Highlights
-
•
Pneumococcus is the most common cause of bacterial pneumonia in children aged <5 years.
-
•
Serotype 19 A is the most frequent, similar to other countries that used pneumococcal conjugate vaccine of 10 serotypes.
-
•
Streptococcus pneumoniae serotype 19 A is associated with multiresistance.
-
•
Non-typeable Haemophilus influenzae is the second most common microorganism.
-
•
Sentinel surveillance is useful to measure the impact of vaccines.
Keywords: Sentinel surveillance, Pneumonia, Streptococcus pneumoniae, Haemophilus influenzae
Abstract
Objectives
Sentinel surveillance for bacterial pneumonia (SSBP) allows the monitoring of immunopreventable diseases. The results of the SSBP carried out at HOMI, Fundación Hospital pediátrico de la Misericordia, are presented.
Methods
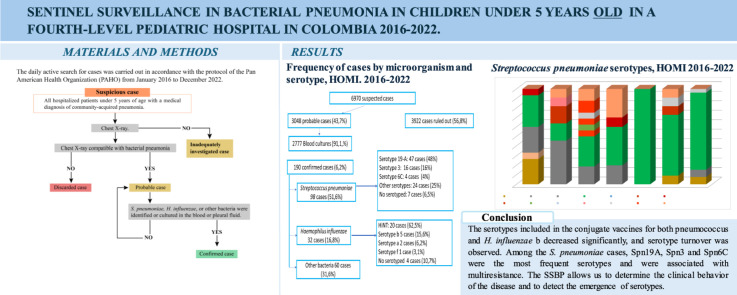
The daily active search for cases was carried out in accordance with the protocol of the Pan American Health Organization from January 2016 to December 2022.
Results
There were 6970 suspected cases of bacterial pneumonia (BP). Among the 3048 (43.7%) patients with probable BP, cultures were obtained from 2777 (91.1%), and BP was confirmed in 190 (6.2%). The causes were Streptococcus pneumoniae in 98 (51.6%) cases, Spn19A in 47 (48%), Spn3 in 16 (16%), and Spn6C in 4 (4%). Haemophilus influenzae was found in 32 (16.8%) cases: non-typeable H. influenzae in 20 (62.5%), H. influenzae type b in five (15.6%), and H. influenzae type a in two (6.2%). Other bacteria were found in 60 (31.6%) cases. A total of 51.6% and 42.9% of patients with S. pneumoniae had decreased sensitivity to penicillin and ceftriaxone, respectively.
Conclusions
The serotypes included in the conjugate vaccines for pneumococcus and H. influenzae b decreased significantly, and serotype turnover was observed. Among the S. pneumoniae cases, Spn19A, Spn3, and Spn6C were the most frequent serotypes and associated with multiresistance. The SSBP allows us to determine the clinical behavior of the disease and to detect the serotypes emergence.
Graphical abstract
Introduction
Community-acquired pneumonia is one of the main causes of hospitalization and death in children under 5 years of age. Over the last three decades, conjugate vaccines have reduced the incidence of pneumonia due to Streptococcus pneumoniae and Haemophilus influenzae type b. Currently, viruses are the main cause of pneumonia; however, the incidence of pneumonia is proportionally higher in low-income countries and the most frequent bacterial agents are S. pneumoniae, H. influenzae type b, Moraxella catarrhalis, and Staphylococcus aureus [1,2].
In Colombia, pneumococcal vaccination began in 2006 with the pneumococcal conjugate vaccine of seven serotypes (PCV7), initially focusing on children under 2 years of age at a high risk of invasive pneumococcal disease. Since 2008, the vaccine has been administered in Bogotá. From 2010 to 2011, given the withdrawal of PCV7 from the market, pneumococcal conjugate vaccine of 13 serotypes (PCV13) was administered in Bogotá and in high-risk areas. Since January 2012, pneumococcal conjugate vaccine of 10 serotypes (PCV10) has been administered universally, using a 2 + 1 scheme administered at 2 months, 4 months, and 12 months of age, with vaccination coverage between 85% and 89%. In July 2022, Colombia switched to PCV13 for the cohort of children born since May 1, 2022, using the same 2 + 1 scheme.
