Abstract
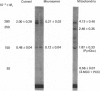
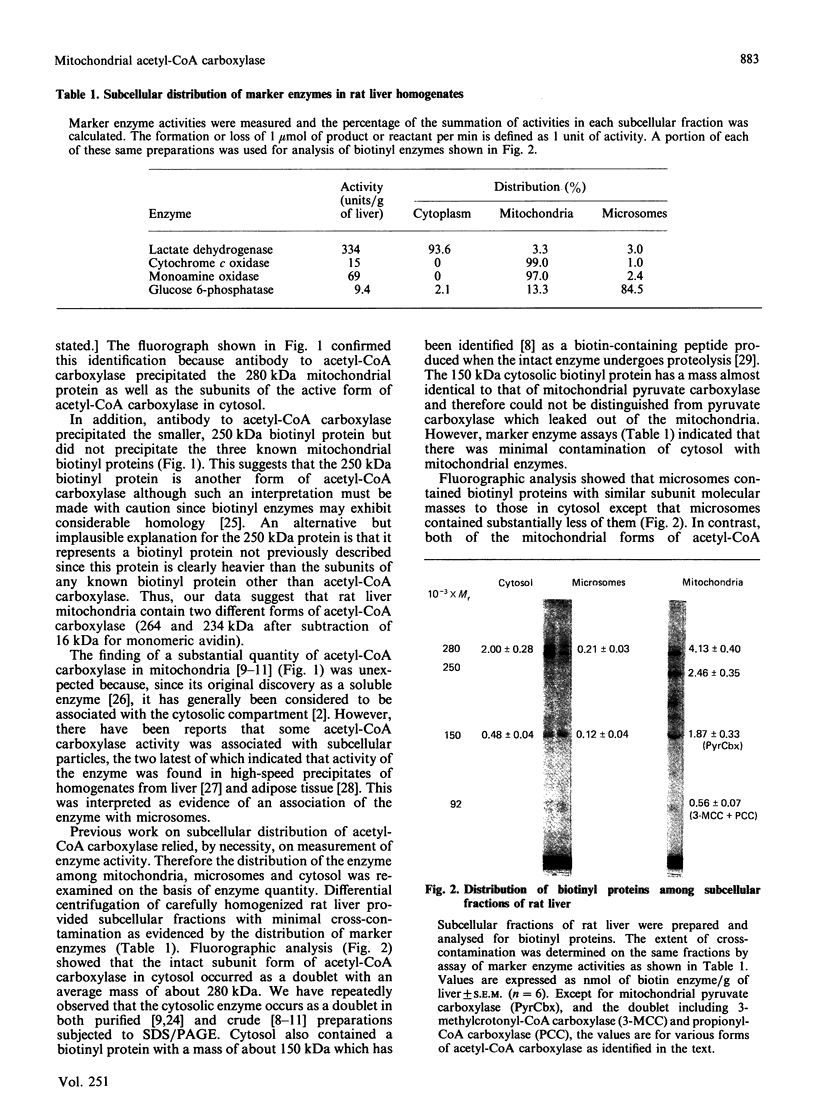
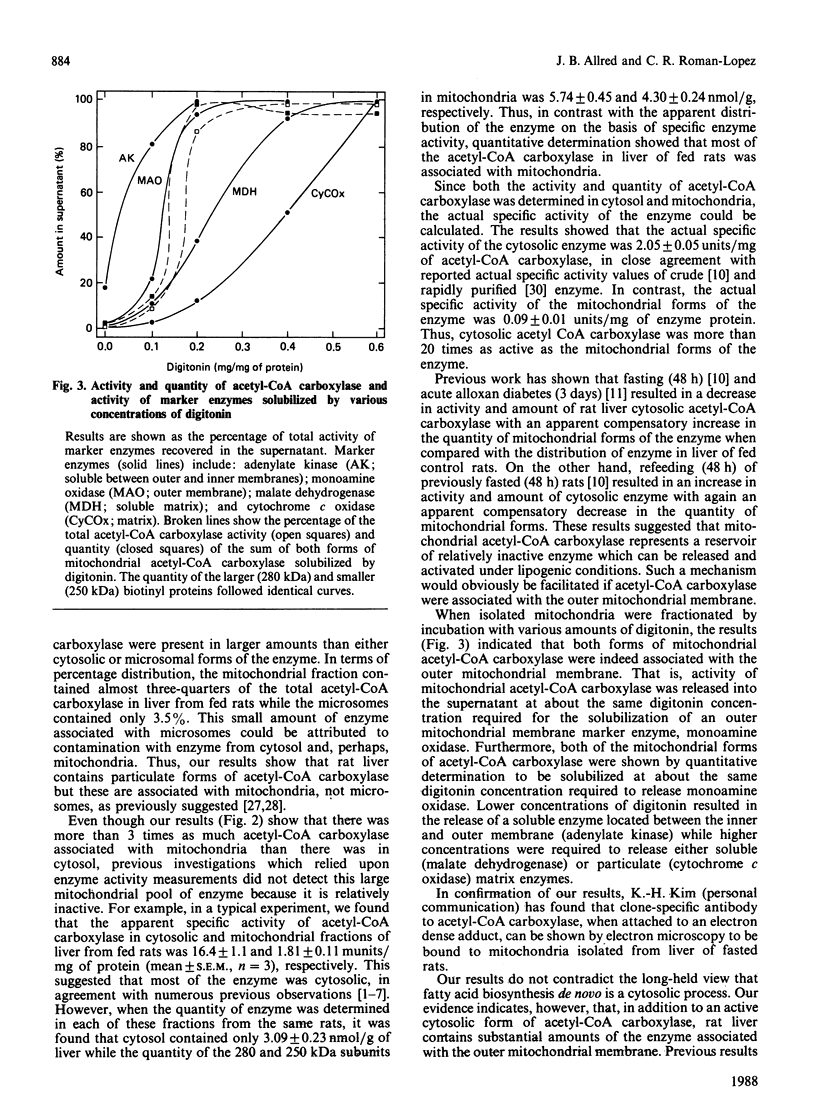
Biotinyl proteins were labelled by incubation of SDS-denatured preparations of subcellular fractions of rat liver with [14C]methylavidin before polyacrylamide-gel electrophoresis. Fluorographic analysis showed that mitochondria contained two forms of acetyl-CoA carboxylase [acetyl-CoA:carbon dioxide ligase (ADP-forming) EC 6.4.1.2], both of which were precipitated by antibody to the enzyme. When both forms were considered, almost three-quarters of the total liver acetyl-CoA carboxylase was found in the mitochondrial fraction of liver from fed rats while only 3.5% was associated with the microsomal fraction. The remainder was present in cytosol, either as the intact active enzyme or as a degradation product. The actual specific activity of the cytosolic enzyme was approx. 2 units/mg of acetyl-CoA carboxylase protein while that of the mitochondrial enzyme was about 20-fold lower, indicating that mitochondrial acetyl-CoA carboxylase was relatively inactive. Fractionation of mitochondria with digitonin showed that acetyl-CoA carboxylase was associated with the outer mitochondrial membrane. The available evidence suggests that mitochondrial acetyl-CoA carboxylase represents a reservoir of enzyme which can be released and activated under lipogenic conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred J. B., Goodson J. Does pyruvate carboxylase interfere with the radioactive bicarbonate fixation assay of acetyl-CoA carboxylase? Biochem J. 1982 Oct 15;208(1):247–248. doi: 10.1042/bj2080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allred J. B., Harris G. J., Goodson J. Regulation of purified rat liver acetyl CoA carboxylase by phosphorylation. J Lipid Res. 1983 Apr;24(4):449–455. [PubMed] [Google Scholar]
- Allred J. B., Roehrig K. L. Heat activation of rat liver acetyl-CoA carboxylase in vitro. J Biol Chem. 1978 Jul 25;253(14):4826–4829. [PubMed] [Google Scholar]
