Abstract
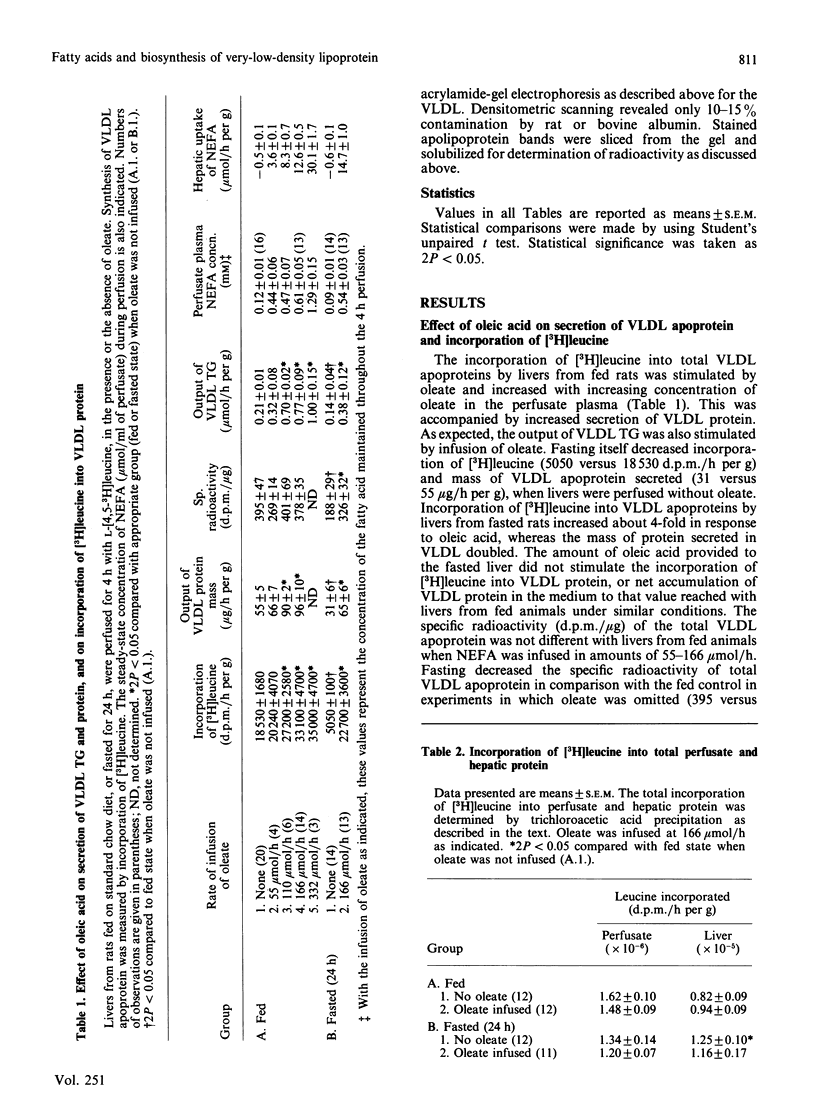
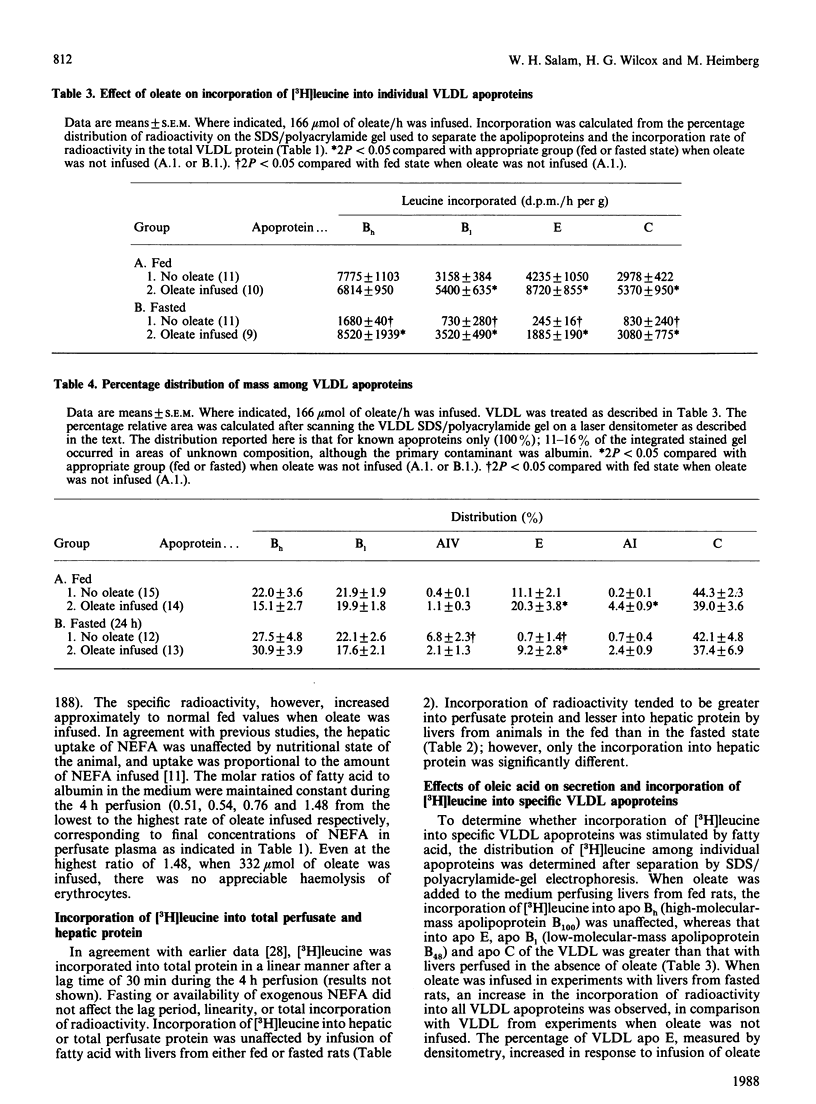
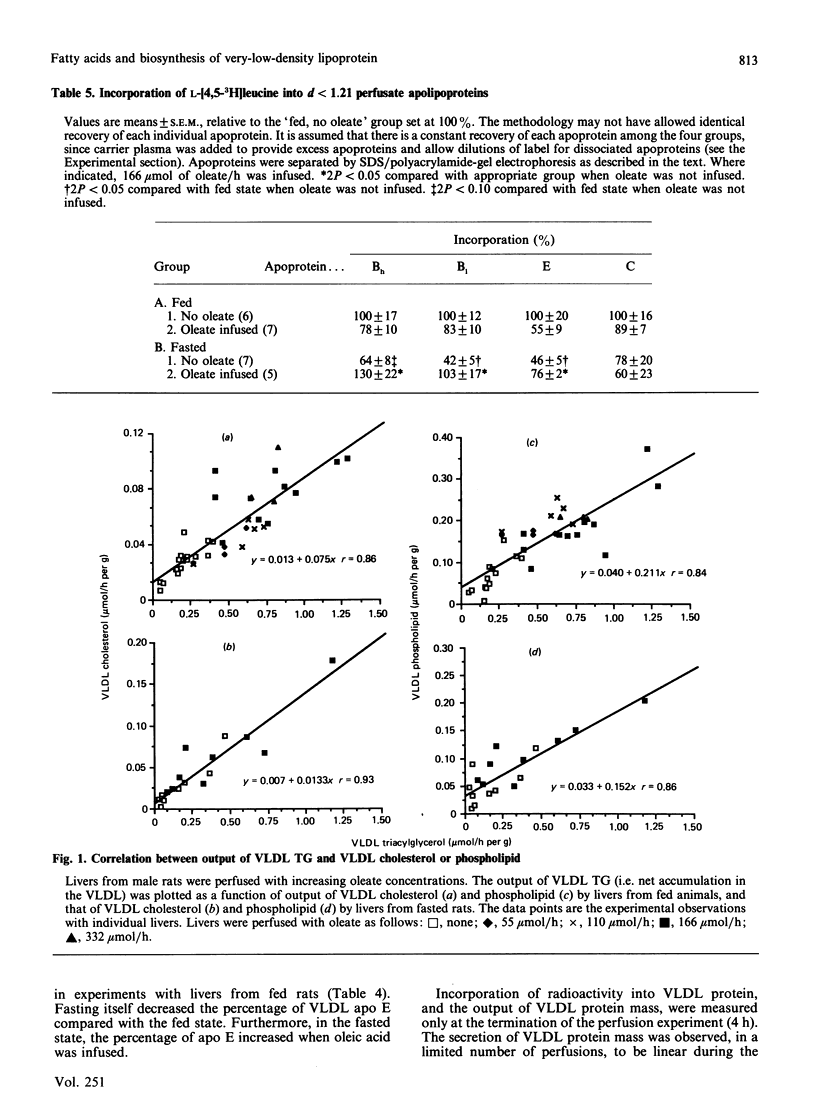
The effects of oleic acid on the biosynthesis and secretion of VLDL (very-low-density-lipoprotein) apoproteins and lipids were investigated in isolated perfused rat liver. Protein synthesis was measured by the incorporation of L-[4,5-3H]leucine into the VLDL apoproteins (d less than 1.006) and into apolipoproteins of the whole perfusate (d less than 1.21). Oleate did not affect incorporation of [3H]leucine into total-perfusate or hepatic protein. The infusion of oleate, however, increased the mass and radioactivity of the VLDL apoprotein in proportion to the concentration of oleate infused. Uptake of oleate was similar with livers from fed or fasted animals. Fasting itself (24 h) decreased the net secretion and incorporation of [3H]leucine into total VLDL apoprotein and decreased the output of VLDL protein by the liver. A linear relationship existed between the output of VLDL triacylglycerol (mumol/h per g of liver) and secretion and/or synthesis of VLDL protein. Net output of VLDL cholesterol and phospholipid also increased linearly with VLDL-triacylglycerol output. Oleate stimulated incorporation of [3H]leucine into VLDL apo (apolipoprotein) E and apo C by livers from fed animals, and into VLDL apo Bh, B1, E and C by livers from fasted rats. The incorporation of [3H]leucine into individual apolipoproteins of the total perfusate lipoprotein (d less than 1.210 ultracentrifugal fraction) was not changed significantly by oleate during perfusion of livers from fed rats, suggesting that the synthesis de novo of each apolipoprotein was not stimulated by oleate. This is in contrast with that observed with livers from fasted rats, in which the synthesis of the total-perfusate lipoprotein (d less than 1.210 fraction) apo B, E and C was apparently stimulated by oleate. The observations with livers from fed rats suggest redistribution of radioactive apolipoproteins to the VLDL during or after the process of secretion, rather than an increase of apoprotein synthesis de novo. It appears, however, that the biosynthesis of apo B1, Bh, E and C was stimulated by oleic acid in livers from fasted rats. Since the incorporations of [3H]leucine into the VLDL and total-perfusate apolipoproteins