Abstract
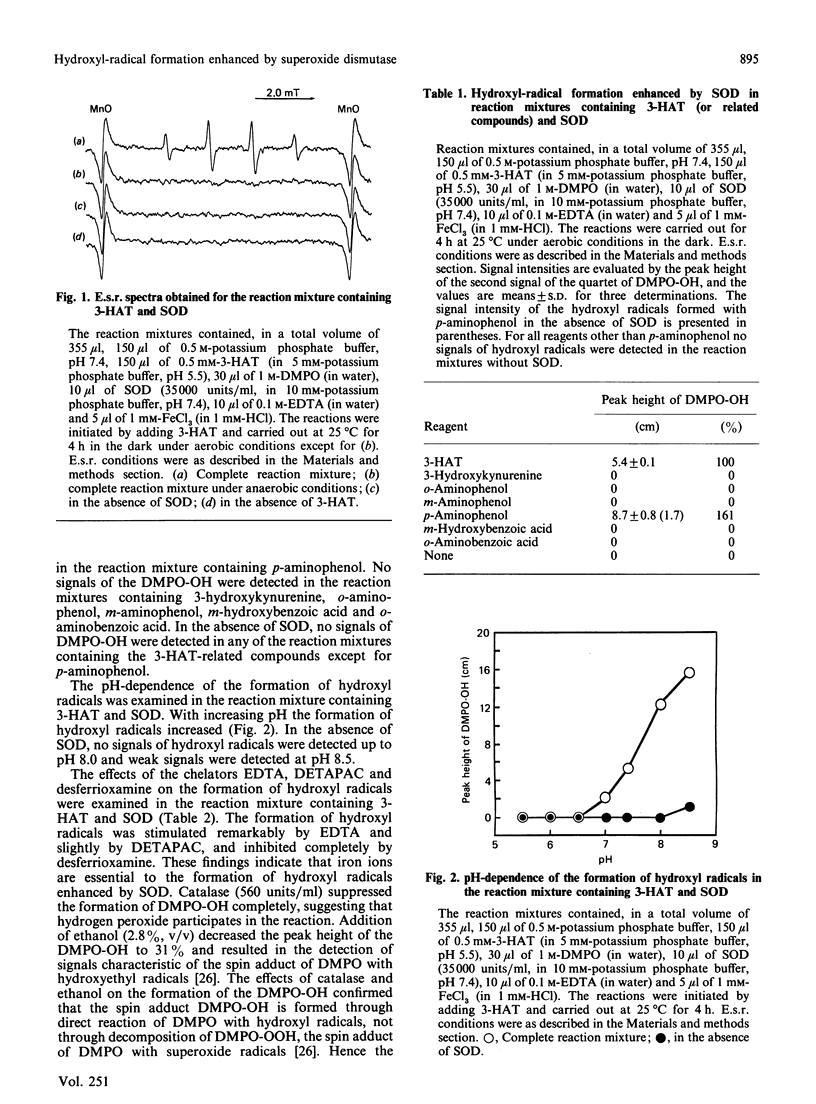
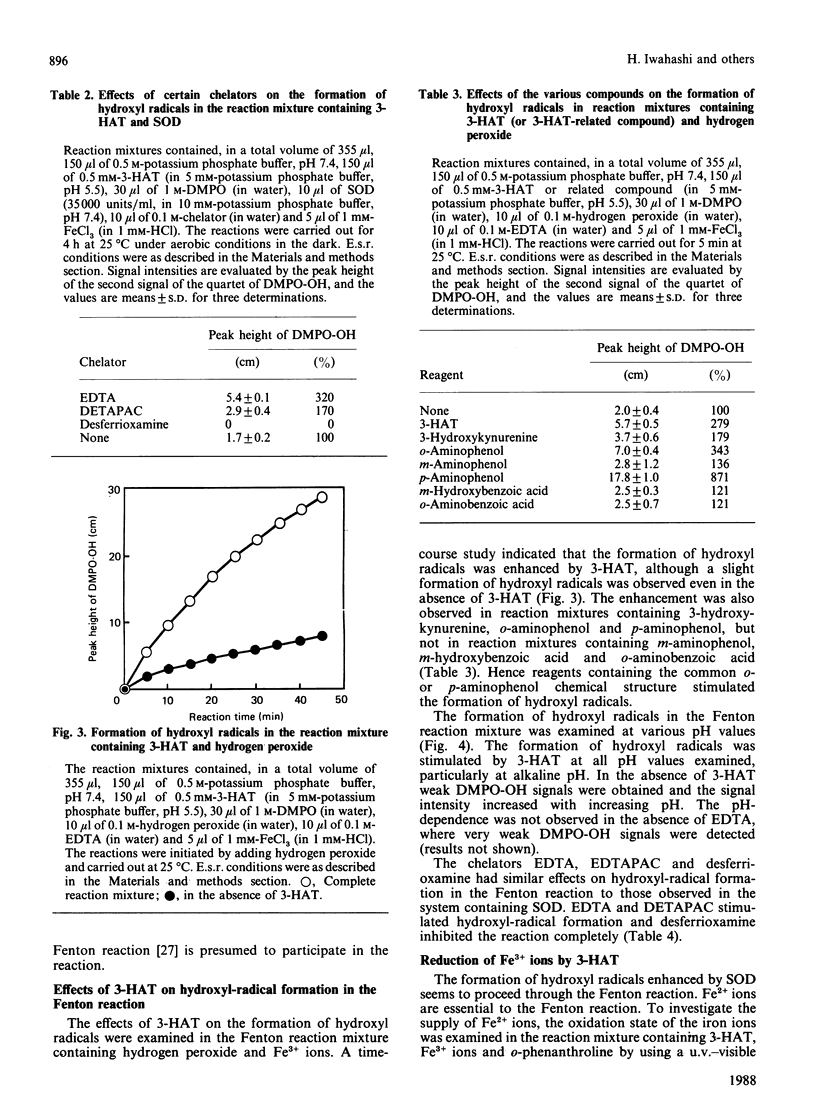
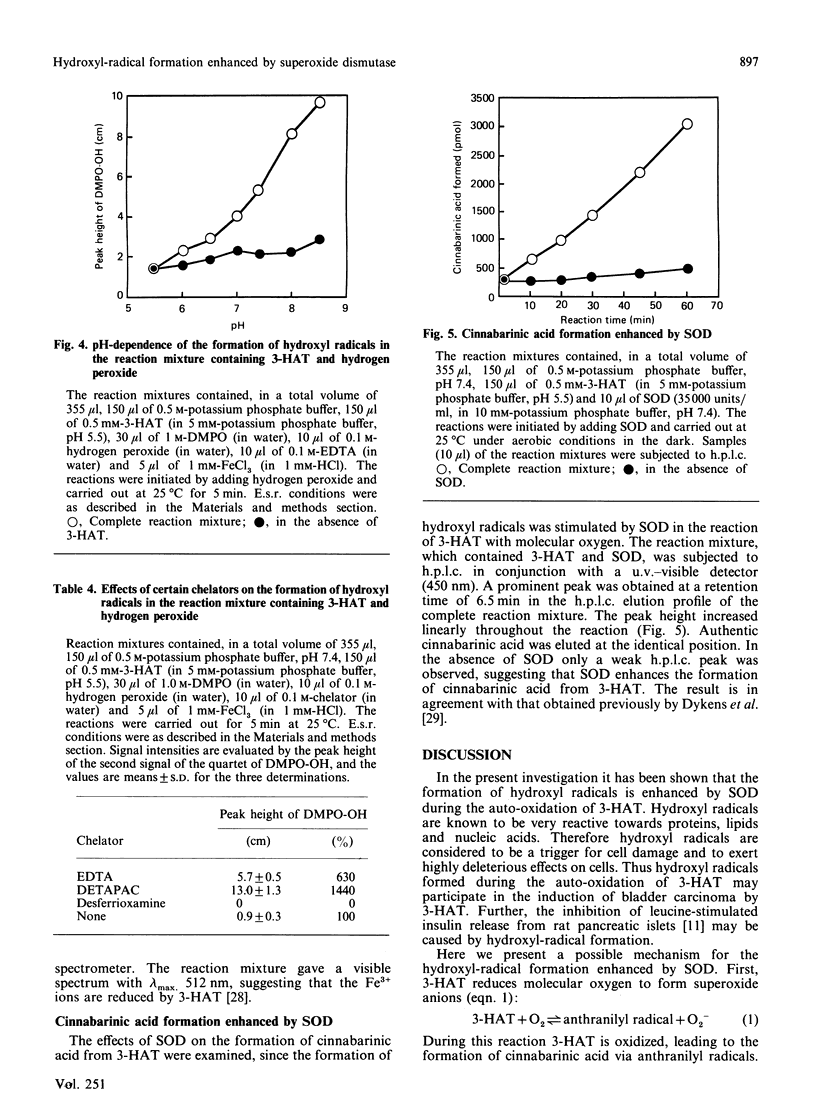
Superoxide dismutase (SOD) enhanced the formation of hydroxyl radicals, which were detected by using the e.s.r. spin-trapping technique, in a reaction mixture containing 3-hydroxyanthranilic acid (or p-aminophenol), Fe3+ ions, EDTA and potassium phosphate buffer, pH 7.4. The hydroxyl-radical formation enhanced by SOD was inhibited by catalase and desferrioxamine, and stimulated by EDTA and diethylenetriaminepenta-acetic acid, suggesting that both hydrogen peroxide and iron ions participate in the reaction. The hydroxyl-radical formation enhanced by SOD may be considered to proceed via the following steps. First, 3-hydroxyanthranilic acid is spontaneously auto-oxidized in a process that requires molecular oxygen and yields superoxide anions and anthranilyl radicals. This reaction seems to be reversible. Secondly, the superoxide anions formed in the first step are dismuted by SOD to generate hydrogen peroxide and molecular oxygen, and hence the equilibrium in the first step is displaced in favour of the formation of superoxide anions. Thirdly, hydroxyl radicals are generated from hydrogen peroxide through the Fenton reaction. In this Fenton reaction Fe2+ ions are available since Fe3+ ions are readily reduced by 3-hydroxyanthranilic acid. The superoxide anions do not seem to participate in the reduction of Fe3+ ions, since superoxide anions are rapidly dismuted by SOD present in the reaction mixture.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABUL-FADL M. A., KHALAFALLAH A. S. Studies on the urinary excretion of certain tryptophan metabolites in bilharziasis and its possible relation to bladder cancer in Egypt. Br J Cancer. 