Abstract
Background
Extravasation (EV), or the leakage of anticancer drugs into perivascular and subcutaneous tissues during intravenous administration, can cause serious conditions that may require surgical intervention. Therefore, updated guidelines for EV based on systematic review are needed. Additionally, classifications for anticancer drugs that cause EV are not standardized across the current guidelines, and some novel drugs have not been classified. Therefore, this study aimed to formulate guidelines using evidence-based information for shared decision making on prevention, early detection, treatment, and care for EV in Japan and provide additional classification for tissue injury based on systematic review.
Materials and methods
The members of the Japanese Society of Cancer Nursing (JSCN), Japanese Society of Medical Oncology (JSMO), and Japanese Society of Pharmaceutical Oncology (JASPO) were surveyed about significant clinical challenges related to EV, and 17 clinical questions (CQs) were formulated. PubMed and ICHUSHI Web were searched using the Patient, Intervention, Comparison, and Outcomes terms listed in each CQ as key words. For the classification of new drugs, articles published through February 2021 were selected using the search terms ‘extravasation’, ‘injection-site reaction’, ‘adverse events’, and the names of individual drugs as key words.
Results
Recommendations based on the results of randomized controlled trials (RCTs) were made with regard to the selection of central venous (CV) devices (CQ2, CQ3a, CQ3b, and CQ3c), regular replacement of peripheral venous catheters (CQ5), and use of fosaprepitant (CQ7). These CQs are novel and were not mentioned in previous guidelines. Warm compression monotherapy (CQ10b) and local injection of steroids (CQ12) are discouraged for the management of EV. Ten new drugs were classified for EV tissue injury.
Conclusions
This study provides updated guidelines for the prevention and treatment of EV, which can be used to help health care providers and patients and their families practice better EV management.
Key words: extravasation, chemotherapy, injection-site reaction, adverse events, clinical practice guideline
Highlights
-
•
This JSCN/JSMO/JASPO EV guideline addressed 17 CQs related to the prevention, early detection, treatment, and care of EV.
-
•
This guideline is based on a systematic review and meta-analysis conforming to GRADE/MINDS 2020.
-
•
Based on RCTs, we recommended to use CV devices rather than peripheral veins, especially CV port for pts with solid tumor.
-
•
We conducted a systematic review on 31 drugs and a classification with 10 additional drugs was proposed.
-
•
Warm compression monotherapy and local injection of steroids were denied for EV based on best available evidence.
Introduction
Extravasation (EV) is the leakage of anticancer drugs into the perivascular and subcutaneous tissues during intravenous administration.1 EV can cause redness, swelling, pain, burning sensation, erosion, and blisters on the skin and surrounding tissues, and it sometimes causes ulceration, tissue necrosis, and other serious skin conditions that require surgical intervention.2 EV occurs in 0.1%-6.5% of patients receiving anticancer drugs.3 Although the overall incidence of EV is extremely low, many patients are at risk of EV, since it is caused by widely prescribed anticancer drugs.2
Anticancer drugs are classified as vesicants, irritants, or non-vesicants based on their potential to cause tissue injury by skin necrosis (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103932).2,4 Vesicants can cause tissue necrosis or formation of blisters. Irritants and non-vesicants are associated with a lower risk of necrosis unless leakage in large quantities occurs; however, they still pose a risk of tissue destruction and can cause inflammation and pain. Therefore, prevention of EV is important for all drugs.
Although prevention, diagnosis, treatment, and care of EV are important, there is limited evidence about the optimal procedures. Multiple guidelines for EV have been published.2,5, 6, 7 However, no EV guideline has been based on a systematic review. Additionally, the classification of tissue injury from anticancer drugs differs between guidelines, which is an important issue in assessing the risk of EV. Therefore, this study aimed to formulate guidelines using evidence-based information for shared decision making on prevention, early detection, treatment, and care for EV in Japan and provide additional classification for tissue injury based on a systematic review.
Materials and methods
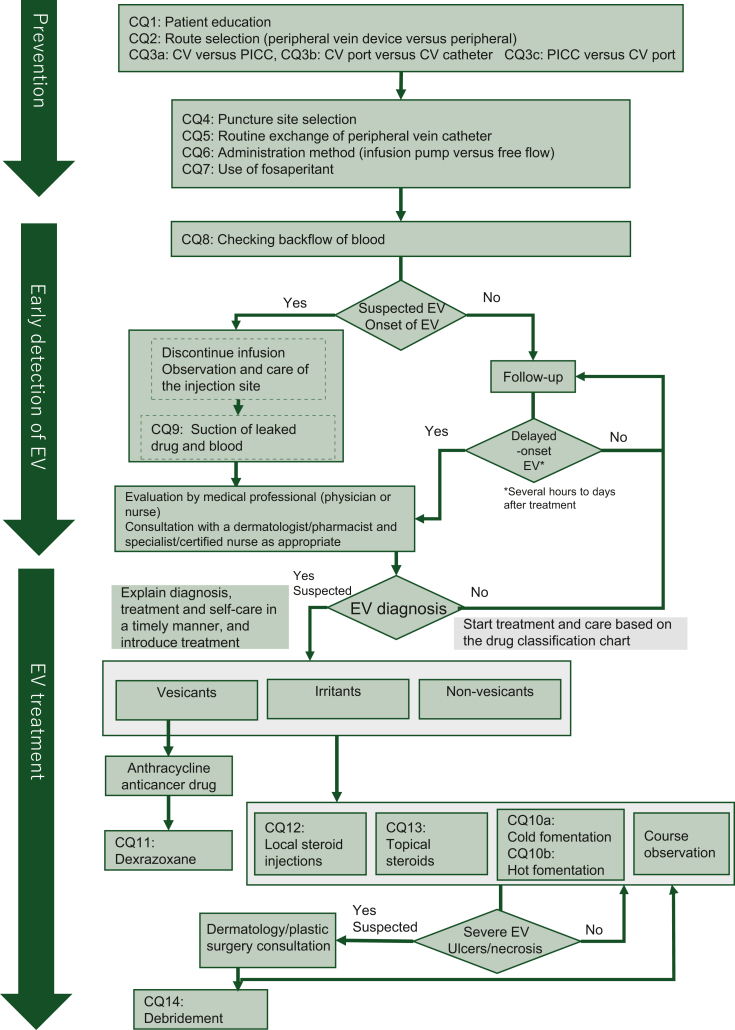
This guideline was created in line with the Grading of Recommendations Assessment, Development, and Evaluations (GRADE)8 and Medical Information Distribution Service (MINDS) Handbook for Clinical Practice Guideline Development 2016 and 2020.9 In addition to a multidisciplinary approach, treatment and care for the prevention of onset and exacerbation of EV require decision making that accounts for the values and intentions of patients and their families. Therefore, this guideline was created via multidisciplinary collaboration of the Japanese Society of Cancer Nursing (JSCN), Japanese Society of Medical Oncology (JSMO), and Japanese Society of Pharmaceutical Oncology (JASPO). The members of the three associations were surveyed about significant clinical challenges related to EV, and 17 clinical questions (CQs) were formulated (Table 1). An algorithm was created to depict the practices implemented by the health care provider for the prevention of onset, early detection, and treatment or care of EV in patients receiving anticancer drugs (Figure 1). This guideline is intended for use by medical staff, patients, and their families.
Table 1.