were increased in fasted-rat liver when the fatty acid was infused, part of the apparent stimulated synthesis of the VLDL apoprotein may be in response to the increased formation and secretion of VLDL lipid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell-Quint J., Forte T., Graham P. Synthesis of two forms of apolipoprotein B by cultured rat hepatocytes. Biochem Biophys Res Commun. 1981 Mar 31;99(2):700–706. doi: 10.1016/0006-291x(81)91800-3. [DOI] [PubMed] [Google Scholar]
- Berry E. M., Aldini R., Bar-On H., Eisenberg S. Role of the liver in the degradation of very low density lipoproteins: a study of lipolysis by heparin releasable liver lipase and uptake during isolated rat liver perfusion. Eur J Clin Invest. 1981 Jun;11(3):151–159. doi: 10.1111/j.1365-2362.1981.tb01834.x. [DOI] [PubMed] [Google Scholar]
- Dashti N., Wolfbauer G. Secretion of lipids, apolipoproteins, and lipoproteins by human hepatoma cell line, HepG2: effects of oleic acid and insulin. J Lipid Res. 1987 Apr;28(4):423–436. [PubMed] [Google Scholar]
- Davis R. A., Boogaerts J. R. Intrahepatic assembly of very low density lipoproteins. Effect of fatty acids on triacylglycerol and apolipoprotein synthesis. J Biol Chem. 1982 Sep 25;257(18):10908–10913. [PubMed] [Google Scholar]
- Dolphin P. J., Forsyth S. J., Krul E. S. Post-secretory acquisition of apolipoprotein E by nascent rat hepatic very-low-density lipoproteins in the absence of cholesteryl ester transfer. Biochim Biophys Acta. 1986 Jan 3;875(1):21–30. doi: 10.1016/0005-2760(86)90006-8. [DOI] [PubMed] [Google Scholar]
- Dolphin P. J. Lipoprotein metabolism and the role of apolipoproteins as metabolic programmers. Can J Biochem Cell Biol. 1985 Aug;63(8):850–869. doi: 10.1139/o85-107. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fainaru M., Felker T. E., Hamilton R. L., Havel R. J. Evidence that a separate particle containing B-apoprotein is present in high-density lipoproteins from perfused rat liver. Metabolism. 1977 Sep;26(9):999–1004. doi: 10.1016/0026-0495(77)90017-8. [DOI] [PubMed] [Google Scholar]
- Feldhoff R. C., Taylor J. M., Jefferson L. S. Synthesis and secretion of rat albumin in vivo, in perfused liver, and in isolated hepatocytes. Effects of hypophysectomy and growth hormone treatment. J Biol Chem. 1977 Jun 10;252(11):3611–3616. [PubMed] [Google Scholar]