1961 Sep;15:479–482. doi: 10.1038/bjc.1961.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN M. J., BOYLAND E., DUKES C. E., HORNING E. S., WATSON J. G. Cancer of the urinary bladder induced in mice with metabolites of aromatic amines and tryptophan. Br J Cancer. 1957 Jun;11(2):212–228. doi: 10.1038/bjc.1957.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B., Nordenskjöld M., Rahimtula A., Moldéus P. Prostaglandin synthetase-catalyzed activation of phenacetin metabolites to genotoxic products. Mol Pharmacol. 1982 Sep;22(2):479–485. [PubMed] [Google Scholar]
- BOYLAND E., WILLIAMS D. C. The metabolism of tryptophan. 2. The metabolism of tryptophan in patients suffering from cancer of the bladder. Biochem J. 1956 Nov;64(3):578–582. doi: 10.1042/bj0640578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYAN G. T., BROWN R. R., PRICE J. M. INCIDENCE OF MOUSE BLADDER TUMORS FOLLOWING IMPLANTATION OF PARAFFIN PELLETS CONTAINING CERTAIN TRYPTOPHAN METABOLITES. Cancer Res. 1964 May;24:582–585. [PubMed] [Google Scholar]
- BRYAN G. T., BROWN R. R., PRICE J. M. MOUSE BLADDER CARCINOGENICITY OF CERTAIN TRYPTOPHAN METABOLITES AND OTHER AROMATIC NITROGEN COMPOUNDS SUSPENDED IN CHOLESTEROL. Cancer Res. 1964 May;24:596–602. [PubMed] [Google Scholar]
- Boyd J. A., Eling T. E. Prostaglandin endoperoxide synthetase-dependent cooxidation of acetaminophen to intermediates which covalently bind in vitro to rabbit renal medullary microsomes. J Pharmacol Exp Ther. 1981 Dec;219(3):659–664. [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Crowe C. A., Yong A. C., Calder I. C., Ham K. N., Tange J. D. The nephrotoxicity of p-aminophenol. I. The effect on microsomal cytochromes, glutathione and covalent binding in kidney and liver. Chem Biol Interact. 1979 Oct;27(2-3):235–243. doi: 10.1016/0009-2797(79)90128-5. [DOI] [PubMed] [Google Scholar]
