Abstract
Background
Anaemia, malaria, and stunting remain health problems, especially in children younger than five years, and those conditions are linked to morbidity and mortality. The main objective was to assess the relationships between anaemia, malaria, and stunting. Also, the current study aimed to understand the complex interrelationships between explanatory factors, and their direct or indirect relationship with childhood malaria, anaemia, and stunting in Burundi.
Methods
The study used secondary data from the Demographic and Health Survey in Burundi (BDHS) conducted on the March 7, 2017, with a weighted sample size of 13611 children younger than five years. A multivariate structural equation model (SEM) was used to evaluate the interrelationships between dependent variables and their direct or indirect relationship with childhood malaria, anaemia, and stunting. SEMs diverge from other techniques, as they look at the effects on hypothesised relationships from both direct and indirect perspectives (Takele et al., 2023) [1]. The variables with statistical significance were set at a p-value <0.05.
Results
The findings from this study indicated an association between anaemia, malaria, and stunting (p < 0.001). The environmental and household factors were statistically significant (p < 0.038 and p < 0.001 respectively) and positively impacted childhood malaria, anaemia, and stunting. The results also indicated that the household factors were statistically significant (p < 0.001) predictors of childhood malaria, anaemia, and stunting. Furthermore, the findings from this study revealed that geophysical factors have a positive significant (p < 0.001) impact on childhood malaria, anaemia, and stunting via the mediating of the household factors. Contrastingly, with the environmental factors as a mediator, we observe a negative significant (p < 0.001) impact on childhood malaria, anaemia, and stunting. Lastly, the results showed that demographic factors had a negative significant (p = 0.004) effect on childhood anaemia, malaria, and stunting via the mediating of household factors.
Conclusion
The findings from this study revealed an association between malaria, anaemia, and stunting, which imply that these conditions could contribute to collaborative improvements in child well-being. In addition, child demographic, household, environmental, and geographic factors were direct and indirect important drivers of childhood malaria, anaemia, and stunting. Therefore, improving sanitation, access to clean water, nutrition practices, and health care, especially for children from rural areas, and uneducated mothers with poor backgrounds could help to control and eliminate stunting, anemia, and malaria in children younger than five years in Burundi.
Keywords: Malaria, Anaemia, Stunting, Interrelationships, SEM, And children younger than five years in Burundi
1. Introduction
Childhood malaria, anaemia, and stunting remain major health problems worldwide, particularly in children younger than five years in developing countries [[1], [2], [3]]. Malaria, anaemia, and stunting are intersecting and associated with morbidity and mortality, with pregnant women and children younger than five years being the most susceptible [[4], [5], [6]]. Anaemia, malaria, and stunting can contribute to children's poor cognitive development, mental health issues, low academic achievement, and susceptibility to diseases. These effects can last until adulthood [[7], [8], [9]].
Malaria is transmitted to individuals through the bites of infected Anopheles mosquitos. Malaria parasites attack red blood cells, decreasing the number of blood cells in the body, which can lead to severe anaemia [3]. Malaria vector breeding and development increase during the rainy and humid seasons accompanied by high temperatures in areas of low altitude [10,11].
Anaemia is defined as a low hemoglobin level in the blood (Hb). In children younger than five years, anaemia is categorized as mild when the Hb is between 10.0 and 10.9 g/dl, moderate, if the Hb is between 7.0 and 9.9 g/dl, severe, for Hb < 7.0 g/dl, and normal if Hb ≥ 11.0 g/dl [[12], [13], [14]]. Iron deficiency is assumed to be the greatest cause of anaemia in roughly 50 % of cases. However, inadequate levels of folate, vitamin B12, protein deficiencies, nutrients, and several illnesses including malaria and diarrhea among others, can increase the risk of anaemia [15,16].
Stunting is long-term or chronic malnutrition, caused by lacking nutrients and other minerals [8]. The nutrition of a child can be estimated using various anthropometric indices in relation to the WHO growth standards [17,18]. The first one is stunting (height-for-age) which implies long-term or chronic malnutrition; wasting (low-weight-for-height) indicates acute malnutrition; and underweight (weight-for-age) which can be either stunting, wasting, or both [[17], [18], [19]]. The nutrition status is calculated using the Z-score for height-for-age (HAZ), weight-for-height (WHZ), and weight-for-age (WAZ). Children younger than five years are treated for stunting, wasting, or being underweight if their HAZ, WAZ, or WHZ are less than minus two standard deviations (<-2 SD) respectively. Moreover, children with Z-scores of less than three standard deviations (<-3 SD) were classified as having severe stunting, wasting, and/or being underweight respectively [14,17,18,20]. The Z-scores are normally obtained from the child's weight, height, or length and calculated as indicated in the following formula:
, where are the mean and standard deviation of the scores, while is the values of length, weight, or height [20,21].
In 2019, the prevalence of anaemia in children younger than five years was 39.8 % globally, with the highest percentage (60.2 %) from Africa [8,14]. In 2017, the prevalence of malaria and stunting in children younger than five years globally was at 61 % and 26.6 % respectively. The highest percentages are from Africa with 92 % of malaria and 22.2 % of stunting cases [17,22]. In Burundi, the prevalence of malaria in children less than five years was 27 % and that of anaemia and stunting were 61 % and 56 % respectively in 2017 [23,24]. These percentages show that malaria, anaemia, and stunting are still health problems in children globally, especially in developing countries, and this includes Burundi. Addressing the anaemia, malaria, and nutritional conditions of children is essential to ensuring a high standard of living for children, especially those younger than five years [16].
Although efforts have been undertaken to control malaria, anaemia, and stunting through humanitarian and government interventions, the percentages show that malaria, anaemia, and stunting are still health problems in children.
The current study used a structural equation model (SEM) to understand the complex interrelationships between dependent variables and their direct or indirect association with childhood malaria, anaemia, and stunting in Burundi. The indirect effect modeling in the SEM allows the inclusion of alternative variables that may not be significant in direct effect models [25]. The direct effects are typically the relationships between the independent and dependent variables without any intermediate variable. However, the indirect effects are the relationships between the independent and dependent variables through a mediated variable [[26], [27]]. The direct and indirect effects can have the same signs or different signs. When the direct and indirect effects have a positive influence, means that there is a complementary mediation. In the case of direct or indirect effects having dissimilarity influence, there is a competitive mediation [27].
Structural equation modelling has become a general method in science for analysing and understanding multivariate relationships among the variables of interest. The analysis of covariance (interaction) using structural equations, also known as latent variable analysis, is a new area of statistics. However, this method has been applied in econometrics and psychometrics for a long time [[28], [29], [30]]. The SEM is usually a multivariate model that links an attribute and unmeasured latent variables [[31], [32], [33]]. The structural equation model can assess complex interrelationships between different variables and related unobserved and observed variables. The assessment can be done by calculating the sample covariance matrix of the observed variables and the population covariance matrix produced by the SEM framework [32,[34], [35], [36], [37], [38]]. The SEM is very important in its extension for calculating measurement errors via latent variables. Structural equation modelling allows the evaluation of numerous sets of observed variables to define the non-measurable variable (latent or construct variables) and allows these latent variables to be related to each other [39,40]. The SEM integrates several variables, which are not measured directly but along their effects or indicators. Furthermore, the SEM methodology involves multivariate data analysis tools that merge features of multiple regression and factor analysis. This allows for the simultaneous calculation of a series of interrelationships between variables and relations of dependency that permit the methodology of directly including a measurement error in the model [37,41,42]. The theoretical model of SEM can be demonstrated by applying mathematical equations and graphs (path diagrams) to summarise a set of hypotheses [33,39]. Moreover, SEM methodology can accommodate both observed and unobserved (latent or constructed) variables, which is one of the most important distinctions between structural equation modelling and other statistical modelling tools [25,37,43].