- Allred J. B., Roman-Lopez C. R., Pope T. S., Goodson J. Dietary dependent distribution of acetyl CoA carboxylase between cytoplasm and mitochondria of rat liver. Biochem Biophys Res Commun. 1985 Jun 14;129(2):453–460. doi: 10.1016/0006-291x(85)90172-x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M. Use of fluorography for sensitive isotope detection in polyacrylamide gel electrophoresis and related techniques. Methods Enzymol. 1983;96:215–222. doi: 10.1016/s0076-6879(83)96019-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Goodson J., Pope T. S., Allred J. B. Molecular weights of subunits of acetyl CoA carboxylase in rat liver cytoplasm. Biochem Biophys Res Commun. 1984 Jul 31;122(2):694–699. doi: 10.1016/s0006-291x(84)80089-3. [DOI] [PubMed] [Google Scholar]
- Greenawalt J. W. The isolation of outer and inner mitochondrial membranes. Methods Enzymol. 1974;31:310–323. doi: 10.1016/0076-6879(74)31033-6. [DOI] [PubMed] [Google Scholar]
- Imesch E., Wolczunowicz M., Rous S. Enzymatic activities of cytoplasmic and of microsomal acetyl-CoA carboxylase of rat epididymal adipose tissue; different regulatory effects of a short-term fasting and palmitoyl-CoA on these two enzymes. Int J Biochem. 1983;15(7):977–980. doi: 10.1016/0020-711x(83)90178-7. [DOI] [PubMed] [Google Scholar]
- Inoue H., Lowenstein J. M. Acetyl coenzyme A carboxylase from rat liver. Purification and demonstration of different subunits. J Biol Chem. 1972 Aug 10;247(15):4825–4832. [PubMed] [Google Scholar]
- Kim K. H. Regulation of acetyl-CoA carboxylase. Curr Top Cell Regul. 1983;22:143–176. doi: 10.1016/b978-0-12-152822-5.50009-9. [DOI] [PubMed] [Google Scholar]
- Lane M. D., Moss J., Ryder E., Stoll E. The activation of acetyl CoA carboxylase by tricarboxylic acids. Adv Enzyme Regul. 1970;9:237–251. doi: 10.1016/s0065-2571(71)80047-x. [DOI] [PubMed] [Google Scholar]
- Majerus P. W., Kilburn E. Acetyl coenzyme A carboxylase. The roles of synthesis and degradation in regulation of enzyme levels in rat liver. J Biol Chem. 1969 Nov 25;244(22):6254–6262. [PubMed] [Google Scholar]