A study conducted in five cities in Colombia revealed that from 2012 to 2016, after the implementation of PCV10, the incidence of pneumonia in children under 2 years of age in Bogotá decreased by 66% (65.6-66.6), that in Medellín decreased by 40.6% (39.3-41.9), and that in Cartagena decreased by 15% (11.2-18.6), with increases in Cali and Barranquilla. A decrease in pneumonia mortality rates in children under 2 years old was also observed, with a national reduction of 48.8% (45.5-51.9), 77.1% (71.1-81.8) in Bogotá, 56.4% (44.1-65.9) in Medellín, 47.8% (33.8-58.9) in Cali, and 46.4% (30.5-58.7) in Barranquilla. No substantial reduction was observed in Cartagena [3].
Sentinel surveillance of bacterial pneumonia (BP) and bacterial meningitis contributes to the generation of standardized and timely data that can be used to characterize the epidemiological behavior of these two pathologies in children under 5 years of age, in addition to providing data that can be used to measure the impact of vaccines on the behavior of the serotypes, the incidence of the disease, and lethality [4]. In 2009, the World Health Organization (WHO) implemented the global sentinel surveillance network for vaccine-preventable invasive bacterial diseases [4]. In 2016, Colombia joined this network. This work presents the results of sentinel surveillance of pneumonia in children under 5 years of age carried out at HOMI, Fundación Hospital pediátrico de la Misericordia, between 2016 and 2022.
Materials and methods
From January 1, 2016 to December 31, 2022, a daily active search for suspected cases of BP was carried out through a system at HOMI, Fundación Hospital pediátrico de la Misericordia, located in Bogotá (Colombia), by checking the International Classification of Diseases 10th Revision codes from J10 to J22 in the database. For the purposes of epidemiological surveillance, in the Sentinel Surveillance Project of Pneumonia, the following case definitions established by the Pan American Health Organization (PAHO) were used [5]:
-
•
Suspicious cases: All hospitalized patients under 5 years of age with a medical diagnosis of community-acquired pneumonia.
-
•
Probable case: Any suspicious case in which the chest radiograph shows a radiological pattern compatible with BP, including the presence of any of the following: alveolar opacity, consolidation, pleural effusion, pneumatoceles, abscesses, or lung cavitation. In addition, it is characterized by a dense image with a cottony appearance (alveolar opacity), which partially or completely involves one or more lung segments or lobes or even an entire lung. These opacities often present areas of air bronchogram and, in some cases, are associated with pleural effusion.
-
•
Confirmed case: Any probable case of BP in which S. pneumoniae, H. influenzae, or other bacteria were identified or cultured in the blood or pleural fluid.
-
•
Discarded case: Any suspected case in which the chest radiograph does not show a radiological pattern compatible with BP.
-
•
Inadequately investigated case: Any suspicious case in which there is no chest X-ray.
The records of patients who were hospitalized at the institution were reviewed daily. A chest X-ray was ordered for suspected cases, and the chest radiographs were interpreted by pediatric radiologists according to the WHO imaging manual [6]. Two peripheral blood cultures were taken for probable cases, and, if there was pleural effusion, a culture of the pleural fluid was taken. The time between the onset of symptoms, the patient's consultation, and the moment of sample collection were analyzed. The proportion of patients who used antibiotics before sample collection was also analyzed, which could influence the microbiological results.
Clinical information and the vaccination history were collected, verifying the web page of Colombia and Bogotá Expanded Program of Immunization (https://paiweb2.paiweb.gov.co/login and https://appb.saludcapital.gov.co).