In the literature, most studies assessed the association between either anaemia and malaria; anaemia and stunting, or malaria and malnutrition using different statistical methods [3,18,28,[29], [44], [45]]. Few studies have tried to evaluate the association between anaemia, malaria, and malnutrition [5,14,30]. However, not all of these studies assessed the association between anaemia, malaria, and stunting simultaneously.
Hence, understanding the relationship between anaemia, malaria, and stunting, as well as other associated risk factors, makes it simpler to control and eradicate the prevalence of anaemia, malaria, and stunting. In addition, it aids in the design of intervention measures by various contributors and policymakers.
Thus, the current study used a structural equation model (SEM) to understand the complex interrelationships between dependent variables and their direct or indirect association with childhood malaria, anaemia, and stunting in Burundi. Furthermore, the study aims to identify if there are associations between anaemia, malaria, and stunting.
2. Methodology and material
2.1. Study area
Burundi is a small (27,834 km2) land-locked and densely populated country located in East Africa with Bujumbura as the capital city [46,47]. Burundi is one of the world's least poor countries, accounting for more than 70 % of the population living in poverty. The economy of the country is based on agriculture, where the majority of the population is employed in the agriculture sector [47,48]. Burundi is bordered by Rwanda to the north, Tanzania to the east and south, Lake Tanganyika to the southwest, and the Democratic Republic of the Congo to the west [46,47,49]. Malaria, anaemia, and stunting are the leading cause of mortality rate in the population, especially, in children younger than five years in the country.
2.2. Data source
This study used secondary data from the Demographic and Health Survey in Burundi (BDHS) conducted on March 7, 2017. The survey was organized by the Institute of Statistics and Economic Studies of Burundi (ISTEEBU) in collaboration with the Ministry of Public Health with the aim of the fight against AIDS. Funding was provided by the Government of Burundi, the United States Agency for International Development (USAID), United Fund for Childhood (UNICEF), the World Health Organization (WHO), The Swiss Cooperation, and the Belgian Cooperation. The ICF gave specialized help to the whole task as a feature of the DHS Program, which was subsidized by USAID, and whose goal is to offer help and technical assistance globally for the realization of population and health surveys.
2.3. Data sampling and design
All Women aged 15–49 years and children from 6 to 59 months who remained in or visited the chosen households the night before the survey were included in the study. The 2017 survey was a national representative of rural and urban areas, Bujumbura-Mairie, and other provinces in the country. The 2017 sample was drawn based on a stratified, two-stage area survey [46]. The first stage of sampling includes a selection of 554 Primary Sampling Units (PSU) or clusters from the enumeration areas (EAs) established during the General Population and Housing Census (GPHC) carried out in 2008. The second stage of sampling involved systematic selection with equal probability and included a sample of 30 households in each cluster from urban and rural areas. A total number of 16620 households were selected and 3180 were from urban areas in 106 clusters, while 13440 were from rural areas in 448 clusters.
2.4. Data weighting
The current study used a weighted sample of 13611 children aged between 6 and 59 months [50].
The weighted sample was used in the study to make inferences, which were representative of the country, and to account for the data set's complex sampling procedure. Individuals surveyed in each region should contribute proportionally to the size of the region's total sample in the sampling procedure. In any case, some regions may have small populations, and this unweighted provision may not accurately represent the population. As a result, the region with a small population is oversampled to tackle these challenges, and thus the weighted sample is used [3,46].
2.5. Study criteria and blood sampling methods
The inclusion criteria for this study were children aged between 6 and 59 months, who remained in or visited the selected households the night before the survey and were guided by their parents or guardians. While the exclusion criteria were children aged less than 6 months, and their parents or guardians were not able to communicate in Kirundi, French, or English [46]. The exclusion and system missing values were regarded as missing data and were removed.
With the parent's or guardian's permission, all children aged between 6 and 59 months were tested for anaemia, malaria, and stunting. The trained laboratory technicians and nurses conducted the process of testing for anaemia, malaria, and stunting. The blood sample for anaemia was collected using a spring-loaded, sterile lancet to make a finger- or heel-prick. The drop of blood was collected in a microcuvette, and the Haemoglobin level was analysed using a portable HemoCue analyzer. The blood sampling for malaria was collected from children's finger- or heel-prick using the SD Biolne Malaria Ag P.f/P, a rapid diagnostic test (RDT). We used a rapid diagnostic test (RDT) as is easy to access and learn, however, microscopy can also be used for malaria testing [11,46].
Lastly, the height measurement was taken using a tape board, where children under two years of age were measured in a lying position, while the older children were measured in a standing position. The weight measurement was taken using electronic scales (SECA) provided by UNICEF. The scale was aligned to zero and parents or guardians were asked to unclothe their children or keep them in light clothing. For the children who were unable to stand, the weight was calculated based on the difference in parent weight compared to the weight of the parent holding the child. Based on the children's weight, height, and age it was easy to calculate their nutritional status (i.e. Weight-for-age, height-for-age, and weight-for-height) based on the WHO guidelines [18,46].
All results were given to the child's parent or guardian in oral and composed structure and were recorded on the Biomarker Questionnaire. Moreover, children who tested positive for malaria, anaemia, or stunting were provided with a full course of medication [46].
Ethics approval
The ethical approval for the 2017 BDHS was organised and assessed by the Institute of Statistics and Economics Studies of Burundi and approved by the Minister of Public Health.
3. Data analysis
3.1. Dependent variable
The dependent (response) variables of interest are malaria, anaemia, and stunting in children younger than five years in Burundi. In this study, a child was considered to have malaria if he/she tested positive or not infected if he/she tested negative using the RDT results. A child can be considered to have either severe (Hb < 7 g/dl), moderate (Hb between 7 and 8.9 g/dl), or mild (Hb between 9 and 10.9 g/dl) anaemia. However, in the current study, the response variable used was binary; hence, the child was considered anaemic only if the Hb level was below the altitude-adjusted threshold of 11 g/dl, otherwise. Lastly, the nutrition status of a child was also binary, and a child was considered malnourished (stunted) when the standard deviation (SD) was less than minus two standard deviations, otherwise, a child is normal (not stunted).
3.2. Independent variables
The independent (exploratory) variables used in this study were also used in previous studies on childhood anaemia, malaria, or malnutrition [18,51]. As a result, this provides the conceptual framework for the current study. These independent variables include demographic, household (socioeconomic), geographical, and environmental factors. The household factors include residence, wealth index, source of drinking water, mother's educational attainment and access to information through television, household access to electricity, type of toilet facility, the household share of toilet facility, as well as the household's main roof, floor, and wall material. The environmental factors include rainfall, proximity to water, land surface temperature, enhanced vegetation index (EVI), aridity, wet days, and clusters. The child's demographic factors include age, gender, and whether or not the child slept under a mosquito net. Finally, the geophysical factors include the geographical regions of the children, travel times, and nightlight composites.
4. Statistical analysis
4.1. Model formulation
The application of structural equation modelling includes various steps such as the development of the theoretical conceptual model; specification of the mathematical model; determination of the model's evidence; and determining the model fit and evaluation of the goodness-of-fit of the model [35,40].