- Nakanishi S., Numa S. Purification of rat liver acetyl coenzyme A carboxylase and immunochemical studies on its synthesis and degradation. Eur J Biochem. 1970 Sep;16(1):161–173. doi: 10.1111/j.1432-1033.1970.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Roman-Lopez C. R., Allred J. B. Acute alloxan diabetes alters the activity but not the total quantity of acetyl CoA carboxylase in rat liver. J Nutr. 1987 Nov;117(11):1976–1981. doi: 10.1093/jn/117.11.1976. [DOI] [PubMed] [Google Scholar]
- Roman-Lopez C. R., Goodson J., Allred J. B. Determination of the quantity of acetyl CoA carboxylase by [14C]methyl avidin binding. J Lipid Res. 1987 May;28(5):599–604. [PubMed] [Google Scholar]
- Romsos D. R., Leveille G. A. Effect of diet on activity of enzymes involved in fatty acid and cholesterol synthesis. Adv Lipid Res. 1974;12(0):97–146. [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. S., Kim K. H. Reevaluation of properties of acetyl-CoA carboxylase from rat liver. J Biol Chem. 1981 Aug 10;256(15):7786–7788. [PubMed] [Google Scholar]
- TABOR C. W., TABOR H., ROSENTHAL S. M. Purification of amine oxidase from beef plasma. J Biol Chem. 1954 Jun;208(2):645–661. [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Mechanisms and regulation of biosynthesis of saturated fatty acids. Physiol Rev. 1976 Apr;56(2):339–417. doi: 10.1152/physrev.1976.56.2.339. [DOI] [PubMed] [Google Scholar]
- WAKIL S. J., TITCHENER E. B., GIBSON D. M. Evidence for the participation of biotin in the enzymic synthesis of fatty acids. Biochim Biophys Acta. 1958 Jul;29(1):225–226. doi: 10.1016/0006-3002(58)90177-x. [DOI] [PubMed] [Google Scholar]
- Wilson J. E. Brain hexokinase, the prototype ambiquitous enzyme. Curr Top Cell Regul. 1980;16:1–54. doi: 10.1016/b978-0-12-152816-4.50005-4. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Friedman S. A., Bacon G. W. Microsomal acetyl-CoA carboxylase: evidence for association of enzyme polymer with liver microsomes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3639–3643. doi: 10.1073/pnas.78.6.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood H. G., Barden R. E. Biotin enzymes. Annu Rev Biochem. 1977;46:385–413. doi: 10.1146/annurev.bi.46.070177.002125. [DOI] [PubMed] [Google Scholar]