Clinical questions and recommendations
| Clinical questions | Recommendation | Desirable outcomes (benefit) | Undesirable outcomes (harm) | Balance between desirable and undesirable outcomes | Recommendation (strength)a | Certainty of evidenceb | Consensus rate | |
|---|---|---|---|---|---|---|---|---|
| CQ1 | Is multiple patient education regarding extravascular invasion recommended for patients receiving anticancer drugs via peripheral or central veins? | No recommendation | Unidentified (probably decreased ulceration and necrosis, decreased EV, and increased telephone consultation) | Unidentified (probably increased medical staff workload) | Not evaluable | None | D | No voting |
| CQ2 | Is placement of a central venous device (CV catheter, PICC, CV port, etc.) recommended in patients scheduled to start anticancer drugs? | CV devices are weakly recommended | Completion of scheduled administration, less pain and anxiety from cannulation | Device complications (8%) | Central venous device is beneficial | Recommend (weak) | B | 9/9 (100%) |
| CQ3a | Which central venous catheter (CV catheter or PICC) is recommended for cancer patients? | PICC is weakly recommended | Decreased complications such as catheter removal, infection, and thrombus | Unidentified | PICC is beneficial (limited to leukemia patients) | Recommend (weak) | B | 9/9 (100%) |
| CQ3b | Which central venous devices, CV catheter or CV port, is recommended for patients with cancer? | CV port is weakly recommended | Decreased device failures, infections, and complications | Thrombus | CV port is beneficial | Recommend (weak) | B | 9/9 (100%) |
| CQ3c | Which central venous device, PICC or CV port, is recommended for patients with solid tumors? | CV port is strongly recommended | Decreased device failures, infections, and complications | Unidentified (probably medical costs) | CV port is beneficial | Recommend (strong) | A | 9/9 (100%) |
| CQ4 | Is it recommended that peripheral intravenous catheters for the administration of anticancer drugs be placed more centrally than at the puncture site? | Central (upstream) placement is weakly recommended | Decreased EV, decrease in skin ulceration at the site of EV | Unidentified (probably decreased puncture vessel options) | Central (upstream) placement is beneficial | Recommend (weak) | C | 8/9 (89%) |
| CQ5 | Is routine replacement of peripheral venous catheters recommended to prevent extravasation in patients receiving continuous (intermittent) administration of anticancer drugs? | Not to do routine replacement is weakly recommended | Decreased EV, decreased phlebitis, decreased skin inflammation due to adhesive plaster | Unidentified (probably increased patient distress, increased medical staff workload) | Routine replacement is harmful | Not recommend (weak) | C | 9/9 (100%) |
| CQ6 | Is free flow recommended over an infusion pump as a method of administration to prevent extravasation? | Balance between strict rate control and EV prevention; free flow is weakly recommended/when strict rate control is required, not to do free flow is weakly recommended. | Decreased EV, decreased skin inflammation, prevention of skin ulceration (necrosis) | Unidentified (probably decreased accuracy of dose rate control, increased medical staff workload) | Unless strict rate control is required, infusion pump is weakly discouraged | Recommend or not recommend (weak) | C | 8/9 (89%) (second vote) |
| CQ7 | Is administration of fosaprepitant recommended considering the risk of extravasation of anticancer drugs? | Fosaprepitant administration is weakly recommended (limited to patients who cannot be administered orally) | Inhibiting nausea and vomiting | Increased EV and injection-site reactions | Fosaprepitant or aprepitant use is beneficial | Recommend (weak) | C | 9/9 (100%) |
| CQ8 | Is it recommended to check blood backflow for early detection of extravasation? | Checks for blood backflow is weakly recommended. | Early detection of EVs, detection of indwelling needle location and breakage, confirmation of intravascular placement, reduction of skin damage (redness and swelling) | Unidentified | Check blood backflow is beneficial | Recommend (weak) | D | 9/9 (100%) |
| CQ9 | Is suction of residual drug solution or blood recommended to prevent exacerbation of skin injury in the event of extravasation? | No recommendation | Decreased areas of skin injury (redness and swelling), pain, ulcers, and shortened recovery days | Unidentified (probably damage to blood vessels due to suction) | Not evaluable | None | D | No voting |
| CQ10a | Is cold compression recommended as local therapy to prevent aggravation/progression of skin injury and inflammation induced by extravasation? | Cold compression is weakly recommended | Decrease in inflammation (dermatitis and vasculitis), pain, and burning sensation at the site of leakage, and shortened recovery days | Skin damage (burns) and exacerbation of inflammation due to low or high temperatures | Cold compresses is beneficial | Recommend (weak) | D | 9/9 (100%) |
| CQ10b | Is warm compression recommended as local therapy to prevent aggravation/progression of skin injury and inflammation induced by extravasation? | Non-use of warm compresses (heat) is weakly recommended | Hot compresses is harmful | Not recommend (weak) | D | 9/9 (100%) (second vote) | ||
| CQ11 | Is the use of dexrazoxane recommended for extravasation induced by anthracycline cancer drug? | Dexrazoxane use is weakly recommended | Decreased surgical procedures (debridements and skin grafts) and shorter recovery days | Dexrazoxane side-effects, hospitalization, and prolongation of hospital days | Dexrazoxane use is beneficial | Recommend (weak) | B | 8/8 (100%) |
| CQ12 | Is local steroid injection recommended for extravasation caused by anticancer drugs? | Not injecting local steroid is weakly recommended. | Decreased surgical procedures (debridements and skin grafts) and shorter recovery days | Local skin damage, local injection pain | Local steroid injection is harmful | Not recommend (weak) | D | 9/9 (100%) |
| CQ13 | Is topical steroid recommended for extravasation caused by anticancer drugs? | Topical steroid application is weakly recommended | Decreased surgical procedures (debridements and skin grafts) and shorter recovery days | Skin damage at the application site (infection, skin atrophy) | Topical steroid application is beneficial | Recommend (weak) | D | 9/9 (100%) |
| CQ14 | Is debridement recommended for skin ulcers without necrosis due to extravasation? | Not to do debridement is weakly recommended | Skin ulcer healing | Skin invasions | Debridement is harmful | Not recommend (weak) | C | 9/9 (100%) |
CV, central venous catheter; CQ, clinical question; EV, extravasation; PICC, peripherally inserted central venous catheter.
Recommend (strong): strongly recommended to do, recommend (weak): weakly recommended to do, not recommend (strong): strongly recommended not to do, not recommend (weak): weakly recommended not to do.
A: High—evidence is a great certainty close to the true effect; B: Moderate—evidence is moderately confident of true effectiveness; C: Low—evidence is limited in its certainty of true near-effect (true effects may differ greatly from evidence estimates); D: Very low—evidence is far from convincing of a true effect (true effects differ significantly from evidence estimates).
Figure 1.
Algorithm of clinical care related to EV. It depicts the role of each CQ in the algorithm of clinical care of EV, such as prevention, early detection, and treatment. CV, central venous catheter; CQ, clinical question; EV, extravasation; PICC, peripherally inserted central venous catheter.
Details of guideline development including systematic review are provided in the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103932. Literature search was mostly carried out from March to April 2021. At the time of literature review, we aimed to distinguish between ‘extravasation’, which refers to the extravascular leakage of anticancer drugs and ‘infiltration’, which refers to the leakage of other drugs. For most of the CQs, we could not find strong evidences. To provide the basement of discussion between medical professionals and patients, or among international colleagues, we preferred to set some direction. For that purpose, we applied the evidence-to-decision framework.8
Tissue injury was classified as follows. The current guideline adopted the National Health Service (NHS)-England EXTRA,6 Oncology Nursing Society (ONS),7 and European Society for Medical Oncology-European Oncology Nursing Society (EONS5) tissue injury classifications for 36 drugs for which the three guidelines were consistent. Four independent reviewers conducted a systematic review of 31 drugs (19 drugs with different classifications according to the three guidelines and 12 drugs that required classification according to a survey of the members of the JSCN, JSMO, and JASPO). The PubMed and ICHUSHI Web databases were used for the search. Literature search was carried out on 23 March 2022. Articles were selected using the search terms ‘extravasation’, ‘injection-site reaction’, ‘adverse events’, and the names of individual drugs as keywords. Tissue injury was classified as vesicant, irritant, or non-vesicant according to the definitions provided (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103932). When tissue injury in multiple reported cases was caused by vesicants or irritants, the guideline panel held a discussion to make the decision.
Results
CQs and recommendations
The recommendations posited in this guideline were determined by a guideline panel based on the results of a systematic review of the 17 CQs. The recommendation, strength of recommendation, certainty of evidence, harm, benefits, and consensus rate are shown in Table 1.
1a. Prevention of EV
[CQ1] Patient education
Recommendation (RC): No recommendation (no vote).
The following benefits were identified: ‘decrease in ulcers/necrosis’ from a case report,10 ‘decrease in the frequency of EV’ from a practice report ,11 and ‘increase in the frequency of telephone consultations’ from a qualitative study,12 all of which were indirect. The balance between benefit and harm was ‘unknown’ and the certainty of evidence was determined to be ‘very low’. Therefore, there were no recommendations regarding patient education.
[CQ2] Device selection: central venous (CV) device [CV catheter, peripherally inserted central catheter (PICC), or CV port] placement
RC: It is weakly recommended to place a CV device in patients planning to receive anticancer drugs.
‘Reduction of administration failure’, a benefit, was investigated in one randomized controlled trial (RCT), which reported a significant decrease in administration failure with a CV port compared to a peripherally inserted catheter.13 No controlled trials investigated the harm. Although the quality of the evidence was high, the certainty of the evidence was rated as ‘moderate’ because there was only one study. Based on this evidence, placement of a CV device in patients planning to receive anticancer drugs is rated as a weak recommendation.
[CQ3] Selection of a CV device
The chosen outcome was device failure (obstruction, infection, thrombosis, or removal). Seven RCTs comparing three devices (two CV catheter versus CV port, four PICC versus CV port, and one PICC versus CV catheter) were included.
1) [CQ3a] Selection of CV catheter or PICC
RC: PICC is weakly recommended in patients with cancer, compared with CV.
One RCT that investigated patients with leukemia receiving induction anticancer chemotherapy14 found that the frequencies of catheter-related infections or thrombi and device failures were significantly lower in the PICC group. Based on this evidence, PICC is weakly recommended over CV catheters as a CV device in patients with cancer. However, because this study included only patients with leukemia, the certainty of the evidence for patients with cancer overall was rated as ‘moderate’.
2) [CQ3b] Selection of CV catheter or CV port
RC: CV port is weakly recommended in patients with cancer, compared with a CV catheter.
Two RCTs, one investigating patients with solid cancer15 and another investigating patients with acute leukemia,16 were extracted. The occurrence of device failure in patients with solid cancer was lower among those with CV ports. However, although complications were more frequent with the use of CV ports in patients with acute leukemia, there was a risk of bias due to numerous exclusions; therefore, the certainty of evidence was rated as ‘moderate’. Therefore, a CV port is weakly recommended over a CV catheter in patients with solid cancer.
3) [CQ3c] Selection of PICC or CV port
RC: CV port is strongly recommended in patients with solid tumors, compared with a PICC.
Four RCTs examining PICCs in patients with solid cancer were extracted. Studies on device failure were limited to one RCT, and the failure rate was significantly lower with the use of CV ports.17 A meta-analysis of other outcomes, including infection, thrombosis, and post-device complications, was reported in all four trials, and these outcomes were significantly less frequent among patients with CV ports.18, 19, 20 Although there was only one RCT investigating device failure, the results of complications were consistent across multiple RCTs; therefore, the certainty of evidence was rated as ‘high’. Placing a CV port over a PICC in patients with solid tumors is strongly recommended.
[CQ4] Site of peripheral venous catheter placement after puncture procedure
RC: It is weakly recommended to place a peripheral venous catheter centrally (upstream) from the site of preceding puncture for patients receiving anticancer drug therapy from the peripheral veins.
One cohort study was extracted, which reported a ‘decrease in EV’, with a catheter placed in a more upstream peripheral vein.21 The certainty of evidence was considered ‘low’ as only one infiltration event was reported. Therefore, the recommendation is rated as weak.