- Forte T. M. Primary hepatocytes in monolayer culture: a model for studies on lipoprotein metabolism. Annu Rev Physiol. 1984;46:403–415. doi: 10.1146/annurev.ph.46.030184.002155. [DOI] [PubMed] [Google Scholar]
- GOODMAN D. S. Preparation of human serum albumin free of long-chain fatty acids. Science. 1957 Jun 28;125(3261):1296–1297. doi: 10.1126/science.125.3261.1296. [DOI] [PubMed] [Google Scholar]
- Goh E. H., Heimberg M. Effects of free fatty acids on activity of hepatic microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase and on secretion of triglyceride and cholesterol by liver. J Biol Chem. 1977 May 10;252(9):2822–2826. [PubMed] [Google Scholar]
- Goh E. H., Heimberg M. Relationship between activity of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A reductase and secretion of very-low-density-lipoprotein cholesterol by the isolated perfused liver and in the intact rat. Biochem J. 1979 Oct 15;184(1):1–6. doi: 10.1042/bj1840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg M., Wilcox H. G. The effect of palmitic and oleic acids on the properties and composition of the very low density lipoprotein secreted by the liver. J Biol Chem. 1972 Feb 10;247(3):875–880. [PubMed] [Google Scholar]
- Kane J. P. Apolipoprotein B: structural and metabolic heterogeneity. Annu Rev Physiol. 1983;45:637–650. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- Keyes W. G., Wilcox H. G., Heimberg M. Formation of the very low density lipoprotein and metabolism of [1-14C]-oleate by perfused livers from rats treated with triiodothyronine or propylthiouracil. Metabolism. 1981 Feb;30(2):135–146. doi: 10.1016/0026-0495(81)90162-1. [DOI] [PubMed] [Google Scholar]
- Kohout M., Kohoutova B., Heimberg M. The regulation of hepatic triglyceride metabolism by free fatty acids. J Biol Chem. 1971 Aug 25;246(16):5067–5074. [PubMed] [Google Scholar]
- Kosykh V. A., Preobrazhensky S. N., Fuki I. V., Zaikina O. E., Tsibulsky V. P., Repin V. S., Smirnov V. N. Cholesterol can stimulate secretion of apolipoprotein B by cultured human hepatocytes. Biochim Biophys Acta. 1985 Oct 2;836(3):385–389. doi: 10.1016/0005-2760(85)90143-2. [DOI] [PubMed] [Google Scholar]
