV virions. Thus, vaccinations with WHcAg or plasmids expressing WHcAg do not protect hepatocytes from viral infection and do not induce a “sterile” immunity. However, viral replication can be suppressed efficiently at an early stage of infection. Roos et al. demonstrated that the suppression of WHV replication in WHcAg-immunized animals is so effective that the virus cannot be detected in liver biopsies (27). Hence the cell-mediated immunity primed by DNA vaccination apparently controlled the infection and significantly reduced the release of virus particles into the periphery. Because there is a strong correlation between anti-surface antibodies and protection in humans and in woodchucks, the appearance of anti-surface antibodies always indicates a termination of the infection. Even though the presence of anti-surface antibodies and virus may occasionally overlap for a short period, significant viral replication has never been observed in the presence of anti-surface antibodies. Woodchucks immunized with inactivated serum derived from chronic WHV carriers do not develop anti-WHsAg (our unpublished observations). Therefore, the presence of antibodies against WHsAg found in woodchucks coimmunized with plasmids expressing WHcAg and IFN-γ after challenge indicates a low-level replication in hepatocytes after challenge with WHV. Neutralizing antibodies, such as anti-HBsAg antibodies, are important to prevent the spread of released WHV to uninfected hepatocytes, while the cellular immune response system primed by DNA vaccination may down regulate intrahepatic WHV replication.
Taken together, our findings demonstrate that DNA covaccination with WHcAg and IFN-γ expression plasmids induces a protective immune response in woodchucks. Coadministration of IFN-γ enhanced the priming of cellular immune responses substantially and appeared to be essential for the control of WHV replication. These observations therefore have implications for the development of novel DNA vaccines for prophylaxis and therapeutic treatments against HBV infection.
ACKNOWLEDGMENTS
We are grateful to Jörg Reimann and Reinhold Schirmbeck for helpful discussions and advice. We thank Thekla Kemper for excellent technical assistance.
This work is supported by grants of the German Bundesministerium für Bildung und Forschung to M.R. and M.L. (BMBF, 01GE96125) and to M.L. and M.R. (BMBF, 01GE9909).
REFERENCES
- 1.Bohm W, Kuhrober A, Paier T, Mertens T, Reimann J, Schirmbeck R. DNA vector constructs that prime hepatitis B surface antigen-specific cytotoxic T lymphocyte and antibody responses in mice after intramuscular injection. J Immunol Methods. 1996;193:29–40. doi: 10.1016/0022-1759(96)00035-x. [DOI] [PubMed] [Google Scholar]
- 2.Bohm W, Mertens T, Schirmbeck R, Reimann J. Routes of plasmid DNA vaccination that prime murine humoral and cellular immune responses. Vaccine. 1998;16:949–954. doi: 10.1016/s0264-410x(97)00302-2. [DOI] [PubMed] [Google Scholar]
- 3.Chow Y H, Chiang B L, Lee Y L, Chi W K, Lin W C, Chen Y T, Tao M H. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 4.Chow Y H, Huang W L, Chi W K, Chu Y D, Tao M H. Improvement of hepatitis B virus DNA vaccines by plasmids coexpressing hepatitis B surface antigen and interleukin-2. J Virol. 1997;71:169–178. doi: 10.1128/jvi.71.1.169-178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cote P J, Roneker C, Cass K, Schodel F, Peterson D, Tennant B, De Noronha F, Gerin J. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 1993;6:161–169. doi: 10.1089/vim.1993.6.161. [DOI] [PubMed] [Google Scholar]
- 6.Davis H L, McCluskie M J, Gerin J L, Purcell R H. DNA vaccine for hepatitis B: evidence for immunogenicity in chimpanzees and comparison with other vaccines. Proc Natl Acad Sci USA. 1996;93:7213–7218. doi: 10.1073/pnas.93.14.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis H L, Whalen R G. DNA-based immunization. Mol Cell Biol Hum Dis. 1995;5:368–387. doi: 10.1007/978-94-011-0547-7_18. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly J J, Ulmer J B, Shiver J W, Liu M A. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 9.Farrar M A, Schreiber R D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 10.Feldman S, Klinger E. Short cut evaluation of Fisher-Yates “excat test.”. Psychometrika. 1963;28:289–291. [Google Scholar]
- 11.Geissler M, Gesien A, Wands J R. Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. J Immunol. 1997;159:5107–5113. [PubMed] [Google Scholar]
- 12.Guidotti L G, Martinez V, Loh Y T, Rogler C E, Chisari F V. Hepatitis B virus nucleocapsid particles do not cross the hepatocyte nuclear membrane in transgenic mice. J Virol. 1994;68:5469–5475. doi: 10.1128/jvi.68.9.5469-5475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston S A, Tang D C. Gene gun transfection of animal cells and genetic immunization. Methods Cell Biol. 1994;43A:353–365. doi: 10.1016/s0091-679x(08)60612-3. [DOI] [PubMed] [Google Scholar]