[CQ5] Routine replacement of peripheral venous catheters
RC: It is weakly recommended not to replace peripheral venous catheters with specific time interval in patients receiving anticancer drugs.
One meta-analysis and one RCT were extracted focusing on the benefit ‘decrease in EV’.22,23 The meta-analysis reported that routine replacement of peripheral venous catheters every 72-96 h resulted in lower infiltration than ad hoc replacement, but not lower EV, as determined by clinical symptoms.22 In contrast, the RCT evaluated EV and found no difference in its incidence based on placement of peripheral venous catheters.23 The certainty of evidence was rated as ‘low’ owing to indirectness caused by inclusion of infiltration among the outcomes. Since there is clear harm associated with peripheral venous catheter replacement (pain for the patient and burden for the medical staff), it was deemed that the harm outweighed the benefit. Therefore, periodic replacement of peripheral venous catheters in patients receiving anticancer drugs is weakly discouraged.
[CQ6] Avoidance of infusion pump
RC: Unless strict dose rate control is required, avoidance of infusion pump is weakly recommended when administering anticancer drugs via the peripheral vein.
One cohort study24 and six case reports25, 26, 27, 28, 29, 30 investigated desirable outcomes, such as ‘decrease in EV’, ‘decrease in skin inflammation’, and ‘avoidance of skin ulceration (necrosis)’. The cohort study examined infiltration in children and reported the inferiority of the infusion pump. Only the case reports described the incidence of EV/infiltration with use of an infusion pump; therefore, the causal relationship between pump use and EV/infiltration was unclear. The strength of evidence of the cohort study was rated as ‘weak’ due to indirectness arising from the lack of inclusion of anticancer drugs. Infusion pumps offer superior rate control, and the balance between benefit and harm varies with circumstances such as the level of drug-induced EV injury and the need for dose rate control. Unless strict dose rate control is required, avoidance of infusion pump is weakly recommended when administering anticancer drugs via the peripheral vein.
[CQ7] Administration of fosaprepitant
RC: Fosaprepitant administration with caution for injection-site reactions is weakly recommended.
A meta-analysis of 14 RCTs with ‘complete control of vomiting’ confirmed the antiemetic efficacy of fosaprepitant.31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 Nineteen retrospective studies reported ‘vasculitis, vascular pain, and injection-site reaction’ with fosaprepitant administration,45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63 and seven studies reporting the ‘leakage of anticancer drugs administered after fosaprepitant’ were extracted.45,48,52,53,62,64 Although multiple studies described EV as an adverse event, none directly examined whether fosaprepitant increases the risk of EV. The certainty of evidence for harm was rated ‘low’ since the results were derived from retrospective studies. The benefit of fosaprepitant administration as a strong antiemetic was determined to outweigh the harm, but fosaprepitant may increase injection-site reaction. Therefore, fosaprepitant administration with caution for injection-site reactions is weakly recommended.
1b. Early detection of EV
[CQ8] Checking blood backflow
RC: Assessing blood backflow for early detection of EV is weakly recommended.
Three case reports assessing ‘early detection of EV’ were extracted.65, 66, 67 No reports of adverse effects associated with assessing blood backflow were found, given that it is beneficial for verifying the position of the indwelling needle, assessing the presence or absence of occlusion, and confirming that the catheter is in the blood vessel. All extracted studies also mentioned concomitant use of interventions, such as observation of the skin at the puncture site of the indwelling needle, subjective symptoms experienced by the patient, and cessation of infusion. However, the timing and method of blood backflow verification were not clear; hence, the certainty of the evidence was rated as ‘low’. Therefore, judging that the benefit outweighs the harm, confirmation of blood backflow for early detection of EV is weakly recommended.
1c. Care at EV onset
[CQ9] Suction of blood or residual drug solution
RC: No recommendation (no vote).
Four studies on ‘reduced severity of skin disorders (redness and swelling)’, ‘reduced skin pain’, ‘reduced incidence of ulceration’, and ‘reduced time to recovery of symptoms’ were extracted.68, 69, 70, 71 There were only single reports of each outcome, and they were deemed to be weak as evidence for the efficacy of suctioning of blood or residual drug solution alone, as the methods and timing of suction were not described and suctioning was combined with other interventions such as steroid ointment application. The certainty of the evidence was rated as ‘very low’ owing to a dearth of studies on the harmful outcomes, which did not allow us to weigh the benefit against the harm. Therefore, there was no recommendation regarding the suctioning of residual drug solution or blood to prevent exacerbation of tissue injury in the EV.
[CQ10] Cold and warm compression
The benefits associated with cold and warm compression were ‘reduced EV site inflammation (dermatitis/vasculitis)’, ‘reduced EV site pain/burning sensation’, and ‘days to recovery of symptoms’. In addition to ‘exacerbation (worsening) of the inflammatory reaction’, the injuries were ‘skin damage due to low temperature’ for the cold compression method and ‘skin damage due to high temperature’ for warm compression.
[CQ10a] Cold compression
RC: Cold compression is weakly recommended as local therapy to prevent aggravation/progression of tissue injury and inflammation induced by EV.
One case-controlled trial and four case reports on cold compression were extracted.72, 73, 74, 75, 76 These studies demonstrated that cold compression results in reduced inflammation at the site of EV, reduced pain and burning sensations, and fewer days until recovery of symptoms. Inflammatory symptoms did recur after temporary reduction in one report; however, both patients were also treated with steroid ointments and topical compresses. Therefore, we judged that the evaluation of the utility of interventions using cold compression alone was poor, and the certainty of the evidence was rated as ‘very low’. Cold compression is effective in reducing pain or inflammation when used in conjunction with other interventions and is noninvasive; therefore, the benefits were deemed to outweigh the risks. Therefore, cold compress is weakly recommended as local therapy to prevent exacerbation or progression of EV.
[CQ10b] Warm compression
RC: Avoidance of warm compresses (heat) is weakly recommended to prevent EV-induced skin damage and aggravation/progression of inflammation.
Since there were no relevant evidences solely studying warm compression in human subjects, one human study and two animal studies were used as references by hand search.
In the human study, subcutaneous injection of hyaluronidase and warm compression to the site of vinorelbine leakage were carried out in addition to the administration of oral antibiotics, which resulted in scarring of erythematous lesions.77 In an experimental study in mice, warm compression (43-45°C) did not aggravate ulcers that arose after subcutaneous injection of vinca alkaloids.78 However, another study observed ulceration in all mice that received subcutaneous injection of vincristine followed by warm compression,79 suggesting the deleterious effect of applying warm compression alone to EV induced by vinca alkaloids. The combination of hyaluronidase and other drugs with warm compression does not cause ulceration and may reduce the inflammatory response, but hyaluronidase is not approved for EV in Japan, and the usefulness of warm compression alone is unknown; hence, the certainty of the evidence was rated as ‘very low’. Therefore, the use of warm compression alone as local therapy to prevent aggravation or progression of skin injury or inflammation induced by EV is weakly not recommended.
1d. Treatment for EV
[CQ11] Dexrazoxane
RC: Administration of dexrazoxane for EV induced by an anthracycline cancer drug is weakly recommended.
One observational study and six case studies were extracted.28,80, 81, 82, 83, 84, 85 Regarding the benefit ‘decrease in the number of surgical procedures (debridement or skin graft)’, administration of dexrazoxane reduced the frequency of surgical procedures.28,80,81 Furthermore, evaluation of the outcome ‘administration of anticancer drugs as scheduled’ revealed that treatment was completed according to schedule after the onset of EV.28,80, 81, 82, 83, 84, 85 The data for the harmful outcome ‘prolonged hospital stay or visit’ varied across studies80,83,85 and could not be homogenized. It was also difficult to eliminate the possibility that the adverse reactions were caused by anticancer drugs.28,80, 81, 82,84,85 Moreover, there is a lack of controlled trials of dexrazoxane, and its efficacy for EV of very small volumes is unknown. Therefore, the certainty of evidence was rated as ‘moderate’, and dexrazoxane administration is weakly recommended for EV induced by anthracycline chemotherapy regimens.
[CQ12] Local steroid injection
RC: It is weakly recommended not to inject local steroids for EV of anticancer drugs.
Seven studies on the ‘decrease in surgical procedures’,75,86, 87, 88, 89, 90, 91 nine studies on the ‘decrease in time to recovery’,75,86, 87, 88, 89, 90, 91, 92, 93 and one study on ‘skin damage at the fomentation site (local infection, skin atrophy, etc.)’ were extracted.88 There were no studies on ‘pain associated with local steroid injection’. The majority of studies were case reports, providing no evidence for local injection of steroids alone. The study by Yamada et al. (M, Yamada, et al. 2016. Conference presentation) compared the severity score and recovery time after vesicant EV between local and non-local steroid injection. The severity score of the local steroid injection group was significantly higher than that of the non-local injection group. Furthermore, the time to recovery was significantly longer in the local steroid injection group. These data suggested the certainty of evidence was ‘very low’; therefore, we weakly discourage local steroid injection for EV. A study by Ohisa et al.,94 which was reported after the guideline was formulated, compared patients who received topical steroids, local anesthetics, and subcutaneous steroids with those who received only topical steroids. The odds ratio for the incidence of skin surgery was significantly higher for patients who underwent subcutaneous steroid injection than for those who underwent topical steroid therapy, supporting the evidence adopted in the guideline.
[CQ13] Topical steroid application
RC: Applying topical steroids for EV of anticancer drugs is weakly recommended.