Identification of microorganisms
At the sentinel institution, the microorganisms isolated from the culture media were identified using the automated technique VITEK 2 system (bioMérieux, Marcy l'Etoile, France). The microorganisms were subsequently sent to the Public Health Laboratory of the District Health Secretariat of Bogotá, and the microorganisms were confirmed via the automated advanced colorimetry system VITEK 2 (bioMérieux, Marcy l'Etoile, France) and sent to the Microbiology Group of the Instituto Nacional de Salud of Colombia for confirmation and phenotypic characterization. The serotyping of S. pneumoniae was carried out via the Quellung reaction, antigen-antibody assays with commercial antisera (BD) and real-time polymerase chain reaction for cerebrospinal fluid and pleural fluid samples that were negative in culture. Serotyping of H. influenzae was performed via real-time polymerase chain reaction. Antimicrobial sensitivity profiles were determined via the disk diffusion method (Kirby-Bauer) and broth microdilution to penicillin (PEN), ceftriaxone (CRO), trimethoprim-sulfamethoxazole, chloramphenicol, tetracycline, erythromycin, and rifampin. The interpretation of the results was carried out according to the Clinical and Laboratory Standards Institute criteria for each year [7]. Multiresistance was defined as resistance to three or more classes of antibiotics.
Statistical analysis
A descriptive, prospective study was conducted. The data were collected via Excel and RStudio databases. Frequency analyses were performed on the included variables, which included sex, age, symptoms, X-ray results, identified microorganisms, vaccination status, serotypes, and the final condition of the patient. In addition to simple frequency analyses, bivariate analyses and statistical significance tests using chi-square tests were conducted. The most relevant findings are presented.
Ethical considerations
This was a risk-free study since the patients did not undergo any intervention or tests other than those indicated for the disease. This study was approved by the ethics and research committee of the HOMI, Fundación Hospital pediátrico de la Misericordia (CEI 2015, 2015).
Results
From 2016 to 2022, there were 75,846 hospital admissions of children under 5 years of age (average 10.835 per year), and, among them, there were 6.970 (9.2%) suspected cases of BP. The average number of suspected cases was 996 per year, with 2016 being the year with the highest number of cases (n = 1.333, representing 15.6% of hospital admissions of children under 5 years of age) and 2020 (n = 556) and 2021 (n = 501) being the years with the lowest number of cases, representing 7% and 4.2% of hospital admissions, respectively (Table 1). Seasonal behavior was observed, with peaks in the months of March, April, and May in all years except 2020 and 2021, during which the COVID-19 pandemic affected the usual behavior of these children (see Graph 1 in the Supplementary material).
Table 1.
Pneumonia indicators from 2016 to 2022.
| Indicator | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | Total |
|---|---|---|---|---|---|---|---|---|
| Number of suspected cases | 1333 | 1041 | 1273 | 1225 | 556 | 501 | 1041 | 6970 |
| Hospitalized | 8857 | 12420 | 10348 | 10653 | 7490 | 14997 | 14081 | 78846 |
| Percentage of hospitalizations for suspected bacterial pneumonia | 15.6% | 8.4% | 12.3% | 11.5% | 7% | 2.6% | 7.4% | 8.8% |
| Number of probable cases | 649 | 618 | 539 | 588 | 134 | 113 | 407 | 3048 |
| Percentage of probable cases | 49% | 59% | 42,26% | 48% | 24% | 22.5% | 39.1% | 43.7% |
| Percentage of probable cases with blood culture | 94% | 90% | 95% | 85% | 87.3% | 90.2% | 93% | 91% |
| Percentage of probable cases with pleural fluid | 0.3% | 3% | 3.7 | 3.4% | 6% | 2.6% | 5.9% | 3.6% |
| Number of laboratory confirmed cases for some bacterial agent | 41 | 26 | 34 | 25 | 6 | 18 | 40 | 190 |
| Percentage of cases confirmed by laboratory for some bacterial agent | 6.3% | 4.2% | 6.3% | 4.3% | 4.4% | 15.9% | 9.8% | 6.2% |