- 14.Kim J J, Nottingham L K, Tsai A, Lee D J, Maguire H C, Oh J, Dentchev T, Manson K H, Wyand M S, Agadjanyan M G, Ugen K E, Weiner D B. Antigen-specific humoral and cellular immune responses can be modulated in rhesus macaques through the use of IFN-gamma, IL-12, or IL-18 gene adjuvants. J Med Primatol. 1999;28:214–223. doi: 10.1111/j.1600-0684.1999.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim J J, Simbiri K A, Sin J I, Dang K, Oh J, Dentchev T, Lee D, Nottingham L K, Chalian A A, McCallus D, Ciccarelli R, Agadjanyan M G, Weiner D B. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J Interferon Cytokine Res. 1999;19:77–84. doi: 10.1089/107999099314441. [DOI] [PubMed] [Google Scholar]
- 16.Kreuzfelder E, Menne S, Ferencik S, Roggendorf M, Grosse-Wilde H. Assessment of peripheral blood mononuclear cell proliferation by [2-3H]adenine uptake in the woodchuck model. Clin Immunol Immunopathol. 1996;78:223–227. doi: 10.1006/clin.1996.0033. [DOI] [PubMed] [Google Scholar]
- 17.Kuhrober A, Pudollek H P, Reifenberg K, Chisari F V, Schlicht H J, Reimann J, Schirmbeck R. DNA immunization induces antibody and cytotoxic T cell responses to hepatitis B core antigen in H-2b mice. J Immunol. 1996;156:3687–3695. [PubMed] [Google Scholar]
- 18.Kuhrober A, Wild J, Pudollek H P, Chisari F V, Reimann J. DNA vaccination with plasmids encoding the intracellular (HBcAg) or secreted (HBeAg) form of the core protein of hepatitis B virus primes T cell responses to two overlapping Kb- and Kd-restricted epitopes. Int Immunol. 1997;9:1203–1212. doi: 10.1093/intimm/9.8.1203. [DOI] [PubMed] [Google Scholar]
- 19.Kupper T S. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Investig Dermatol. 1990;94:S146–S150. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- 20.Leitner W W, Seguin M C, Ballou W R, Seitz J P, Schultz A M, Sheehy M J, Lyon J A. Immune responses induced by intramuscular or gene gun injection of protective deoxyribonucleic acid vaccines that express the circumsporozoite protein from Plasmodium berghei malaria parasites. J Immunol. 1997;159:6112–6119. [PubMed] [Google Scholar]
- 21.Lohrengel B, Lu M, Roggendorf M. Molecular cloning of the woodchuck cytokines: TNF-alpha, IFN-gamma, and IL-6. Immunogenetics. 1998;47:332–335. doi: 10.1007/s002510050366. [DOI] [PubMed] [Google Scholar]
- 22.Lu M, Hilken G, Kruppenbacher J, Kemper T, Schirmbeck R, Reimann J, Roggendorf M. Immunization of woodchucks with plasmids expressing woodchuck hepatitis virus (WHV) core antigen and surface antigen suppresses WHV infection. J Virol. 1999;73:281–289. doi: 10.1128/jvi.73.1.281-289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menne S, Maschke J, Lu M, Grosse-Wilde H, Roggendorf M. T-cell response to woodchuck hepatitis virus (WHV) antigens during acute self-limited WHV infection and convalescence and after viral challenge. J Virol. 1998;72:6083–6091. doi: 10.1128/jvi.72.7.6083-6091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menne S, Maschke J, Tolle T K, Lu M, Roggendorf M. Characterization of T-cell response to woodchuck hepatitis virus core protein and protection of woodchucks from infection by immunization with peptides containing a T-cell epitope. J Virol. 1997;71:65–74. doi: 10.1128/jvi.71.1.65-74.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomery D L, Ulmer J B, Donnelly J J, Liu M A. DNA vaccines. Pharmacol Ther. 1997;74:195–205. doi: 10.1016/s0163-7258(97)82003-7. [DOI] [PubMed] [Google Scholar]
- 26.Pertmer T M, Eisenbraun M D, McCabe D, Prayaga S K, Fuller D H, Haynes J R. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13:1427–1430. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 27.Roos S, Fuchs K, Roggendorf M. Protection of woodchucks from infection with woodchuck hepatitis virus by immunization with recombinant core protein. J Gen Virol. 1989;70:2087–2095. doi: 10.1099/0022-1317-70-8-2087. [DOI] [PubMed] [Google Scholar]
- 28.Schodel F, Neckermann G, Peterson D, Fuchs K, Fuller S, Will H, Roggendorf M. Immunization with recombinant woodchuck hepatitis virus nucleocapsid antigen or hepatitis B virus nucleocapsid antigen protects woodchucks from woodchuck hepatitis virus infection. Vaccine. 1993;11:624–628. doi: 10.1016/0264-410x(93)90307-j. [DOI] [PubMed] [Google Scholar]
- 29.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 30.Tang D C, DeVit M, Johnston S A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992;356:152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- 31.Torres C A, Iwasaki A, Barber B H, Robinson H L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J Immunol. 1997;158:4529–4532. [PubMed] [Google Scholar]
- 32.Triyatni M, Jilbert A R, Qiao M, Miller D S, Burrell C J. Protective efficacy of DNA vaccines against duck hepatitis B virus infection. J Virol. 1998;72:84–94. doi: 10.1128/jvi.72.1.84-94.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whalen R G. DNA vaccines for emerging infectious diseases: what if? Emerg Infect Dis. 1996;2:168–175. doi: 10.3201/eid0203.960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whalen R G, Leclerc C, Deriaud E, Schirmbeck R, Reimann J, Davis H L. DNA-mediated immunization to the hepatitis B surface antigen. Activation and entrainment of the immune response. Ann N Y Acad Sci. 1995;772:64–76. doi: 10.1111/j.1749-6632.1995.tb44732.x. [DOI] [PubMed] [Google Scholar]
- 35.Wild J, Gruner B, Metzger K, Kuhrober A, Pudollek H P, Hauser H, Schirmbeck R, Reimann J. Polyvalent vaccination against hepatitis B surface and core antigen using a dicistronic expression plasmid. Vaccine. 1998;16:353–360. doi: 10.1016/s0264-410x(97)80913-9. [DOI] [PubMed] [Google Scholar]