One study on ‘reduction of surgical procedures’,95 nine studies on ‘time to recovery’,71,74,75,90,92,95, 96, 97, 98 and one study on ‘skin disorder at the fomentation site (local infection, skin atrophy, etc.)’97 were extracted. All were case reports; none reported monotherapy with topical steroid application but rather reported its use combined with another intervention. The certainty of evidence was rated as ‘very low’. However, since its efficacy in combination with other interventions was demonstrated, it was judged to be effective based on indirect evidence. In general, the use of topical steroids for the purpose of inflammation control at EV sites is considered effective, and judging that the benefit outweighs the harm, topical application of steroids to EV sites is weakly recommended.
[CQ14] Debridement
RC: It is weakly recommended not to debride EV-induced skin ulcer lesions without necrosis.
Five observational studies were extracted.99, 100, 101, 102, 103 Surgical and conservative therapies within 72 h of leakage of drugs other than anticancer drugs were compared for the beneficial outcome of ‘healing of skin ulcer’, and both elicited good results.99 Other studies reported the efficacy of surgical procedures for ulcerous lesions; however, they did not describe the timing of ulcer development or their conditions.100, 101, 102, 103 ‘Postoperative sequelae’, a harmful outcome, was unevaluable for ulcers without necrosis. The certainty of evidence was classified as ‘low’. Skin ulcers without EV-induced necrosis may be cured even with conservative treatment. Therefore, the disadvantages of uniformly recommending debridement were deemed to outweigh the advantages. Given the characteristic of EV-related tissue injury, i.e. its origin in subcutaneous tissue, estimating the extent of tissue damage early and determining the depth of debridement is challenging. Therefore, for EV-induced skin ulcer lesions without necrosis, it is weakly recommended not to debride them.
Tissue injury classification table
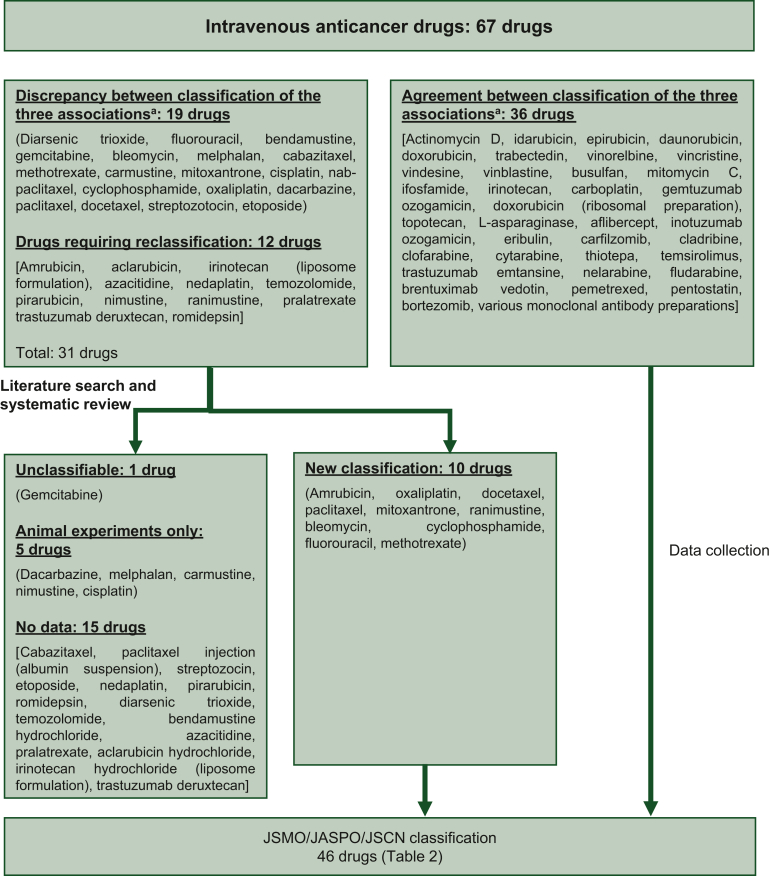
The flowchart of the systematic review for EV tissue injury is shown in Figure 2. Ten classifiable drugs were added, such that EV injury caused by 46 drugs was classified (Table 2). Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103932, enumerates the drug classifications of the three previous guidelines and the present systematic review.
Figure 2.
Flowof the systematic review on EV drug classification. Oncology Nursing Society (aONS); Chemotherapy and Immunotherapy Guidelines and Recommendations for Practice [2019, ESMO-European Oncology Nursing Society (EONS)]; Management of chemotherapy extravasation: ESMO – EONS Clinical Practice Guidelines (2012), National Health Survice (NHS)-England EXTRA Guidelines for the Management of Extravasation of a Systemic Anti-Cancer Therapy including Cytotoxic Agents (Last update: 2018, Review date: 2020). JSMO, Japanese Society of Medical Oncology; JASPO, Journal of Japanese Society of Pharmaceutical Oncology; JSCN, Japanese Society of Cancer Nursing.
Table 2.
Drug classification table
| Vesicants | Irritants | Non-vesicants |
|---|---|---|
| Actinomycin D | Ifosfamide | L-asparaginase |
| Idarubicin | Irinotecan | Aflibercept |
| Epirubicin | Carboplatin | Inotuzumab ozogamicin |
| Daunorubicin | Gemtuzumab ozogamicin | Eribulin |
| Doxorubicin | Doxorubicin (ribosomal preparation) | Carfilzomib |
| Trabectedin | Topotecan (nogitecan) | Cladribine |
| Vinorelbine | Bleomycina | Clofarabine |
| Vincristine | Cyclophosphamideb | Cytarabine |
| Vindesine | Fluorouracilc | Thiotepa |
| Vinblastine | Temsirolimus | |
| Busulfan | Trastuzumab emtansine | |
| Mitomycin C | Nelarabine | |
| Amrubicina | Fludarabine | |
| Oxaliplatina | Brentuximab vedotin | |
| Docetaxela | Pemetrexed | |
| Paclitaxela | Pentostatin | |
| Mitoxantronea | Bortezomib | |
| Ranimustinea | Various monoclonal antibody preparations | |
| Methotrexatea |
JASPO, Japanese Society of Pharmaceutical Oncology; JSMO, Japanese Society of Medical Oncology; JSCN, Japanese Society of Cancer Nursing.
Classification according to the systematic review by JSMO/JASPO/JSCN.
Potential vesicant when combined with anthracycline anticancer agents.
Potential vesicant in prolonged therapy and large doses.
Of the 10 drugs, amrubicin, oxaliplatin, docetaxel, paclitaxel, mitoxantrone, and ranimustine were classified as vesicants because of reported EV-related necrosis in humans and necrotic anticancer drug-related symptoms (Supplementary Table S3,70,72,73,86,104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134 available at https://doi.org/10.1016/j.esmoop.2024.103932).
Bleomycin had only been reported to cause inflammatory reactions, even after intradermal injection, and was classified as an irritant. Methotrexate was classified as a non-vesicant drug because local reactions to subcutaneous injection were limited to erythema and crusts.
Necrosis was observed in all studies of cyclophosphamide, but the effects of concomitant drugs could not be ruled out as the patients were receiving combination therapy with anthracycline anticancer drugs.113, 114, 115 Therefore, it was conditionally classified as an inflammatory anticancer drug, under the caveat that it could become a vesicant in the event of EV owing to cyclophosphamide administered after an anthracycline anticancer drug.
Blistering71 and grade 3 EV requiring surgical intervention due to ulceration or necrosis132 have been reported with fluorouracil, but the effects of regimens requiring long-term and high-dose administration of fluorouracil cannot be ruled out. Therefore, fluorouracil was conditionally classified as an irritant, under the caveat that it could become a vesicant in the event of long-term administration or large quantities of EV.
Discussion
This JSCN/JSMO/JASPO EV guideline addressed 17 CQs related to the prevention, early detection, treatment, and care of EV. This guideline is based on a systematic review and meta-analysis conforming to GRADE/MINDS 2020. Further, we conducted a systematic review on 31 drugs for which there was no consensus among existing guidelines or new drugs with insufficient evaluation regarding the classification of the potential of leaked anticancer drugs to cause damage to surrounding tissues, and a classification with 10 additional drugs was proposed. Among the 17 CQs, recommendations based on the results of RCTs were made with regard to the selection of CV devices (CQ2, CQ3a, CQ3b, and CQ3c), regular replacement of peripheral venous catheters (CQ5), and administration of fosaprepitant (CQ7). These CQs are novel and were not mentioned in previous guidelines. Several practices that have been instituted conventionally without clear evidence, such as warm compression monotherapy (CQ10b) and local injection of steroids (CQ12), were investigated based on the best available evidence, and consequently, their use is discouraged for the management of EV.
Compared with the four preceding guidelines regarding EV, NHS and ONS guidelines classified agents by DNA binding or non-DNA binding, speculated by the pharmacological background. As far as the systematic research carried out in this guideline is concerned, we could not identify clinical evidence to support this classification. Also, the ESMO-EONS guideline recommended warm compression to vinca alkaloids, taxane, and platinum salts and hyaluronidase to vinca alkaloids and taxane. Our systematic review identified evidence to support warm compression with hyaluronidase only for vinca alkaloids and no clinical evidence to support warm compression monotherapy. Rather, evidences in mice suggest potential harm of warm compression monotherapy for vinca alkaloids EV. Because neither dimethyl sulfoxide (DMSO) nor hyaluronidase are approved in Japan, our guideline recommended to avoid warm compression monotherapy. Also, we recommended to avoid topical injection of steroids and this is compatible with the ESMO guideline. As a result, our approach to EV became very simple, which is applicable to many countries even with restriction for medical resources. Another novel topic of this guideline is refutation to devices for the prevention of EV (for CV devices, CQ2, CQ3a, CQ3b, CQ3c, and for pomp, CQ6).