- DUNNING W. F., CURTIS M. R., MAUN M. E. The effect of added dietary tryptophane on the occurrence of 2-acetylaminofluorene-induced liver and bladder cancer in rats. Cancer Res. 1950 Jul;10(7):454–459. [PubMed] [Google Scholar]
- Dykens J. A., Sullivan S. G., Stern A. Oxidative reactivity of the tryptophan metabolites 3-hydroxyanthranilate, cinnabarinate, quinolinate and picolinate. Biochem Pharmacol. 1987 Jan 15;36(2):211–217. doi: 10.1016/0006-2952(87)90691-5. [DOI] [PubMed] [Google Scholar]
- Finazzi-Agró A., Di Giulio A., Amicosante G., Crifó C. Photohemolysis of erythrocytes enriched with superoxide dismutase, catalase and glutathione peroxidase. Photochem Photobiol. 1986 Apr;43(4):409–412. doi: 10.1111/j.1751-1097.1986.tb05622.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein E., Rosen G. M., Rauckman E. J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch Biochem Biophys. 1980 Mar;200(1):1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- Goshima N., Wadano A., Miura K. 3-Hydroxykynurenine as O2-. scavenger in the blowfly, Aldrichina grahami. Biochem Biophys Res Commun. 1986 Sep 14;139(2):666–672. doi: 10.1016/s0006-291x(86)80042-0. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Job D., Dunford H. B. Substituent effect on the oxidation of phenols and aromatic amines by horseradish peroxidase compound I. Eur J Biochem. 1976 Jul 15;66(3):607–614. doi: 10.1111/j.1432-1033.1976.tb10588.x. [DOI] [PubMed] [Google Scholar]
- Josephy P. D., Eling T. E., Mason R. P. Oxidation of p-aminophenol catalyzed by horseradish peroxidase and prostaglandin synthase. Mol Pharmacol. 1983 Mar;23(2):461–466. [PubMed] [Google Scholar]