4.1.1. Theoretical conceptual and path models
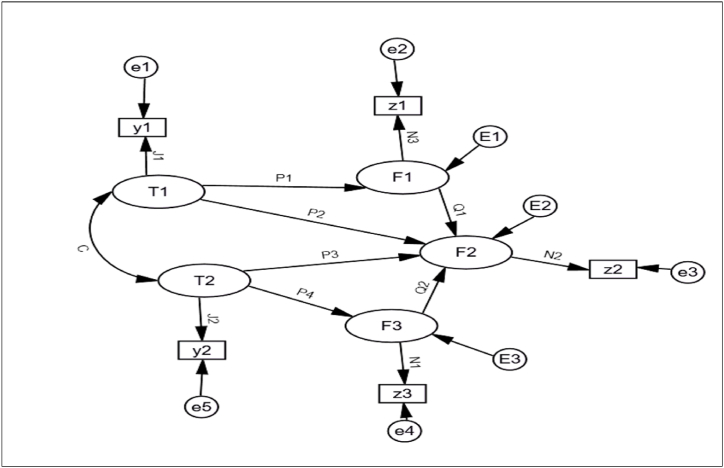
The latent (constructs) variables cannot be measured directly and are known as theoretical concepts. The latent variable is measured by observed (indicator) variables, and they assist the expansion and estimation of casual relationships in SEM [35,52]. The latent variables related to each other in the model must be indicated first, and the impact that these variables apply to each other is categorized as exogenous and endogenous [39,40].
In the model are the latent endogenous variables; the latent exogenous variables; are the observed endogenous variables; the observed exogenous variables; is the measurement errors, are structural errors; is the coefficient correlation between the latent exogenous variable, while , , , and are the coefficients as indicated in Fig. 1.
Fig. 1.
Graphical path of a structural model.
The exogenous (predictive) variables are not manipulated by the effect of other variables in the model and are measured without error. However, the endogenous (dependent) variables are influenced by the effect of other variables included in the model [43].
Structural equation modelling offers the unique capacity to describe latent or unobserved variables in a linear model, contrary to other statistical approaches where observed variables in a specific data set are employed for statistical analysis [34,35,53,54].
The SEM includes the measurement and structural models which can be written as follows:
| (4.1) |
where denotes a vector of latent endogenous variables, denotes a vector of latent endogenous variables, represents an matrix of coefficients connecting the latent endogenous variables to each order, denotes an coefficients of the matrix that links the endogenous variables to the exogenous variables, and denotes an vector of the structural disturbances or errors. The main diagonal components of are often zeros with viewed as mutually independent and normally distributed [39,43,55]. The measurement model, which is theoretically described independently for the endogenous and the exogenous variables, linking the observable and latent variables together is expressed by:
| (4.2) |
Where (p×m) and (q×k) are coefficient matrices illustrating the relationship between latent endogenous and exogenous factors and the observable variables, respectively. Consequently, are p×1 and q×1 vectors of measurement errors in Y and X respectively.
In order to generate a scale for the related latent variables, each column of the matrices normally has a value that is set to one. As an alternative, this can also be accomplished by setting the variances of the latent exogenous variables in a matrix defined as , and the matrix corresponding to the exogenous variable's covariance matrix to zero. Furthermore, the measurement model's fit to the confirmatory factor analysis (CFA) application, which identifies the latent components, must be addressed [35,39,40].
The CFA examines the theoretical measurement model and determines whether the hypothesised measurement model produces a variance-covariance matrix that is identical to the sample variance-covariance matrix. Based on equation (4.2), the measurement errors and , each having a multivariate normal distribution, are considered to have zero expectations.
The errors presume independent of each other, independent of latent endogenous variables , latent exogenous variables , and independent of the disturbances . Additionally, it is assumed that the latent exogenous variables have a multivariate normal distribution and that the observations are independently sampled. However, for the endogenous variables that are accurately measured, this assumption is irrelevant [39,40].
The structural errors , on the other hand, are unaffected by the latent exogenous variables and have zero expectation with a multivariate normal distribution. The observed indicators X and Y in this case exhibit a multivariate normal distribution and can be expressed as follows:
| (4.3) |
where , is the indicators' population covariance matrix, which is a function of the model's parameter , and can be calculated as:
| (4.5) |
where , indicates a k×k covariance matrix of the latent exogenous variables denotes the m×m covariance matrix of the disturbance term, and represents the covariance matrices of the measurement errors μ and θ.
4.2. Model estimation
In order to obtain the matrix , related to the confirmatory factor analysis (CFA) of Equation (1.5), it should be expected that ; ; ; and [43]. In any given model, the restriction is essential in certain components of the matrix , which incorporates setting a few parameters to zero. With adequate limitation, the maximum likelihood estimates (MLE) might be achieved for the parameters of the model and the log-likelihood related to the model can be portrayed as a function of the model's parameters of and , as the sampling covariance matrix between the observable variables. In the structural equation model, this method focuses on estimating the parameters , in order to minimise the inconsistency of function . The inconsistency function is a scalar that estimates the distance between the examining covariance and the adjusted covariance matrix [53,56,57]. In structural equation modelling, the MLE and generalised least squares (GLS) are the most commonly used estimation methods [35,53,58]. The MLE methods are characterised by parameters so that the two matrices and are pretty much as close as could really be expected, for the likelihood logarithm estimates the vicinity between the two matrices. The asymptotic standard errors are calculated from the square root of the matrix diagonals, in the parameter estimates. It is also expected that the structural relationships between the latent endogenous variables and the latent exogenous variables are linear, as they are the interrelationships exuding between the indicator variables and the latent constructs.
4.3. Model identifiability
A principal step in model evaluation is to check the model's identifiability of latent variables, which is a complicated task in SEM without a straightforward answer [32,57]. The model can be reported as none identifiable when the system of equations cannot be solved. The counting rule for identifiability is generally the number of free parameters in the model, and must not be more than the number of variance and covariance between the observable variables, and can be shown as:
| (4.6) |
where, represents the number of endogenous variables and represents the number of exogenous variables in the model [43,59].
However, the counting rule is not generally a sufficient condition, as the condition can be met yet still obtain a non-identifiable model. Hence, more conditions allow a model to be identified such as when measurement errors are not correlated when at least two exclusive indicators exist for each of the latent variables when a single indicator for latent variables is equally assumed without error is possible, and when the structural model includes only observed variables [35,40]. The SEM is not stable with a small sample size, and the minimum sample size depends on the involvement of the model, the degree of freedom, and the size effect [40,43].
4.4. Model diagnostics
In the structural equation model, the verification of the model fit is based on various goodness-of-fit-model criteria and is created to assess the model under several assumptions. In addition, verifying the goodness-of-fit is not a direct process as accessible in other multivariate techniques [39,43]. The chi-square ( the test is widely regarded as the only statistical significance test commonly used to evaluate the theoretical model in SEM. The current study used a p-value less than 5 % as the cutoff for statistically significant variables.
The insignificant results indicate a resemblance between the original sample variance-covariance matrix and the variance-covariance matrix estimated by the model. Although, the application of is very difficult when the sample size is large, and rejecting the null hypothesis becomes hard [60,58]. When the chi-square has a zero value, it means there is a good fit or no difference between the values in the sample covariance matrix and the model-suggested covariance matrix generated based on a theoretical model. However, in structural equation modelling, it is recommended that various goodness-of-fit criteria be used in conjunction with overall fit measurements [43,50,61]. As a result, the measurement indices range from poor fit to perfect fit, and various structural equation modelling programs report a range of the most common model fit as follows:
-
➢
Goodness of fit index (GIF) = 1- , where 1 indicates perfect fit
-
➢
Root mean square error of approximation (RMSEA) = , as a value less than 0.05 indicates the model's good fit.
-
➢
Comparative fit index (CFI) = 1-, where the good fit the value greater than 0.9 is expected.