The main limitation of this guideline is that the best available evidence for many CQs was obtained from case reports and relatively small retrospective studies. Moreover, even in the prospective studies, some important factors which can influence the device choice such as the goal of therapy (curable or palliative), costs, and burden for the medical staff, are scarcely reported. Hopefully, future studies in this field capture and report these data. Another limitation is the lack of recommendation regarding antidotes, because hyaluronidase or DMSO are not approved in Japan. The limitations for drug classification include small sample size, reporting bias, and effect of confounding factors such as combined treatment with other anticancer drugs or treatment for EV. Some drugs such as gemcitabine may increase risk of EV following drugs.74 There is an urgent need for accumulation of well-designed clinical studies in this field. Another limitation is the focus on EV in the peripheral veins, not for EV from CV devices. This was based on questionnaires filled out by the members of JSCN/JSMO/JASPO. This could change in the future as a result of the adaptation of this guideline, which recommends CV devices.
In conclusion, we provided a guideline for EV management based on a systematic review with a multidisciplinary approach. Currently we are in the process of evaluating the adaptation of this guideline and developing further refined guidelines in collaboration with the JSCN/JSMO/JASPO working group. We hope this guideline will help health care providers and patients and their families in practicing better EV management.
Acknowledgements
The systematic review team was also involved in the development of the guideline. The team members were H. Aoyagi, K. Asano, T. Arahata, K. Akachi, K. Kunitomo, M. Koyama, R. Hashimoto, and M. Miura from JSCN, S. Aihara, T. Ogata, T. Onoe, M. Takahashi, M. Nishimura, and W. Munakata from JSMO; and H. Kado, M. Kaneko, O. Kuniyoshi, Y. Kumakura, Y. Kurata, M. Komuro, D. Suzuki, S. Sumikawa, A. Soejima, A. Tsuboya, T. Hayama, and M. Yoshida from JASPO. The following literature search specialists, dermatologists, and patient groups also assisted in the development of the guidelines: K. Kato, M. Yamazaki, T. Takai, A. Nishizawa, N. Sakurai, and K. Hiruma. Our guidelines were completed with suggestions from the following external evaluation experts: K. Ozawa and K. Hirai from JSCN; E. Sakaida and A. Shimomura from JSMO; and K. Suzuki and Y. Makino form JASPO. We are deeply grateful to all these members.
Funding
This work was supported by the Japanese Society of Cancer Nursing, Japanese Society of Medical Oncology, and Japanese Society of Pharmaceutical Oncology (no grant number).
Disclosure
KM received funding for clinical trial to the institution from AstraZeneca, Bristol Myers-Squib, Chugai, Daiichi-Sankyo, Eisai, Eli-Lilly, Gilead Sciences, MSD, and Novartis. He also received honoraria from Bayer, Chugai, Daiichi-Sankyo, Eisai, Eli-Lilly, Kyowa-Kirin, MSD, Pfizer, Taiho, and Takeda. RO served as advisor for Kissei Pharma. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Kim J.T., Park J.Y., Lee H.J., Cheon Y.J. Guidelines for the management of extravasation. J Educ Eval Health Prof. 2020;17:21. doi: 10.3352/jeehp.2020.17.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wengström Y., Margulies A. European Oncology Nursing Society extravasation guidelines. European Oncology Nursing Society Task Force. Eur J Oncol Nurs. 2008;12(4):357–361. doi: 10.1016/j.ejon.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Dorr R.T. Antidotes to vesicant chemotherapy extravasations. Blood Rev. 1990;4(1):41–60. doi: 10.1016/0268-960x(90)90015-k. [DOI] [PubMed] [Google Scholar]
- 4.Sauerland C., Engelking C., Wickham R., Corbi D. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33(6):1134–1141. doi: 10.1188/06.ONF.1134-1141. [DOI] [PubMed] [Google Scholar]
- 5.Pérez Fidalgo J.A., García Fabregat L., Cervantes A., et al. ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 7) doi: 10.1093/annonc/mds294. vii167-173. [DOI] [PubMed] [Google Scholar]
- 6.NHS-England EXTRA Guidelines for the management of extravasation of a systemic anti-cancer therapy including cytotoxic agents (Last update: 2018, Rev Review date: 2020) https://www.england.nhs.uk/midlands/wp-content/uploads/sites/46/2019/05/management-extravasation-of-a-systemic-anti-cancer-therapy-including-cytotoxic-agents.pdf Available at.
- 7.Olsen M.M., LeFebvre K.B., Walker S.L., Dunphy E.P. Oncology Nursing Society; Pittsburgh, PA: 2019. Chemotherapy and Immunotherapy Guidelines and Recommendations for Practice. [Google Scholar]
- 8.Schünemann H., Brożek J., Guyatt G., Oxman A., editors. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. The GRADE Working Group; 2013. https://gdt.gradepro.org/app/handbook/handbook.html Available at: [Google Scholar]
- 9.Hashimoto R. [Minds Guide for Developing Clinical Practice Guidelines Ver. 2.0 by Japan Council for Quality Health Care -Practice of EBM-] Seishin Shinkeigaku Zasshi. 2017;119(3):158–165. [PubMed] [Google Scholar]
- 10.González T. Chemotherapy extravasations: prevention, identification, management, and documentation. Clin J Oncol Nurs. 2013;17(1):61–66. doi: 10.1188/13.CJON.61-66. [DOI] [PubMed] [Google Scholar]
- 11.Coyle C.E., Griffie J., Czaplewski L.M. Eliminating extravasation events: a multidisciplinary approach. J Infus Nurs. 2015;38(suppl 6):S43–S50. doi: 10.1097/NAN.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett D.J., Childs D.S., Breitkopf C.R., et al. Chemotherapy acute infusion reactions: a qualitative report of the perspectives of patients with cancer. Am J Hosp Palliat Care. 2018;35(11):1384–1389. doi: 10.1177/1049909118773995. [DOI] [PubMed] [Google Scholar]
- 13.Bow E.J., Kilpatrick M.G., Clinch J.J. Totally implantable venous access ports systems for patients receiving chemotherapy for solid tissue malignancies: a randomized controlled clinical trial examining the safety, efficacy, costs, and impact on quality of life. J Clin Oncol. 1999;17(4):1267. doi: 10.1200/JCO.1999.17.4.1267. [DOI] [PubMed] [Google Scholar]
- 14.Picardi M., Della Pepa R., Cerchione C., et al. A frontline approach with peripherally inserted versus centrally inserted central venous catheters for remission induction chemotherapy phase of acute myeloid leukemia: a randomized comparison. Clin Lymphoma Myeloma Leuk. 2019;19(4):e184–e194. doi: 10.1016/j.clml.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Wu O., Boyd K., Paul J., et al. Hickman catheter and implantable port devices for the delivery of chemotherapy: a phase II randomised controlled trial and economic evaluation. Br J Cancer. 2016;114(9):979–985. doi: 10.1038/bjc.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansson E., Björkholm M., Björvell H., et al. Totally implantable subcutaneous port system versus central venous catheter placed before induction chemotherapy in patients with acute leukaemia – a randomized study. Support Care Cancer. 2004;12(2):99–105. doi: 10.1007/s00520-003-0558-1. [DOI] [PubMed] [Google Scholar]
- 17.Taxbro K., Hammarskjöld F., Thelin B., et al. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: an open-label, randomised, two-centre trial. Br J Anaesth. 2019;122(6):734–741. doi: 10.1016/j.bja.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Patel G.S., Jain K., Kumar R., et al. Comparison of peripherally inserted central venous catheters (PICC) versus subcutaneously implanted port-chamber catheters by complication and cost for patients receiving chemotherapy for non-haematological malignancies. Support Care Cancer. 2014;22(1):121–128. doi: 10.1007/s00520-013-1941-1. [DOI] [PubMed] [Google Scholar]
- 19.Clatot F., Fontanilles M., Lefebvre L., et al. Randomised phase II trial evaluating the safety of peripherally inserted catheters versus implanted port catheters during adjuvant chemotherapy in patients with early breast cancer. Eur J Cancer. 2020;126:116–124. doi: 10.1016/j.ejca.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Clemons M., Stober C., Kehoe A., et al. A randomized trial comparing vascular access strategies for patients receiving chemotherapy with trastuzumab for early-stage breast cancer. Support Care Cancer. 2020;28(10):4891–4899. doi: 10.1007/s00520-020-05326-y. [DOI] [PubMed] [Google Scholar]
- 21.Chan R.J., Alexander A., Bransdon M., et al. Challenging the distal-to-proximal cannulation technique for administration of anticancer therapies: a prospective cohort study. Cancer Nurs. 2012;35(5):E35–E40. doi: 10.1097/NCC.0b013e3182352916. [DOI] [PubMed] [Google Scholar]