- MUSAJO L., BENASSI C. A., PARPAJOLA A. Isolation of Kynurenine and 3-hydroxykynurenine from human pathological urine. Nature. 1955 May 14;175(4463):855–856. doi: 10.1038/175855a0. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Ogawa H., Nagamura Y., Ishiguro I. Cinnabarinic acid formation in Malpighian tubules of the silkworm, Bombyx mori. Participation of catalase in cinnabarinic acid formation in the presence of manganese ion. Hoppe Seylers Z Physiol Chem. 1983 Aug;364(8):1059–1066. doi: 10.1515/bchm2.1983.364.2.1059. [DOI] [PubMed] [Google Scholar]
- Rogers K. S., Evangelista S. J. 3-Hydroxykynurenine, 3-hydroxyanthranilic acid, and o-aminophenol inhibit leucine-stimulated insulin release from rat pancreatic islets. Proc Soc Exp Biol Med. 1985 Feb;178(2):275–278. doi: 10.3181/00379727-178-42010. [DOI] [PubMed] [Google Scholar]
- Rutkowski J. V., Ferm V. H. Comparison of the teratogenic effects of the isomeric forms of aminophenol in the Syrian golden hamster. Toxicol Appl Pharmacol. 1982 Apr;63(2):264–269. doi: 10.1016/0041-008x(82)90048-5. [DOI] [PubMed] [Google Scholar]
- Scott M. D., Meshnick S. R., Eaton J. W. Superoxide dismutase-rich bacteria. Paradoxical increase in oxidant toxicity. J Biol Chem. 1987 Mar 15;262(8):3640–3645. [PubMed] [Google Scholar]
- Tomoda A., Shirasawa E., Nagao S., Minami M., Yoneyama Y. Involvement of oxidoreductive reactions of intracellular haemoglobin in the metabolism of 3-hydroxyanthranilic acid in human erythrocytes. Biochem J. 1984 Sep 15;222(3):755–760. doi: 10.1042/bj2220755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenser T. V., Mattammal M. B., Armbrecht H. J., Davis B. B. Benzidine binding to nucleic acids mediated by the peroxidative activity of prostaglandin endoperoxide synthetase. Cancer Res. 1980 Aug;40(8 Pt 1):2839–2845. [PubMed] [Google Scholar]