- Krul E. S., Dolphin P. J., Rubinstein D. Secretion of nascent lipoproteins by isolated rat hepatocytes. Can J Biochem. 1981 Aug;59(8):676–686. doi: 10.1139/o81-094. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Regulation of fat metabolism of the liver. Nature. 1967 Aug 12;215(5102):716–718. doi: 10.1038/215716a0. [DOI] [PubMed] [Google Scholar]
- Nestruck A. C., Rubinstein D. The synthesis of apoproteins of very low density lipoproteins isolated from the Golgi apparatus of rat liver. Can J Biochem. 1976 Jul;54(7):617–628. doi: 10.1139/o76-091. [DOI] [PubMed] [Google Scholar]
- Ontko J. A. Metabolism of free fatty acids in isolated liver cells. Factors affecting the partition between esterification and oxidation. J Biol Chem. 1972 Mar 25;247(6):1788–1800. [PubMed] [Google Scholar]
- Patsch W., Tamai T., Schonfeld G. Effect of fatty acids on lipid and apoprotein secretion and association in hepatocyte cultures. J Clin Invest. 1983 Jul;72(1):371–378. doi: 10.1172/JCI110977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersburg S. J., Madeley A., Robinson D. S. A study of the interrelationship between the triacylglycerol and protein components of very-low-density lipoproteins using the perfused rat liver. Biochem J. 1975 Sep;150(3):315–321. doi: 10.1042/bj1500315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash J. M., Rothblat G. H., Sparks C. E. Lipoprotein apolipoprotein synthesis by human hepatoma cells in culture. Biochim Biophys Acta. 1981 Nov 23;666(2):294–298. doi: 10.1016/0005-2760(81)90120-x. [DOI] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Ruderman N. B., Richards K. C., Valles de Bourges V. Regulation of production and release of lipoprotein by the perfused rat liver. J Lipid Res. 1968 Sep;9(5):613–619. [PubMed] [Google Scholar]
- Salam W. H., Wilcox H. G., Heimberg M. Stimulation by oleic acid of incorporation of L-[4,5-3H]leucine into very low density lipoprotein apoprotein by the isolated perfused rat liver. Biochem Biophys Res Commun. 1985 Oct 15;132(1):28–34. doi: 10.1016/0006-291x(85)90983-0. [DOI] [PubMed] [Google Scholar]
- Soler-Argilaga C., Wilcox H. G., Heimberg M. The effect of sex on the quantity and properties of the very low density lipoprotein secreted by the liver in vitro. J Lipid Res. 1976 Mar;17(2):139–145. [PubMed] [Google Scholar]
- Stajner A., Sůva J. The determination of nonesterified fatty acids in blood serum using a stable cupric reagent. J Clin Chem Clin Biochem. 1977 Sep;15(9):513–514. doi: 10.1515/cclm.1977.15.1-12.513. [DOI] [PubMed] [Google Scholar]