- 22.Webster J., Osborne S., Rickard C.M., Marsh N. Clinically-indicated replacement versus routine replacement of peripheral venous catheters. Cochrane Database Syst Rev. 2019;1(1) doi: 10.1002/14651858.CD007798.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vendramim P., Avelar A.F.M., Rickard C.M., Pedreira M.D.L.G. The RESPECT trial-Replacement of peripheral intravenous catheters according to clinical reasons or every 96 hours: a randomized, controlled, non-inferiority trial. Int J Nurs Stud. 2020;107 doi: 10.1016/j.ijnurstu.2019.103504. [DOI] [PubMed] [Google Scholar]
- 24.de Lima Jacinto A.K., Avelar A.F., Pedreira M.L. Predisposing factors for infiltration in children submitted to peripheral venous catheterization. J Infus Nurs. 2011;34(6):391–398. doi: 10.1097/NAN.0b013e3182306491. [DOI] [PubMed] [Google Scholar]
- 25.Mateu-de Antonio J., Acuña-Reina L., Pons J.C., Buisán M.J. Lack of toxicity in a cladribine extravasation. Ann Pharmacother. 1999;33(7-8):873. doi: 10.1345/aph.18333. [DOI] [PubMed] [Google Scholar]
- 26.Conde-Estévez D., Saumell S., Salar A., Mateu-de Antonio J. Successful dexrazoxane treatment of a potentially severe extravasation of concentrated doxorubicin. Anticancer Drugs. 2010;21(8):790–794. doi: 10.1097/CAD.0b013e32833d9032. [DOI] [PubMed] [Google Scholar]
- 27.Bebawy J.F., Gupta D.K., Koht A. Compartment syndrome caused by a properly functioning infusion pump. J Clin Anesth. 2011;23(2):134–136. doi: 10.1016/j.jclinane.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Muthuramalingam S., Gale J., Bradbury J. Dexrazoxane efficacy for anthracycline extravasation: use in UK clinical practice. Int J Clin Pract. 2013;67(3):244–249. doi: 10.1111/ijcp.12103. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki R., Oyama T., Morimoto M. Three cases of skin necrosis from extravasation of intravenous fluids in children. Jpn J Surg Wound Care. 2016;7(2):99–104. [Google Scholar]
- 30.Abe-Doi M., Murayama R., Yabunaka K., Tanabe H., Komiyama C., Sanada H. Ultrasonographic assessment of an induration caused by extravasation of a nonvesicant anticancer drug: a case report. Medicine (Baltimore) 2019;98(14) doi: 10.1097/MD.0000000000015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinstein C., Jordan K., Green S., et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy regimens: a subgroup analysis from a randomized clinical trial of response in subjects by cancer type. BMC Cancer. 2020;20(1):918. doi: 10.1186/s12885-020-07259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z., Yang Y., Lu P., et al. Fosaprepitant versus aprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving cisplatin-based chemotherapy: a multicenter, randomized, double-blind, double-simulated, positive-controlled phase III trial. Ann Transl Med. 2020;8(5):234. doi: 10.21037/atm.2019.12.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radhakrishnan V., Joshi A., Ramamoorthy J., et al. Intravenous fosaprepitant for the prevention of chemotherapy-induced vomiting in children: a double-blind, placebo-controlled, phase III randomized trial. Pediatr Blood Cancer. 2019;66(3) doi: 10.1002/pbc.27551. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein C., Jordan K., Green S.A., et al. Evaluation of factors contributing to the response to fosaprepitant in a heterogeneous, moderately emetogenic chemotherapy population: an exploratory analysis of a randomized phase III trial. Support Care Cancer. 2018;26(11):3773–3780. doi: 10.1007/s00520-018-4242-x. [DOI] [PubMed] [Google Scholar]
- 35.Yang L.Q., Sun X.C., Qin S.K., et al. Efficacy and safety of fosaprepitant in the prevention of nausea and vomiting following highly emetogenic chemotherapy in Chinese people: a randomized, double-blind, phase III study. Eur J Cancer Care. 2017;26(6) doi: 10.1111/ecc.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navari R.M., Nagy C.K., Le-Rademacher J., Loprinzi C.L. Olanzapine versus fosaprepitant for the prevention of concurrent chemotherapy radiotherapy-induced nausea and vomiting. J Commun Support Oncol. 2016;14(4):141–147. doi: 10.12788/jcso.0245. [DOI] [PubMed] [Google Scholar]
- 37.Ruhlmann C.H., Herrstedt J. New treatments on the horizon for chemoradiotherapy-induced nausea and vomiting. Expert Opin Pharmacother. 2016;17(12):1623–1629. doi: 10.1080/14656566.2016.1202923. [DOI] [PubMed] [Google Scholar]
- 38.Micha J.P., Rettenmaier M.A., Brown J.V., III, et al. A randomized controlled pilot study comparing the impact of aprepitant and fosaprepitant on chemotherapy induced nausea and vomiting in patients treated for gynecologic cancer. Int J Gynecol Cancer. 2016;26(2):389–393. doi: 10.1097/IGC.0000000000000593. [DOI] [PubMed] [Google Scholar]
- 39.Weinstein C., Jordan K., Green S.A., et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: results of a randomized, double-blind phase III trial. Ann Oncol. 2016;27(1):172–178. doi: 10.1093/annonc/mdv482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ando Y., Hayashi T., Ito K., et al. Comparison between 5-day aprepitant and single-dose fosaprepitant meglumine for preventing nausea and vomiting induced by cisplatin-based chemotherapy. Support Care Cancer. 2016;24(2):871–878. doi: 10.1007/s00520-015-2856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maru A., Gangadharan V.P., Desai C.J., et al. A phase 3, randomized, double-blind study of single-dose fosaprepitant for prevention of cisplatin-induced nausea and vomiting: results of an Indian population subanalysis. Ind J Cancer. 2013;50(4):285–291. doi: 10.4103/0019-509X.123580. [DOI] [PubMed] [Google Scholar]
- 42.Saito H., Yoshizawa H., Yoshimori K., et al. Efficacy and safety of single-dose fosaprepitant in the prevention of chemotherapy-induced nausea and vomiting in patients receiving high-dose cisplatin: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Ann Oncol. 2013;24(4):1067–1073. doi: 10.1093/annonc/mds541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grunberg S., Chua D., Maru A., et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol--EASE. J Clin Oncol. 2011;29(11):1495–1501. doi: 10.1200/JCO.2010.31.7859. [DOI] [PubMed] [Google Scholar]
- 44.Kitayama H., Tsuji Y., Sugiyama J., Doi A., Kondo T., Hirayama M. Efficacy of palonosetron and 1-day dexamethasone in moderately emetogenic chemotherapy compared with fosaprepitant, granisetron, and dexamethasone: a prospective randomized crossover study. Int J Clin Oncol. 2015;20(6):1051–1056. doi: 10.1007/s10147-015-0823-6. [DOI] [PubMed] [Google Scholar]
- 45.Sasaki N., Yoshino M., Kurematsu N., et al. Survey of injection site reactions in chemotherapy with peripheral administration of fosaprepitant and investigation of factors contributing to their occurrence. Jpn J Pharm Palliat Care Sci. 2020;13(4):111–117. (Japanese) [Google Scholar]
- 46.Abe-Doi M., Murayama R., Komiyama C., Sanada H. Incidence, risk factors, and assessment of induration by ultrasonography after chemotherapy administration through a peripheral intravenous catheter) Jpn J Nurs Sci. 2020;17(3):1–12. doi: 10.1111/jjns.12329. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe Y., Kikuchi M., Kisara S., et al. Comparison of effectiveness and safety between fosaprepitant and aprepitant in patients with breast cancer receiving the FEC100 regimen. J Drug Interact Res. 2015;39(1):29–35. [Google Scholar]
- 48.Kurematsu N., Yoshino M., Sasaki N., et al. Survey of injection site injury in fosaprepitant. Med J Niigata PH. 2014;62:30–35. (Japanese) [Google Scholar]
- 49.Abe T., Dogami M., Sato M., et al. The investigation about the incidence of phlebitis or vasalgia due to the administration of Anthracycline and Fosaprepitant Meglumine. Jpn J Cancer Care. 2014;19(1):83–86. [Google Scholar]
- 50.Imazu K., Nishikawa C., Nishi Y., et al. Investigation into appearance situation of injection site reaction by anthracyclines using fosaprepitant. Jpn Soc Hosp Pharm. 2013;49(11):1187–1191. (Japanese) [Google Scholar]
- 51.Miyazaki K., Sudo R., Murata Y. Injection site reaction after peripheral intravenous injection of fosaprepitant. Jpn J Cancer Clin. 2013;59(4):455–458. (Japanese) [Google Scholar]
- 52.Candelario N., Lu M.L. Fosaprepitant dimeglumine for the management of chemotherapy-induced nausea and vomiting: patient selection and perspectives. Cancer Manage Res. 2016;8:77–82. doi: 10.2147/CMAR.S93620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamasaki M., Kimura R., Mayahara S., et al. Study on the infusion-site adverse events and vascular distribution of epirubicin in chemotherapy with epirubicin and fosaprepitant. Mol Clin Oncol. 2019;11(1):43–49. doi: 10.3892/mco.2019.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boccia R., Geller R.B., Clendeninn N., Ottoboni T. Hypersensitivity and infusion-site adverse events with intravenous fosaprepitant after anthracycline-containing chemotherapy: a retrospective study. Future Oncol. 2019;15(3):297–303. doi: 10.2217/fon-2018-0662. [DOI] [PubMed] [Google Scholar]