-
➢
Tucker-Lewis index (TLI) = .
-
➢
Incremental fit index (IFI) = , and
-
➢
Normal fit index (NFI) = .
These mentioned above goodness-of-fit criteria are based on differences in variance-covariance matrices between observed (original, ) and model-implied (replicate, ) [43,53,57,59]. In addition, to check the validity and reliability of the internal consistency between various items, Cronbach's alpha (coefficient alpha) method was used in this study. Cronbach's alpha coefficient varies between 0 and 1, and the acceptable coefficient is ≥ 0.7 [[62], [63], [64]].
5. Model fit and data analysis
Initially, we evaluated a theoretical model for individual variables (household, environmental, child demography, and geophysical factors) to guarantee that the theoretical relationships between the observable variables and their corresponding factors were upheld by the data.
The CFA was used to evaluate whether the measurement model and relationships between all the latent and manifest variables are relevant. Numerous model fit indices were used in the analysis of this study, however, the common technique for assessing model fit is and should not be significant for a good model [43,65]. Among the model fit indices and their conditions, the incorporated use of CFI, GFI, IFI, TLI, and NFI should be greater than 0.90 for a good model [53,57,61].
The RMSEA ≤0.05 indicates a best-fit model, however, the values between 0.05 and 0.08 show a reasonable fit model [50,59,61]. The validity of the structural model used a cross-validation method, which includes categorizing the data into two different sample sizes [40,59]. The first sample was considered as an adjustment sample, while the second was the validation sample. We first tested the SEM on the adjustment sample and analysed the goodness-of-fit model, once the model reached a good fit for the adjustment sample, the model could be assessed on the validation sample [40,50]. The validity of the model was obtained when the covariance structure of the model reached the best fit in both the adjustment and validation samples. The maximum likelihood estimation techniques are used for full structural equation modelling in the calibration sample. In general, the less value of the better goodness of the fit to the data. However, we cannot depend only on , as the test statistics are sensitive when the sample size is large and tend to reject the model [43,50,66]. Therefore, other indices, which are not depending on the sample size, are included. These indices and their cut-off indicating a good fit are NFI ≥0.95, CFI ≥0.95, AGFI ≥0.90, and RMSEA ≤0.07 [35,40,50,67].
In the theoretical model, we used the household factors (residence, wealth index, source of drinking water, type of toilet facility, the household share of toilet facility, mother's educational attainment, mother's access to information through television, household access to electricity, household's main roof, floor, and wall material); environmental factors (rainfall, proximity to water, land surface temperature, enhanced vegetation index (EVI), Aridity, wet days, and cluster); and child demographic factors (child's age, child's gender, and child slept under a mosquito net) to determine whether they directly or indirectly influence childhood malaria, anaemia, and stunting. The geophysical factors (geographical regions of the children, travel times, and nightlight composites) were determined whether they are directly or indirectly related to childhood malaria, anaemia, and stunting through the mediating effects of household factors.
Relying upon those assumptions, the conceptual framework can be defined by latent variables, which are deduced through observable variables because they cannot be directly measured, to assess their impact on childhood malaria, anaemia, and stunting co-morbidity. In a full SEM, the latent factors are then normalized on other factors based on the theory, empirical research, and suitable observed indicators [43].
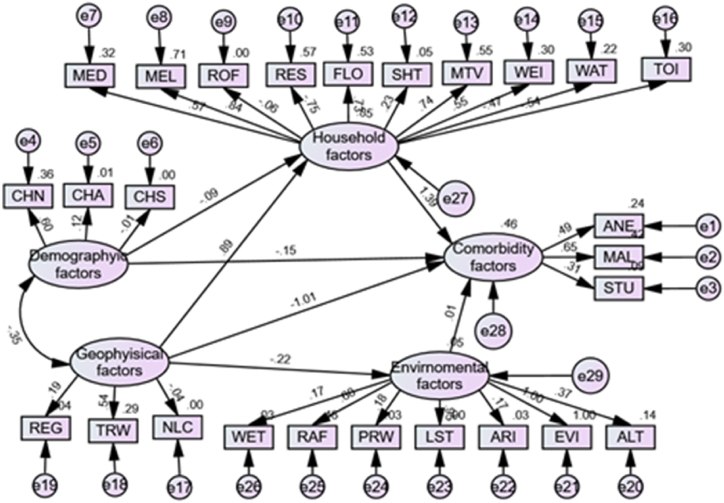
In order to assess the model fit and evaluate the variables of interest (child demography, household-level, environmental, and geophysical factors), we used the two approaches of equations (4.1), (4.2) by conducting a confirmatory factor analysis (CFA) to test the hypothesised associations among these variables. We then created a conceptual path model diagram (Fig. 2) that comprised the endogenous and exogenous variables to represent the causal structure shown in the figure and define all of the conceptual relationships between these factors with respect to childhood co-morbidity. Thereafter, we evaluated the estimation model with all latent and observed factors included in the full model. The association between the direct and indirect variables, together with childhood malaria, anaemia, and stunting was assessed using structural techniques. For the acceptance or rejection of the model, we performed a goodness of fit test using SPSS AMOS software version 27.0 [66,68].
Fig. 2.
Theoretical full structural model.
6. Results and interpretations
All exploratory variables were included in the model and were removed one by one to get the best-fit model. Fig. 2 indicates the results of the full model, after deleting some items in the model for the best fit. The household constructs had 11 factor higher order construct comprising: residence, wealth index, source of drinking water, type of toilet facility, the household share of toilet facility, mother's educational attainment, and mother's access to information through television, household access to electricity, household's main roof, floor, and wall material, the Cronbach's score (0.84) was acceptable after deleting one variable (household's main wall material) from the model, and 10 items left as indicated in Fig. 2.
For the environmental factors, Cronbach's score (0.79) was acceptable after deleting two variables (maximum and minimum temperature) from the model and left with seven. The geophysical factors resulted in a higher order of three factors in the model comprising geographical regions of the children, travel times, and nightlight composites; and Cronbach's score (0.90) was acceptable. The child demographic factors (child's age, child gender, and child sleeping under mosquito net) also had an acceptable Cronbach's score (0.81).
The non-significant Bollen-Stine p statistic, together with the underlying model statistics, demonstrated that the model was a good fit for the data in each testing model case, illustrating factor validity [59,67,68]. The Cronbach's score in every case was above the recommended cut-off of 0.75. This shows an adaptable model, internal consistency, and good scale reliability between the constructs (items) in the model [59,27].
Then we improved the CFA model to get a better model with the highest goodness-of-fit as indicated in Table 1. The calibration sample model fit results showed that changing the model is not effective. The standardized regression weights for this model were all significant at a 5 % significant level. The full path results of SEM are indicated in Fig. 2.
Table 1.
The goodness-of-fit indices in the two models (CFA and SEM).
| MODEL | GFI | CFI | IFI | TLI | NFI | RMSEA | |
|---|---|---|---|---|---|---|---|
| Conceptual CFA model | 732.025 | 0.651 | 0.645 | 0.640 | 0.545 | 0.648 | 0.046 |
| Full structural model | 435.020 | 0.968 | 0.976 | 0.926 | 0.912 | 0.913 | 0.04 |
Both the direct and indirect effects of geophysical, and demographic factors are statistically significant (p < 0.001) on childhood malaria, anaemia, and stunting.
The direct and indirect interrelationships between contextual factors and their impact on childhood malaria, anaemia, and stunting are summarised in Table 2 and 3.
Table 2.