| Number of laboratory confirmed cases for Streptococcus pneumoniae and Haemophilus influenzae | 25 | 15 | 27 | 16 | 4 | 11 | 32 | 130 |
| Number of serotyped cases | 23 | 14 | 25 | 12 | 4 | 10 | 31 | 119 |
| Percentage of cases with serotype | 92% | 93% | 92.6% | 75% | 100% | 90.9% | 96.9% | 91.5% |
| Number of deaths in probable cases | 14 | 11 | 7 | 10 | 4 | 4 | 6 | 56 |
| Lethality probable cases | 2.2% | 1.8% | 1.3 | 1.7% | 3% | 3.5% | 1.5% | 1.8% |
| Number of deaths in confirmed cases by microorganisms under sentinel surveillance | 3 | 2 | 2 | 1 | 1 | 1 | 2 | 12 |
| Lethality in confirmed cases | 7.3% | 7.7% | 5.9% | 4% | 25% | 8.3% | 5.0% | 6.6% |
Among the total number of suspected cases, 3048 (43.7%) met the criterion of probable cases. The annual average was 435 cases; in 2016, there were 649 cases, and in 2020 and 2021, there were 133 and 113 cases, respectively (Table 1). The frequency of males was greater than that of females at 1.743 (57%). Regarding the age distribution of the probable cases, 954 (31.3%) were <12 months of age, 829 (27.2%) were between 12 and 23 months, 577 (18.9%) were between 24 and 35 months, 423 (13.9%) were between 36 and 47 months, and 265 (8.7%) were between 48 and 59 months. The median time between the onset of symptoms and hospital admission was 4 days (interquartile range [IQR] 3-7). The most frequent symptoms were cough (99.7%), fever (86%), respiratory distress (68%), intercostal retractions (52%), and vomiting (24.8%). A total of 570 (18.7%) patients received antibiotics during the week before hospitalization (Table 1s, Supplementary material). The most frequent radiological findings were consolidation (1.794, 58.9%), pleural effusion (193, 6.3%), interstitial opacities (259, 8.5%), and air bronchogram (130, 4.3%). There was an increase in pneumonia with empyema and an increase in patients whose pleural fluid was obtained (6% in 2020 and 2022). Since 2018, the median hospital stay was 7 days (IQR 5-13), and the proportion of patients admitted to the pediatric intensive care unit (PICU) was 35.6% (615 of 1780), with a median stay of 5 days (IQR 3-10). Between 2016 and 2022, 56 probable cases died, with a lethality rate of 1.8% (Table 1).
Among the 2777 (91.1%) probable cases, blood cultures were obtained, the median time between admission and blood culture collection was 0.6 days (IQR 0-1.2), and the median time between the onset of symptoms and blood culture collection was 6 days (IQR 3-8). The results were positive for a pathogen in 190 (6.2%) cases. Comparing blood culture positivity, a decrease from 7% in patients who did not receive antibiotics to 5% in those who did was observed (P = 0.03). The proportion of confirmed cases compared with probable cases was greater in 2021 (16.8%) and 2022 (9.8%) and lower in 2020 (3.7%). The average number of confirmed cases per year was 27.1. The years with the highest number of confirmed cases were 2016 and 2022, with 40 and 39 cases, respectively, and the years with the lowest number of confirmed cases were 2020 and 2021, with five and 19 cases, respectively (Table 1). The most common microorganisms were S. pneumoniae (98, 51.6%) and H. influenzae (32, 16.8%). Among these, 28 (87.5%) were analyzed in the reference laboratory, 20 (7.14%) were non-typeable H. influenzae, five (17, 8%) were H. influenzae type b, two (7.1%) were H. influenzae type a, and one (3.6%) was H. influenzae type f (Tables 2 and 3). The lethality rate among the confirmed patients was 6.3% (12 of 190), and, among them, eight had S. pneumoniae (three Spn3, two Spn 19A, one Spn 6C, one Spn 11A, and one not serotyped), one had H. influenzae non-typeable, one had Proteus mirabilis, one had Klebsiella pneumoniae, and one had Klebsiella oxytoca. The lethality ratio according to the microorganism was 8.1% (eight of 98) for S. pneumoniae, 3.1% (one of 32) for H. influenzae (typeable and non-typeable), and 5% (three of 60) for the other microorganisms.