- 55.Chau E., Lundberg J., Phillips G., Berger M., Wesolowski R. Updated report on incidence of infusion-site reactions associated with peripheral intravenous administration of fosaprepitant. J Oncol Pharm Pract. 2019;25(5):1053–1057. doi: 10.1177/1078155218769347. [DOI] [PubMed] [Google Scholar]
- 56.Gonçalves S.C., Sanches S.M., Bueno C.T., Villela de Castro D.L., Damascena A., Santos G.R.C. Incidence of infusion site reactions in peripheral fosaprepitant infusions. J Infus Nurs. 2017;40(6):380–383. doi: 10.1097/NAN.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 57.Tsuda T., Kyomori C., Mizukami T., et al. Infusion site adverse events in breast cancer patients receiving highly emetic chemotherapy with prophylactic anti-emetic treatment with aprepitant and fosaprepitant: a retrospective comparison. Mol Clin Oncol. 2016;4(4):603–606. doi: 10.3892/mco.2016.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujii T., Nishimura N., Urayama K.Y., et al. Differential impact of fosaprepitant on infusion site adverse events between cisplatin- and anthracycline-based chemotherapy regimens. Anticancer Res. 2015;35(1):379–383. [PubMed] [Google Scholar]
- 59.Hegerova L.T., Leal A.D., Grendahl D.C., et al. An analysis of fosaprepitant-induced venous toxicity in patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2015;23(1):55–59. doi: 10.1007/s00520-014-2326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sato Y., Kondo M., Inagaki A., et al. Highly frequent and enhanced injection site reaction induced by peripheral venous injection of fosaprepitant in anthracycline-treated patients. J Cancer. 2014;5(5):390–397. doi: 10.7150/jca.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lundberg J.D., Crawford B.S., Phillips G., Berger M.J., Wesolowski R. Incidence of infusion-site reactions associated with peripheral intravenous administration of fosaprepitant. Support Care Cancer. 2014;22(6):1461–1466. doi: 10.1007/s00520-013-2106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leal A.D., Kadakia K.C., Looker S., et al. Fosaprepitant-induced phlebitis: a focus on patients receiving doxorubicin/cyclophosphamide therapy. Support Care Cancer. 2014;22(5):1313–1317. doi: 10.1007/s00520-013-2089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ishihama H., Toyama T., Umagami H., et al. Study of pH adjustment for preventing fosaprepitant-induced Angialgia. Prog Med. 2018;38(12):1363–1368. (Japanese) [Google Scholar]
- 64.Segna A.N., Baron R.H., Cohen B. Infusion site reactions: classification in the setting of fosaprepitant administration with chemotherapy. Clin J Oncol Nurs. 2020;24(6):E79–E84. doi: 10.1188/20.CJON.E79-E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murayama R., Oya M., Abe-Doi M., Oe M., Komiyama C., Sanada H. Characteristics of subcutaneous tissues at the site of insertion of peripheral infusion in patients undergoing paclitaxel and carboplatin chemotherapy. Drug Discov Ther. 2019;13(5):288–293. doi: 10.5582/ddt.2019.01064. [DOI] [PubMed] [Google Scholar]
- 66.Nishimori H., Kouge N., Nishimoto H., et al. Risk factor for occlusion of central venous access port system in colon cancer patients. Palliative Care Res. 2013;8(1):135–141. [Google Scholar]
- 67.Hadaway L.C. Preventing and managing peripheral extravasation. Nursing. 2004;34(5):66–67. doi: 10.1097/00152193-200405000-00056. [DOI] [PubMed] [Google Scholar]
- 68.Schulmeister L. Managing vesicant extravasations. Oncologist. 2008;13(3):284–288. doi: 10.1634/theoncologist.2007-0191. [DOI] [PubMed] [Google Scholar]
- 69.Camp-Sorrell D. Developing extravasation protocols and monitoring outcomes. J Intraven Nurs. 1998;21(4):232–239. [PubMed] [Google Scholar]
- 70.Vandeweyer E., Deraemaecker R. Early surgical suction and washout for treatment of cytotoxic drug extravasations. Acta Chir Belg. 2000;100(1):37–38. [PubMed] [Google Scholar]
- 71.Kondo S., Matsuzaki D., Hasegawa T., et al. A case of delayed extravascular leakage of 5-FU with blistering. Teishin igaku. 2018;70(1):20–23. (Japanese) [Google Scholar]
- 72.Berghammer P., Pöhnl R., Baur M., et al. Docetaxel extravasation. Support Care Cancer. 2001;9(2):131–134. doi: 10.1007/s005200000182. [DOI] [PubMed] [Google Scholar]
- 73.Chang A. A case of mitoxantrone extravasation. J Oncol Pharm Pract. 2020;26(5):1270–1273. doi: 10.1177/1078155219893736. [DOI] [PubMed] [Google Scholar]
- 74.Okuda H., Masatsugu A., Sijimaya T., Arai R. Skin necrosis due to the extravasation of irritant anticancer agents. Intern Med. 2018;57(5):757–760. doi: 10.2169/internalmedicine.9329-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitsuma A., Sawaki M., Shibata T., et al. Extravasation of pegylated-liposomal doxorubicin: favorable outcome after immediate subcutaneous administration of corticosteroids. Nagoya J Med Sci. 2012;74(1-2):189–192. (Japanese) [PMC free article] [PubMed] [Google Scholar]
- 76.Bertelli G., Gozza A., Forno G.B., et al. Topical dimethylsulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13(11):2851–2855. doi: 10.1200/JCO.1995.13.11.2851. [DOI] [PubMed] [Google Scholar]
- 77.Das C.K., Gogia A. Vinorelbine-induced chemotherapy port extravasation. Lancet Oncol. 2016;17(12):e568. doi: 10.1016/S1470-2045(16)30556-3. [DOI] [PubMed] [Google Scholar]
- 78.Dorr R.T., Alberts D.S. Vinca alkaloid skin toxicity: antidote and drug disposition studies in the mouse. J Natl Cancer Inst. 1985;74(1):113–120. [PubMed] [Google Scholar]
- 79.Ishida Y., Oyama N., Takeda T. Experimental study on effects of applying a poultice to skin lesions produced by extravasation of vinca alkaloids. Jpn J Nurs Art Sci. 2005;4(2):38–41. (Japanese) [Google Scholar]
- 80.Mouridsen H.T., Langer S.W., Buter J., et al. Treatment of anthracycline extravasation with Savene (dexrazoxane): results from two prospective clinical multicentre studies. Ann Oncol. 2007;18(3):546–550. doi: 10.1093/annonc/mdl413. [DOI] [PubMed] [Google Scholar]
- 81.Fontaine C., Noens L., Pierre P., De Grève J. Savene® (dexrazoxane) use in clinical practice. Support Care Cancer. 2012;20(5):1109–1112. doi: 10.1007/s00520-012-1382-2. [DOI] [PubMed] [Google Scholar]
- 82.Kazakova V., Vanegas Y.A.M., Torres T.A., Kozyreva O. Delayed presentation of doxorubicin extravasation into pleural space: case report and review of literature. J Oncol Pharm Pract. 2021;27(6):1520–1527. doi: 10.1177/1078155220975848. [DOI] [PubMed] [Google Scholar]
- 83.Chang R., Murray N. Management of anthracycline extravasation into the pleural space. Oxf Med Case Rep. 2016;2016(10) doi: 10.1093/omcr/omw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aigner B., Bauernhofer T., Petru E., et al. Complete recovery of a wide local reaction by the use of dexrazoxane 72 hours after epirubicin extravasation: case report and review of the literature. Dermatology. 2014;229(4):288–292. doi: 10.1159/000365391. [DOI] [PubMed] [Google Scholar]
- 85.Uges J.W., Vollaard A.M., Wilms E.B., et al. Intrapleural extravasation of epirubicin, 5-fluouracil, and cyclophosphamide, treated with dexrazoxane. Int J Clin Oncol. 2006;11(6):467–470. doi: 10.1007/s10147-006-0598-x. [DOI] [PubMed] [Google Scholar]
- 86.Nakagawa Y., Kaneko E., Morita H. A case of extravascular leakage of an anticancer drug treated with dexrazoxane. Rinsho derma (Tokyo) 2016;58(9):1462–1463. [Google Scholar]
- 87.Takagi M., Kohama M., Takeuchi M. Experience in the treatment of forearm skin necrosis caused by extravasation leakage of epirubicin. Jpn J Surg Wound Care. 2017;8(2):57–60. (Japanese) [Google Scholar]
- 88.Muto J., Yoshioka T., Saito T., et al. A case of skin and soft tissue injury due to extravasation leakage of chemotherapy drug for breast cancer. J Obihiro Kosei Hosp. 2014;17:86–90. (Japanese) [Google Scholar]
- 89.Kitamura A., Fukumoto S., Goto S., et al. Treatment and management of skin ulcers caused by extravasation of anticancer drugs. Minami Osaka Med J. 1994;42(1):1–17. (Japanese) [Google Scholar]
- 90.Ishihara K., Yamazaki N. Extravasation leakage of anticancer drugs and its management. Skin Cancer. 1992;7(1):117–128. (Japanese) [Google Scholar]
- 91.Lawrence H.J., Walsh D., Zapotowski K.A., et al. Topical dimethylsulfoxide may prevent tissue damage from anthracycline extravasation. Cancer Chemother Pharmacol. 1989;23(5):316–318. doi: 10.1007/BF00292411. [DOI] [PubMed] [Google Scholar]
- 92.Haseida Y., Yamaguchi H. Initial treatment of extravasation of anticancer drugs. J Jpn P R S. 1992;12(5):299–306. (Japanese) [Google Scholar]
- 93.Tsavaris N.B., Karagiaouris P., Tzannou I., et al. Conservative approach to the treatment of chemotherapy-induced extravasation. J Dermatol Surg Oncol. 1990;16(6):519–522. doi: 10.1111/j.1524-4725.1990.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 94.Ohisa K., Yamana H., Morita K., Matsui H., Fushimi K., Yasunaga H. Association between subcutaneous steroid injection for extravasation of vesicant anticancer drugs and skin ulcers requiring surgery. Eur J Oncol Nurs. 2022;58 doi: 10.1016/j.ejon.2022.102119. [DOI] [PubMed] [Google Scholar]