Standardised direct and indirect effects of factors on childhood malaria, anaemia, and stunting. Note: the ∗ on the numbers indicates the p-values. p∗≤ 0.05; p∗∗≤ 0.01, and p∗∗∗≤ 0.001.
| Factors | Total effect | Direct effect | Indirect effect |
|---|---|---|---|
| Geophysical factors → Household | 0.889∗ | 0.889∗ | – |
| Geophysical factors → Environmental | −0.217∗∗ | −0.217∗∗ | – |
| Geophysical factors →Co-morbidity | 0.231∗ | −1.006∗ | 1.237∗ |
| Household factor→Co-morbidity | 1.394∗ | 1.394∗ | – |
| Child demography factor→Co-morbidity | −0.271∗ | −0.150∗ | −0.121∗ |
| Child demography factor→Household | −0.087∗∗∗ | −0.087∗∗∗ | – |
| Environmental → Co-morbidity | 0.014∗∗ | 0.014∗∗ | – |
Table 3.
Standardised regression coefficients for the full structured model.
| Parameters | Estimate | 95%CI Lower | 95%CI Upper | P-value | |
|---|---|---|---|---|---|
| Environmental factors | Geophysical factors | −0.217 | 0.802 | 0.908 | <0.001 |
| Household factors | Demographic factors | −0.087 | 0.881 | 0.956 | 0.004 |
| Household factors | Geophysical factors | 0.889 | 2.305 | 2.573 | <0.001 |
| Comorbidity factors | Environmental factors | 0.014 | 0.967 | 1.062 | 0.038 |
| Comorbidity factors | Household factors | 1.394 | 3.193 | 5.150 | <0.001 |
| Comorbidity factors | Demographic factors | −0.150 | 0.820 | 0.904 | 0.010 |
| Comorbidity factors | Geophysical factors | 1.006 | 0.286 | 0.461 | <0.001 |
| ANE | Comorbidity factors | 0.493 | 1.481 | 1.809 | <0.001 |
| MAL | Comorbidity factors | 0.650 | 1.691 | 2.164 | <0.001 |
| STU | Comorbidity factors | 0.306 | 1.256 | 1.468 | <0.001 |
| FLO | Household factors | 0.731 | 1.958 | 2.203 | <0.001 |
| WAT | Household factors | −0.465 | 0.608 | 0.649 | <0.001 |
| MEL | Household factors | 0.842 | 2.259 | 2.385 | <0.001 |
| ROF | Household factors | −0.062 | 0.929 | 0.951 | <0.001 |
| TOI | Household factors | −0.544 | 0.563 | 0.599 | <0.001 |
| WEI | Household factors | 0.549 | 1.649 | 1.818 | <0.001 |
| MTV | Household factors | 0.739 | 2.052 | 2.136 | <0.001 |
| SHT | Household factors | 0.230 | 1.246 | 1.271 | <0.001 |
| RES | Household factors | −0.752 | 0.458 | 0.485 | <0.001 |
| MED | Household factors | 0.567 | 1.659 | 1.874 | <0.001 |
| ALT | Environmental factors | 0.371 | 0.916 | 2.293 | <0.001 |
| EVI | Environmental factors | 0.999 | 1.442 | 5.114 | <0.001 |
| ARI | Environmental factors | 0.171 | 0.709 | 1.986 | <0.001 |
| LST | Environmental factors | 0.951 | 1.598 | 4.191 | <0.001 |
| PRW | Environmental factors | 0.178 | 0.685 | 2.085 | <0.001 |
| RAF | Environmental factors | 0.680 | 1.239 | 3.146 | <0.001 |
| WET | Environmental factors | 0.170 | 0.725 | 1.939 | <0.001 |
| REG | Geophysical factors | 0.194 | 1.096 | 1.344 | <0.001 |
| TRW | Geophysical factors | 0.535 | 1.099 | 2.654 | <0.001 |
| NLC | Geophysical factors | −0.036 | 0.603 | 1.543 | <0.001 |
| CHN | Demographic factors | 0.603 | 1.702 | 1.962 | <0.001 |
| CHS | Demographic factors | −0.007 | 0.963 | 1.024 | 0.043 |
| CHA | Demographic factors | 0.120 | 1.033 | 1.231 | <0.001 |
ANE: Anaemia,MAL: Malaria,STU: Stunting, FLO: Type of floor material,WAT: Type of drinking water,MEL: Mother's access to electricity,ROF: Type of roof material,TOI: Household has toilet facility,WEI:Wealth index,MTV: Mother's access to television,SHT: Whether household share toilet facility,RES: Type of place of residence,MED: Mother's education,ALT: Cluster altitude, EVI: Enhanced vegetation index,ARI:Aridity,LST:Land surface temperature,PRW: Proximity to water,RAF:Rainfall,WET: Wet days,REG: Region,TRW:Travel times,NLC: Night light composite,CHN: Child slept under a mosquito net, CHS:Child's sex,CHA: Child's age.
In this study, the testing for the partial mediation effect assumed the estimation of two phases. The direct effect was used in phase one to estimate the effect of predictors (household, demographic, environmental, and geophysical factors) on childhood malaria, anaemia, and stunting. The direct path coefficient from both geophysical and demographic factors on malaria, anaemia, and stunting was statistically significant (p = 0.001) and revealed a negative direct effect on childhood malaria, anaemia, and stunting as indicated in Table 2. The negative sign shows the opposite directions between dependent and independent variables. The possible reason might be increasing the number of children sleeping under a mosquito bed net in the malaria region can reduce malaria, anaemia, and stunting. Furthermore, as children grow the chances of having malaria, anaemia, and stunting reduce [[3], [4], [5]].
However, the direct path coefficient from both household and environmental factors on malaria, anaemia, and stunting was statistically significant (p = 0.004) and revealed a positive direct effect on childhood malaria, anaemia, and stunting. The previous studies showed that household and environmental factors used in this study could have a positive impact on childhood malaria, anaemia, and stunting [17,29,49]. This is due to some variables from household and environmental factors presented in this study such as residence area, wealth index, altitude, toilet, and water facilities contribute positively to childhood anaemia, malaria, and stunting [3,17,29,49].
There was a negative direct association between geophysical and environmental factors (β = −0.217, p = 0.008). The geophysical factors (regions of a child, travel times, and nightlight composites.) are assumed to be related to environmental factors [68,69].
We also observed a negative direct association between child demographic and household factors (β = −0.087, p < 0.001). This might be because demographic factors (child age, gender, and child sleeping under mosquito net) are from household factors [50]. Furthermore, we observed a positive interrelationship between geophysical and child demographic factors.
In the second phase, we involved the testing the indirect relationship between geophysical and demographic factors on childhood anaemia, malaria, and stunting.
The geophysical factors had a positive indirect association with childhood malaria, anaemia, and stunting via the mediating effect of the household factors. The positive indirect effect it might be addressed by the socio-economic factors in the household. This is confirmed by previous studies [1,70,71], indicating that improving socio-economic, results in a reduction of childhood anaemia, malaria, and stunting.
Contrastingly, this indirect association was negative with the mediating effect of environmental factors. Environmental factors such as low altitude (<2400 m) and high (≥2400 m) temperature in previous studies have shown a negative effect on childhood malaria, anaemia, or malnutrition [2,10,29,72]. This is because childhood anaemia, malaria, and stunting are high in children living in low-altitude areas [2].
The demographic factors had a negative indirect association with childhood naemia, malaria, and stunting via the mediating effect of household factors. In this study, we used age and children sleeping under mosquito bed nets as demographic factors and these variables are from household factors. As child age increases, children are not likely to sleep under a mosquito bed net, and this increases childhood malaria, anaemia, and stunting. These results are in line with the previous studies [2,15,71,73].