- Swift L. L., Padley R. J., Getz G. S. Differential labeling of rat hepatic Golgi and serum very low density lipoprotein apoprotein B variants. J Lipid Res. 1987 Feb;28(2):207–215. [PubMed] [Google Scholar]
- Vance J. E., Vance D. E. The role of phosphatidylcholine biosynthesis in the secretion of lipoproteins from hepatocytes. Can J Biochem Cell Biol. 1985 Aug;63(8):870–881. doi: 10.1139/o85-108. [DOI] [PubMed] [Google Scholar]
- Wilcow H. G., Dunn G. D., Heimberg M. Effects of several common long chain fatty acids on the properties and lipid composition of the very low density lipoprotein secreted by the perfused rat liver. Biochim Biophys Acta. 1975 Jul 22;398(1):39–54. doi: 10.1016/0005-2760(75)90168-x. [DOI] [PubMed] [Google Scholar]
- Wilcox H. G., Dishmon G., Helmberg M. Hepatic lipid metabolism in experimental diabetes. IV. Incorporation of amino acid 14-C into lipoprotein-protein and triglyceride. J Biol Chem. 1968 Feb 10;243(3):666–675. [PubMed] [Google Scholar]
- Wilcox H. G., Dunn G. D., Heimberg M. Effects of a mixture of a saturated with an unsaturated fatty acid on secretion of the very low density lipoprotein by the liver. Biochem Biophys Res Commun. 1976 Dec 6;73(3):733–740. doi: 10.1016/0006-291x(76)90871-8. [DOI] [PubMed] [Google Scholar]
- Wilcox H. G., Heimberg M. Secretion and uptake of nascent hepatic very low density lipoprotein by perfused livers from fed and fasted rats. J Lipid Res. 1987 Apr;28(4):351–360. [PubMed] [Google Scholar]
- Windler E., Chao Y., Havel R. J. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J Biol Chem. 1980 Jun 10;255(11):5475–5480. [PubMed] [Google Scholar]
- Windmueller H. G., Herbert P. N., Levy R. I. Biosynthesis of lymph and plasma lipoprotein apoproteins by isolated perfused rat liver and intestine. J Lipid Res. 1973 Mar;14(2):215–223. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Regulated biosynthesis and divergent metabolism of three forms of hepatic apolipoprotein B in the rat. J Lipid Res. 1985 Jan;26(1):70–81. [PubMed] [Google Scholar]
- Woodside W. F., Heimberg M. The metabolism of oleic acid by the perfused rat liver in experimental diabetes induced by antiinsulin serum. Metabolism. 1978 Dec;27(12):1763–1777. doi: 10.1016/0026-0495(78)90262-7. [DOI] [PubMed] [Google Scholar]