- 95.Nagata K., Fujii N., Kishida M., et al. Two cases of skin ulceration due to leakage of anticancer drug: a surgical case after localisation of the ulcer. Rinsho Derma. 2005;47(13):1845–1848. (Japanese) [Google Scholar]
- 96.Kamiya T., Omine Y., Toyomiyama K., et al. A case of cellulitis 14 days after oxaliplatin leakage. Med. J. Okinawa Red Cross Hosp. 2008;16(1):27–28. (Japanese) [Google Scholar]
- 97.Uña E., Cuadrillero F., López-Lara F. Drug extravasation: a dreaded complication. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.09.2008.0887. bcr09.2008.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.El Saghir N.S., Otrock Z.K. Docetaxel extravasation into the normal breast during breast cancer treatment. Anticancer Drugs. 2004;15(4):401–404. doi: 10.1097/00001813-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 99.Loth T.S., Eversmann W.W., Jr. Extravasation injuries in the upper extremity. Clin Orthop Relat Res. 1991;272:248–254. [PubMed] [Google Scholar]
- 100.Scuderi N., Onesti M.G. Antitumor agents: extravasation, management, and surgical treatment. Ann Plast Surg. 1994;32(1):39–44. [PubMed] [Google Scholar]
- 101.Cedidi C., Hierner R., Berger A. Plastic surgical management in tissue extravasation of cytotoxic agents in the upper extremity. Eur J Med Res. 2001;6(7):309–314. [PubMed] [Google Scholar]
- 102.Langstein H.N., Duman H., Seelig D., et al. Retrospective study of the management of chemotherapeutic extravasation injury. Ann Plast Surg. 2002;49(4):369–374. doi: 10.1097/00000637-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 103.Linder R.M., Upton J., Osteen R. Management of extensive doxorubicin hydrochloride extravasation injuries. J Hand Surg Am. 1983;8(1):32–38. doi: 10.1016/s0363-5023(83)80048-3. [DOI] [PubMed] [Google Scholar]
- 104.Tagashira H., Izushi Y., Ikuta T., et al. Regimen of 5-fluorouracil and cisplatin increases the incidence of extravasation in patients undergoing chemotherapy. In Vivo. 2021;35(2):1147–1150. doi: 10.21873/invivo.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morisada S., Yanagi Y., Kashiwazaki Y., et al. Toxicological aspects of a novel 9-aminoanthracycline, SM-5887. Jpn J Cancer Res. 1989;80(1):77–82. doi: 10.1111/j.1349-7006.1989.tb02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Langer S.W., Thougaard A.V., Sehested M., et al. Treatment of experimental extravasation of amrubicin, liposomal doxorubicin, and mitoxantrone with dexrazoxane. Cancer Chemother Pharmacol. 2012;69(2):573–576. doi: 10.1007/s00280-011-1794-6. [DOI] [PubMed] [Google Scholar]
- 107.Bahadori F., Demiray M. Management of extravasation of oxaliplatin by mimicking its biotransformation. Clin Transl Oncol. 2018;20(10):1353–1357. doi: 10.1007/s12094-018-1854-z. [DOI] [PubMed] [Google Scholar]
- 108.Kretzschmar A., Pink D., Thuss-Patience P., et al. Extravasations of oxaliplatin. J Clin Oncol. 2003;21(21):4068–4069. doi: 10.1200/JCO.2003.99.095. [DOI] [PubMed] [Google Scholar]
- 109.Masters B., Hickish T., Cidon E.U. A midline for oxaliplatin infusion: the myth of safety devices. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-204360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pericay C., López A., Soler J.R., Bonfill T., Dotor E., Saigí E. Extravasation of oxaliplatin: an infrequent and irritant toxicity. Clin Transl Oncol. 2009;11(2):114–116. doi: 10.1007/s12094-009-0324-z. [DOI] [PubMed] [Google Scholar]
- 111.Kennedy J.G., Donahue J.P., Hoang B., et al. Vesicant characteristics of oxaliplatin following antecubital extravasation. Clin Oncol (R Coll Radiol) 2003;15(5):237–239. doi: 10.1016/s0936-6555(02)00338-2. [DOI] [PubMed] [Google Scholar]
- 112.Foo K.F., Michael M., Toner G., et al. A case report of oxaliplatin extravasation. Ann Oncol. 2003;14(6):961–962. doi: 10.1093/annonc/mdg252. [DOI] [PubMed] [Google Scholar]
- 113.Kamiya C., Ohmine Y., Tomiya T., et al. A case of cellulitis 14 days after oxaliplatin leakage. Med J Okinawa Red Cross Hosp. 2008;16(1):27–28. [Google Scholar]
- 114.Wickham R. Vesicant extravasation from an implanted venous access port. Oncology (Williston Park) 2009;23(suppl 4):34–38. [PubMed] [Google Scholar]
- 115.Lossos I.S., Ben-Yehuda D. Cutaneous and subcutaneous necrosis following dexrazoxane-CHOP therapy. Ann Pharmacother. 1999;33(2):253–254. doi: 10.1345/aph.18131. [DOI] [PubMed] [Google Scholar]
- 116.Muto J., Yoshioka T., Saito T., et al. A case of skin and soft tissue injury due to extravascular leakage of chemotherapeutic drug for breast cancer. J Obihiro Kosel Hospital. 2014;17:86–90. (Japanese) [Google Scholar]
- 117.Cifuentes L., Ring J., Brockow K. Extravasation of docetaxel. J Dtsch Dermatol Ges. 2012;10(9):662–663. doi: 10.1111/j.1610-0387.2012.07973.x. [DOI] [PubMed] [Google Scholar]
- 118.Barceló R., Viteri A., Muñoz A., Carrera S., Rubio I., López-Vivanco G. Extravasation of docetaxel: a red hand syndrome. Arch Dermatol. 2005;141(10):1326–1327. doi: 10.1001/archderm.141.10.1326. [DOI] [PubMed] [Google Scholar]
- 119.Bicher A., Levenback C., Burke T.W., et al. Infusion site soft-tissue injury after paclitaxel administration. Cancer. 1995;76(1):116–120. doi: 10.1002/1097-0142(19950701)76:1<116::aid-cncr2820760118>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 120.Barutca S., Kadikoylu G., Bolaman Z., Meydan N., Yavasoglu I. Extravasation of paclitaxel into breast tissue from central catheter port. Support Care Cancer. 2002;10(7):563–565. doi: 10.1007/s00520-002-0372-1. [DOI] [PubMed] [Google Scholar]
- 121.Bailey W.L., Crump R.M. Taxol extravasation: a case report. Can Oncol Nurs J. 1997;7(2):96–99. doi: 10.5737/1181912x729697. [DOI] [PubMed] [Google Scholar]
- 122.Stein M.E., Drumea K., Abu-Rasmi R., Haim N. Taxol-induced cellulitis after extravasation: a rarely reported event. Am J Clin Oncol. 1997;20(5):540. doi: 10.1097/00000421-199710000-00023. [DOI] [PubMed] [Google Scholar]
- 123.Beri R., Rosen F.R., Pacini M.J., Desai S.R. Severe dermatologic reactions at multiple sites after paclitaxel administration. Ann Pharmacother. 2004;38(2):238–241. doi: 10.1345/aph.1D206. [DOI] [PubMed] [Google Scholar]
- 124.Ajani J.A., Dodd L.G., Daugherty K., Warkentin D., Ilson D.H. Taxol-induced soft-tissue injury secondary to extravasation: characterization by histopathology and clinical course. J Natl Cancer Inst. 1994;86(1):51–53. doi: 10.1093/jnci/86.1.51. [DOI] [PubMed] [Google Scholar]
- 125.Meehan J.L., Sporn J.R. Case report of Taxol administration via central vein producing a recall reaction at a site of prior Taxol extravasation. J Natl Cancer Inst. 1994;86(16):1250–1251. doi: 10.1093/jnci/86.16.1250. [DOI] [PubMed] [Google Scholar]
- 126.Herrington J.D., Figueroa J.A. Severe necrosis due to paclitaxel extravasation. Pharmacotherapy. 1997;17(1):163–165. [PubMed] [Google Scholar]
- 127.Reddy S.S., Somayaji S., Krishna Murthy M., et al. 5-Fluorouracil induced extravasation injury. Indian J Cancer. 2020;57(4):467–469. doi: 10.4103/ijc.IJC_281_19. [DOI] [PubMed] [Google Scholar]
- 128.Luke E. Mitoxantrone-induced extravasation. Oncol Nurs Forum. 2005;32(1):27–29. doi: 10.1188/05.ONF.27-29. [DOI] [PubMed] [Google Scholar]
- 129.Levin M., Caravone D., Geiser C. Mitoxantrone extravasation and tissue necrosis. Am J Health Syst Pharm. 1996;53(10):1192–1194. doi: 10.1093/ajhp/53.10.1192. [DOI] [PubMed] [Google Scholar]
- 130.Templeton S.F., Solomon A.R., Swerlick R.A. Intradermal bleomycin injections into normal human skin. A histopathologic and immunopathologic study. Arch Dermatol. 1994;130(5):577–583. [PubMed] [Google Scholar]
- 131.Tanigaki T., Endo H. A case of squamous cell carcinoma treated by intralesional injection of oil bleomycin. Dermatologica. 1985;170(6):302–305. doi: 10.1159/000249555. [DOI] [PubMed] [Google Scholar]
- 132.Muthiah S., Charlton F., Farr P.M. Localized inflammatory reactions at sites of subcutaneous methotrexate injections during treatment with ultraviolet B. Br J Dermatol. 2018;179(1):192–194. doi: 10.1111/bjd.16357. [DOI] [PubMed] [Google Scholar]
- 133.Sadoghi B., Kränke B., Cerroni L., et al. Unusual cutaneous reaction at site of methotrexate injection in two patients with psoriasis and psoriatic. Arthritis Acta Derm Venereol. 2021;101(11) doi: 10.2340/actadv.v101.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Priego-Recio C.M., Camacho-Martínez F.M. Local reaction after subcutaneous injection of methotrexate: uncommon side effect. J Eur Acad Dermatol Venereol. 2016;30(3):523–524. doi: 10.1111/jdv.12920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.