The present study also indicated a positive association between malaria (0.65), anaemia (0.49), and stunting (0.31) (p < 0.001). This means that any change in either one or two disease(s) has a positive impact on the other (s). The findings from the current study are in line with the previous studies [3,18,74,75].
7. Strength and limitations
Structural Equation Model (SEM) was good to fit the data and indicated the complex interrelationships between geographical, child demography, household, and environmental factors, as well as their direct or indirect relationship with childhood anaemia, malaria, and stunting in Burundi. In addition, the sample size used in the current study is quite large.
However, SEM could not address the trends of the association between the predictors and response variables if they were linearly or nonlinearly associated. The present study also did not consider wasting and being underweight as a measurement of nutrition status. Lastly, the dataset was cross-sectional and could not study the change in factors and prevalence over time. Hence, a further study should be conducted to address these issues.
8. Summary and conclusion
The present study used the structural equation model, to demonstrate the complex interrelationships between the variables of interest and fitted our data well. We assumed that the observed variables are dependent on the latent variables. The model fits our data well and explains the complex interrelationships between variables in a dataset. The findings from this study indicate an association between anaemia, malaria, and stunting. In addition, results from the current study revealed that the geographical factors were statistically significant determinants of childhood malaria, anaemia, and stunting, and have a direct and indirect effect on childhood anaemia, malaria, and stunting. The estimated indirect path for the effect of geophysical factors on childhood anaemia, malaria, and stunting, as mediated by household factors was statistically significant and positive.
However, the estimated indirect paths for the effect of geophysical factors on childhood anaemia, malaria, and stunting factors, as mediated by environmental factors were statistically significant but negative. The child demography factors such as the child's age, child's gender, and child sleeping under a mosquito net, were statistically significant predictors of childhood anaemia, malaria, and stunting. The estimated indirect path effect on childhood anaemia, malaria, and stunting via the mediating effect of household factors was statistically significant and negative.
The household factors comprising residence, wealth index, source of drinking water, type of toilet facility, the household share of toilet facility, mother's educational attainment, mother's access to information through television, the household has access to electricity, household's main roof, floor, and wall material were also statistically significant predictors in childhood anaemia, malaria, and stunting. The study also indicates that environmental factors such as rainfall, proximity to water, land surface temperature, enhanced vegetation index, Aridity, wet days, and cluster were statistically significant predictors of childhood anaemia, malaria, and stunting. The findings from this study will assist Burundian policymakers and healthcare professionals in developing protective measures and planning mediation systems that target children younger than five years.
In order to develop effective intervention strategies to help reduce anaemia, malaria, and stunting in children younger than five years. The authors recommended that the Burundian government and policymakers should focus on fighting against anaemia, malaria, and stunting in children younger than five years. To achieve this, each household should be educated on measures and prevention of anaemia, malaria, and stunting, through social media, and workshops. In addition, the healthcare, toilet facilities, sleeping under mosquito bed nets, and use of clean water should be improved; especially, for individuals from rural areas, uneducated mothers, and poorer quantile index households. Furthermore, improving the nutritional status of children mostly from rural areas, uneducated mothers, and poorer quantile index households will reduce anaemia, malaria, and stunting in children younger than five years.
Funding statement
The authors declare that there was no funding associated with this study.
Institutional review board Statement
Not applicable.
Additional information
No additional information is available for this paper.
Data availability statement
The dataset used during this study can be accessed for free after registration on the DHS programme using the following link:
https://www.dhsprogram.com/data/dataset_admin/login_main.cfm.
CRediT authorship contribution statement
Rugiranka Tony Gaston: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Shaun Ramroop: Validation, editing, Project administration, Resources, Supervision. Faustin Habyarimana: Validation, editing, Project administration, Resources, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors acknowledge the National Malaria Control Programme and the ICF through the DHS programme for providing the data.
References
- 1.Leal L.P., Batista Filho M., Lira P.I.C.D., Figueiroa J.N., Osório M.M. Prevalence of anemia and associated factors in children aged 6-59 months in Pernambuco, Northeastern Brazil. Rev. Saude Publica. 2011;45(3):457–466. doi: 10.1590/s0034-89102011000300003. [DOI] [PubMed] [Google Scholar]
- 2.Aheto J.M.K., Keegan T.J., Taylor B.M., Diggle P.J. Childhood malnutrition and its determinants among under‐five children in Ghana. Paediatr. Perinat. Epidemiol. 2015;29(6):552–561. doi: 10.1111/ppe.12222. [DOI] [PubMed] [Google Scholar]
- 3.Gaston R.T., Ramroop S., Habyarimana F. Joint modelling of malaria and anaemia in children less than five years of age in Malawi. Heliyon. 2021;7(5) doi: 10.1016/j.heliyon.2021.e06899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osterbauer B., Kapisi J., Bigira V., Mwangwa F., Kinara S., Kamya M.R., Dorsey G. Factors associated with malaria parasitaemia, malnutrition, and anaemia among HIV-exposed and unexposed Ugandan infants: a cross-sectional survey. Malar. J. 2012;11(1):1–6. doi: 10.1186/1475-2875-11-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kateera F., Ingabire C.M., Hakizimana E., Kalinda P., Mens P.F., Grobusch M.P., Mutesa L., van Vugt M. Malaria, anaemia and under-nutrition: three frequently co-existing conditions among preschool children in rural Rwanda. Malar. J. 2015;14(1):1–11. doi: 10.1186/s12936-015-0973-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumbele I.U.N., Kimbi H.K., Ndamukong-Nyanga J.L., Nweboh M., Anchang-Kimbi J.K., Lum E., Nana Y., Ndamukong K.K., Lehman L.G. Malarial anaemia and anaemia severity in apparently healthy primary school children in urban and rural settings in the Mount Cameroon area: cross sectional survey. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam M.A., Richard S.A., Fahim S.M., Mahfuz M., Nahar B., Das S., Shrestha B., Koshy B., Mduma E., Seidman J.C., Murray-Kolb L.E. Impact of early-onset persistent stunting on cognitive development at 5 years of age: results from a multi-country cohort study. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0227839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assaf S., Juan C. Stunting and anemia in children from urban poor environments in 28 low and middle-income countries: a meta-analysis of demographic and health survey data. Nutrients. 2020;12(11):3539. doi: 10.3390/nu12113539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman A., De Sanctis V., Alaaraj N., Ahmed S., Alyafei F., Hamed N., Soliman N. Early and long-term consequences of nutritional stunting: from childhood to adulthood. Acta Biomed.: Atenei parmensis. 2021;92(1) doi: 10.23750/abm.v92i1.11346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirombo J., Lowe R., Kazembe L. Using structured additive regression models to estimate risk factors of malaria: analysis of 2010 Malawi malaria indicator survey data. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaston R.T., Ramroop S. Prevalence of and factors associated with malaria in children under five years of age in Malawi, using malaria indicator survey data. Heliyon. 2020;6(5) doi: 10.1016/j.heliyon.2020.e03946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . vol. 3. World Health Organization; 2015. (Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Tropical Diseases 2015). [Google Scholar]
- 13.Kejo D., Petrucka P.M., Martin H., Kimanya M.E., Mosha T.C. Prevalence and predictors of anemia among children under 5 years of age in Arusha District, Tanzania. Pediatr. Health Med. Therapeut. 2018;9:9. doi: 10.2147/PHMT.S148515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakwe N., Bigoga J., Ngondi J., Njeambosay B., Esemu L., Kouambeng C., Nyonglema P., Seumen C., Gouado I., Oben J. Relationship between malaria, anaemia, nutritional and socio-economic status amongst under-ten children, in the North Region of Cameroon: a cross-sectional assessment. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0218442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCuskee S., Brickley E.B., Wood A., Mossialos E. Malaria and macronutrient deficiency as correlates of anemia in young children: a systematic review of observational studies. Annals of global health. 2014;80(6):458–465. doi: 10.1016/j.aogh.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Gaston R.T., Ramroop S., Habyarimana F. Determinants of factors associated with anaemia among children under five years in Lesotho. Afr. Popul. Stud. 2018;32(1) [Google Scholar]
- 17.World Health Organization . Switzerland World Health Organization; Geneva: 2007. WHO Child Growth Standards: Head Circumference-For-Age, Arm Circumference-For-Age, Triceps Skinfold-For-Age and Subscapular Skinfold-For-Age: Methods and Development. 2007. [Google Scholar]
- 18.Gaston R.T., Habyarimana F., Ramroop S. Joint modelling of anaemia and stunting in children less than five years of age in Lesotho: a cross-sectional case study. BMC Publ. Health. 2022;22(1):1–11. doi: 10.1186/s12889-022-12690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Das D., Grais R.F., Okiro E.A., Stepniewska K., Mansoor R., Van Der Kam S., Terlouw D.J., Tarning J., Barnes K.I., Guerin P.J. Complex interactions between malaria and malnutrition: a systematic literature review. BMC Med. 2018;16(1):1–14. doi: 10.1186/s12916-018-1177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cormack B.E., Embleton N.D., van Goudoever J.B., Hay W.W., Bloomfield F.H. Comparing apples with apples: it is time for standardized reporting of neonatal nutrition and growth studies. Pediatr. Res. 2016;79(6):810–820. doi: 10.1038/pr.2016.26. [DOI] [PubMed] [Google Scholar]
- 21.Urbano J., Lima H., Hanjalic A. Proceedings of the 42nd International ACM SIGIR Conference on Research and Development in Information Retrieval. 2019, July. A new perspective on score standardization; pp. 1061–1064. [Google Scholar]
- 22.Gardner W., Kassebaum N. Global, regional, and national prevalence of anemia and its causes in 204 countries and territories, 1990–2019. Curr. Dev. Nutr. 2020;4(2):830. [Google Scholar]
- 23.Wali N., Agho K.E., Renzaho A.M. Factors associated with stunting among children under 5 years in five South Asian countries (2014–2018): analysis of demographic health surveys. Nutrients. 2020;12(12):3875. doi: 10.3390/nu12123875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moise I.K. Causes of morbidity and mortality among neonates and children in post-conflict Burundi: a cross-sectional retrospective study. Children. 2018;5(9):125. doi: 10.3390/children5090125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takele K., Zewotir T., Ndanguza D. A combined model of child malnutrition and morbidity in Ethiopia using structural equation models. Sci. Rep. 2023;13(1):471. doi: 10.1038/s41598-023-27440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes A.F. Partial, conditional, and moderated moderated mediation: quantification, inference, and interpretation. Commun. Monogr. 2018;85(1):4–40. [Google Scholar]
- 27.Vinodh S., Aravindraj S., Pushkar B., Kishore S. Estimation of reliability and validity of agility constructs using structural equation modelling. Int. J. Prod. Res. 2012;50(23):6737–6745. [Google Scholar]
- 28.Verhoef H., West C.E., Veenemans J., Beguin Y., Kok F.J. Stunting may determine the severity of malaria-associated anemia in African children. Pediatrics. 2002;110(4) doi: 10.1542/peds.110.4.e48. [DOI] [PubMed] [Google Scholar]
- 29.Calis J.C., Phiri K.S., Faragher E.B., Brabin B.J., Bates I., Cuevas L.E., de Haan R.J., Phiri A.I., Malange P., Khoka M., Hulshof P.J. Research article (new england journal of medicine) severe anemia in Malawian children. Malawi Med. J. 2016;28(3):99–107. [PMC free article] [PubMed] [Google Scholar]
- 30.Teh R.N., Sumbele I.U.N., Meduke D.N., Ojong S.T., Kimbi H.K. Malaria parasitaemia, anaemia and malnutrition in children less than 15 years residing in different altitudes along the slope of Mount Cameroon: prevalence, intensity and risk factors. Malar. J. 2018;17(1):1–13. doi: 10.1186/s12936-018-2492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gould J., Golob T.F. Shopping without travel or travel without shopping, an investigation of electronic home shopping. Transport Rev. 1997;17(4):355–376. [Google Scholar]
- 32.Golob T.F. Structural equation modeling for travel behavior research. Transp. Res. Part B Methodol. 2003;37(1):1–25. [Google Scholar]
- 33.Lowry P.B., Gaskin J. Partial least squares (PLS) structural equation modeling (SEM) for building and testing behavioral causal theory: when to choose it and how to use it. IEEE transactions on professional communication. 2014;57(2):123–146. [Google Scholar]
- 34.Bollen K.A. John Wiley & Sons; New York: 1989. Structural Equations with Latent Variables. [Google Scholar]
- 35.Fan Y., Chen J., Shirkey G., John R., Wu S.R., Park H., Shao C. Applications of structural equation modeling (SEM) in ecological studies: an updated review. Ecological processes. 2016;5(1):1–12. [Google Scholar]
- 36.Caraka R.E., Noh M., Chen R.C., Lee Y., Gio P.U., Pardamean B. Connecting climate and communicable disease to penta helix using hierarchical likelihood structural equation modelling. Symmetry. 2021;13(4):657. [Google Scholar]
- 37.Bollen K.A. John Wiley & Sons; New York: 1989. Structural Equations with Latent Variables. [Google Scholar]
- 38.Austin J.T., Wolfle L.M. Annotated bibliography of structural equation modelling: technical work. Br. J. Math. Stat. Psychol. 1991;44(1):93–152. doi: 10.1111/j.2044-8317.1991.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 39.Kaplan D. second ed. SAGE publications; California, USA: 2008. Structural Equation Modeling: Foundations and Extensions. [Google Scholar]
- 40.Byrne B.M. Routledge; New York: 2013. Structural Equation Modeling with Mplus: Basic Concepts, Applications, and Programming. [Google Scholar]
- 41.Feasey N.A., Everett D., Faragher E.B., Roca-Feltrer A., Kang’ombe A., Denis B., Kerac M., Molyneux E., Molyneux M., Jahn A., Gordon M.A. Modelling the contributions of malaria, HIV, malnutrition and rainfall to the decline in paediatric invasive non-typhoidal Salmonella disease in Malawi. PLoS Neglected Trop. Dis. 2015;9(7) doi: 10.1371/journal.pntd.0003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollen K.A., Hoyle R.H. In: Handbook of Structural Equation Modeling. Hoyle R.H., editor. The Guilford Press; New York: 2012. Latent variables in structural equation modeling; pp. 56–67. [Google Scholar]
- 43.Schumacker R.E., Lomax R.G. fourth ed. Routledge; New York, USA: 2016. A Beginner's Guide to Structural Equation Modeling. [Google Scholar]
- 44.Tine R.C., Ndiaye M., Hansson H.H., Ndour C.T., Faye B., Alifrangis M., Sylla K., Ndiaye J.L., Magnussen P., Bygbjerg I.C., Gaye O. The association between malaria parasitaemia, erythrocyte polymorphisms, malnutrition and anaemia in children less than 10 years in Senegal: a case control study. BMC Res. Notes. 2012;5(1):1–10. doi: 10.1186/1756-0500-5-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinyoki D.K., Moloney G.M., Uthman O.A., Odundo E.O., Kandala N.B., Noor A.M., Snow R.W., Berkley J.A. Co-morbidity of malnutrition with falciparum malaria parasitaemia among children under the aged 6–59 months in Somalia: a geostatistical analysis. Infectious diseases of poverty. 2018;7(1):1–9. doi: 10.1186/s40249-018-0449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ministry at the Presidency in charge of good governance and planning [Burundi] (MPBGP) Ministry of health and the fight against AID [Burundi] (MPLS) Institute of Statistics and Economic Studies of Burundi (ISTEEBU), and ICF. ISTEEBU, MSPLS, ICF; Bujumbura, Burundi: 2017. Third Demographic and Health Survey 2016-17; pp. 1–668. [Google Scholar]
- 47.Sinzinkayo D., Baza D., Gnanguenon V., Koepfli C. The lead-up to epidemic transmission: malaria trends and control interventions in Burundi 2000 to 2019. Malar. J. 2021;20(1):1–7. doi: 10.1186/s12936-021-03830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moise I.K., Roy S.S., Nkengurutse D., Ndikubagenzi J. Seasonal and geographic variation of pediatric malaria in Burundi: 2011 to 2012. Int. J. Environ. Res. Publ. Health. 2016;13(4):425. doi: 10.3390/ijerph13040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nimpagaritse M., Korachais C., Meessen B. Effects in spite of tough constraints-A theory of change based investigation of contextual and implementation factors affecting the results of a performance based financing scheme extended to malnutrition in Burundi. PLoS One. 2020;15(1) doi: 10.1371/journal.pone.0226376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bentler P.M. Comparative fit indexes in structural models. Psychol. Bull. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 51.Bliznashka L., Arsenault J.E., Becquey E., Ruel M.T., Olney D.K. Using structural equation modelling to understand the contributors to anaemia among young Burkinabe children. Matern. Child Nutr. 2020;16(1) doi: 10.1111/mcn.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chavance M., Escolano S., Romon M., Basdevant A., de Lauzon-Guillain B., Charles M.A. Latent variables and structural equation models for longitudinal relationships: an illustration in nutritional epidemiology. BMC Med. Res. Methodol. 2010;10(1):1–10. doi: 10.1186/1471-2288-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ainur A.K., Sayang M.D., Jannoo Z., Yap B.W. Sample size and non-normality effects on goodness of fit measures in structural equation models. Pertanika journal of science & technology. 2017;25(2):575–586. [Google Scholar]
- 54.Grace J.B., Anderson T.M., Olff H., Scheiner S.M. On the specification of structural equation models for ecological systems. Ecol. Monogr. 2010;80(1):67–87. [Google Scholar]
- 55.Jöreskog K.G., Sörbom D. Recent developments in structural equation modeling. J. Market. Res. 1982;19(4):404–416. [Google Scholar]
- 56.Marsh H.W., Hocevar D. Application of confirmatory factor analysis to the study of self-concept: first-and higher order factor models and their invariance across groups. Psychol. Bull. 1985;97(3):562. [Google Scholar]
- 57.West S.G., Taylor A.B., Wu W. Model fit and model selection in structural equation modeling. Handbook of structural equation modeling. 2012;1:209–231. [Google Scholar]
- 58.Raykov T., Marcoulides G.A. second ed. Routledge; New York, USA: 2012. A First Course in Structural Equation Modeling. [Google Scholar]
- 59.Kline R.B. fourth ed. Guilford press; New York, USA: 2015. Principles and Practice of Structural Equation Modeling. [Google Scholar]
- 60.Ditlevsen S., Christensen U., Lynch J., Damsgaard M.T., Keiding N. The mediation proportion: a structural equation approach for estimating the proportion of exposure effect on outcome explained by an intermediate variable. Epidemiology. 2005;16(1):114–120. doi: 10.1097/01.ede.0000147107.76079.07. [DOI] [PubMed] [Google Scholar]
- 61.Kock N., Lynn G. Lateral collinearity and misleading results in variance-based SEM: an illustration and recommendations. J. Assoc. Inf. Syst. Online. 2012;13(7):546–580. [Google Scholar]
- 62.Bryman A., Bell E. Reliability and validity in qualitative research. Business Research Methods. 2011;2:215–243. [Google Scholar]
- 63.Bonett D.G., Wright T.A. Cronbach's alpha reliability: interval estimation, hypothesis testing, and sample size planning. J. Organ. Behav. 2015;36(1):3–15. [Google Scholar]
- 64.Bujang M.A., Omar E.D., Baharum N.A. A review on sample size determination for Cronbach's alpha test: a simple guide for researchers. Malays. J. Med. Sci.: MJMS. 2018;25(6):85. doi: 10.21315/mjms2018.25.6.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagozzi R.P., Foxall G.R. Construct validation of a measure of adaptive-innovative cognitive styles in consumption. Int. J. Res. Market. 1996;13(3):201–213. [Google Scholar]
- 66.Carpentier J., Mageau G.A., Vallerand R.J. Ruminations and flow: why do people with a more harmonious passion experience higher well-being? J. Happiness Stud. 2012;13(3):501–518. [Google Scholar]
- 67.Hooper D., Coughlan J., Mullen M.R. Structural equation modelling: guidelines for determining model fit. Electron. J. Bus. Res. Methods. 2008;6(1):53–60. [Google Scholar]
- 68.Bentler P.M., Wu E.J. Multivariate Software; Encino, CA: 2005. EQS 6.1 for Windows. Structural Equations Program Manual. [Google Scholar]
- 69.Cassarino M., Setti A. Environment as ‘Brain Training’: a review of geographical and physical environmental influences on cognitive ageing. Ageing Res. Rev. 2015;23:167–182. doi: 10.1016/j.arr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Yu H., Peng Z.R. Exploring the spatial variation of ridesourcing demand and its relationship to built environment and socioeconomic factors with the geographically weighted Poisson regression. J. Transport Geogr. 2019;75:147–163. [Google Scholar]
- 71.Adebayo S.B., Gayawan E., Heumann C., Seiler C. Joint modeling of Anaemia and Malaria in children under five in Nigeria. Spatial and spatio-temporal epidemiology. 2016;17:105–115. doi: 10.1016/j.sste.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 72.Soria R., Egger M., Scherrer U., Bender N., Rimoldi S.F. Pulmonary artery pressure and arterial oxygen saturation in people living at high or low altitude: systematic review and meta-analysis. Journal of applied physiology. 2016;121(5):1151–1159. doi: 10.1152/japplphysiol.00394.2016. [DOI] [PubMed] [Google Scholar]
- 73.Seyoum S. Analysis of prevalence of malaria and anemia using bivariate probit model. Annals of Data Science. 2018;5(2):301–312. [Google Scholar]
- 74.Das D., Grais R.F., Okiro E.A., Stepniewska K., Mansoor R., Van Der Kam S., Terlouw D.J., Tarning J., Barnes K.I., Guerin P.J. Complex interactions between malaria and malnutrition: a systematic literature review. BMC Med. 2018;16(1):1–14. doi: 10.1186/s12916-018-1177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price R.N., Simpson J.A., Nosten F., Luxemburger C., Hkirjaroen L., ter Kuile F.E.I.K.O., Chongsuphajaisiddhi T., White N.J. Factors contributing to anemia after uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 2001;65(5):614. doi: 10.4269/ajtmh.2001.65.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used during this study can be accessed for free after registration on the DHS programme using the following link:
https://www.dhsprogram.com/data/dataset_admin/login_main.cfm.