Table 2.
Microorganisms most frequently isolated from patients with bacterial pneumonia.
| Microorganisms | 2016-2022 |
|
|---|---|---|
| n | % | |
| Streptococcus pneumoniae | 98 | 51.6 |
| Haemophilus influenzae | 32 | 16.8 |
| Staphylococcus aureus | 24 | 12.6 |
| Other | 19 | 10 |
| Klebsiella pneumoniae | 5 | 2.7 |
| Salmonella | 4 | 2.2 |
| Enterobacter cloacae | 3 | 1.6 |
| Klebsiella oxytoca | 2 | 1.0 |
| Serratia marcescens | 2 | 1.0 |
| Neisseria meningitidis | 1 | 0.5 |
| Total | 190 | 100 |
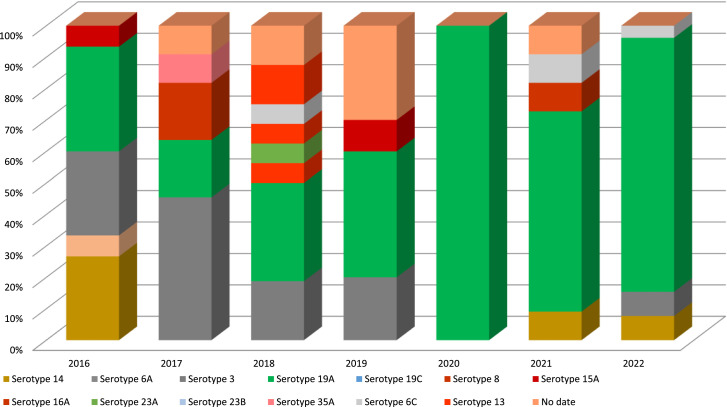
S. pneumoniae serotypes were detected in 91 (92.9%) cases, and the most frequent serotypes were 19A (n = 47; 51.6%), three (n = 16; 17.5%), 14 (n = 7; 7.7%), 6C (n = 4; 4.4%), and 9N (n = 4; 4.4%) (Table 4). Figure 1 shows the serotypes of S. pneumoniae by year; there was a decrease in the serotypes included in PCV10 and a predominance of Spn19A. The lethality rates for each pneumococcal serotype were 4.2% (two of 47) for 19A, 18.7% (three of 16) for 3, 25% (one of four) for 6C, and 100% (one of one) for 11A.
Table 4.
Antibiotic susceptibility of Streptococcus pneumoniae, Haemophilus influenzae, and Staphylococcus aureus.
|
Streptococcus pneumoniae (total) | ||||
|---|---|---|---|---|
| Antibiotic | Sensitive | Intermediate | Resistant | Total |
| n (%) | n (%) | n (%) | n (%) | |
| Penicillin | 44 (48,4) | 35 (38.5) | 12 (13) | 91 (100) |
| Ceftriaxone | 52 (57,1) | 32 (35,2) | 7 (7.7) | |
| Trimethoprim sulfa | 38 (41.8) | 2 (2.2) | 51 (56) | |
| Macrolides | 43 (47.3) | 0 | 48 (52.7) | |
| Chloramphenicol | 91 (100) | 0 | 0 | |
| Vancomycin | 91 (100) | 0 | 0 | |
| Streptococcus pneumoniae serotype 19A | ||||
| Penicillin | 7 (15.6) | 29 (64.4) | 9 (20) | 45 (100) |
| Ceftriaxone | 14 (31.1) | 27 (60) | 4 (8.9) | |
| Trimethoprim sulfa | 5 (11.1) | 1 (2.2) | 39 (86.7) | |
| Macrolides | 6 (13.3) | 0 | 39 (86.7) | |
| Chloramphenicol | 45 (100) | 0 | 0 | |
| Vancomycin | 45 (100) | 0 | 0 | |
| Haemophilus influenzae | ||||
| Ampicillin | 22 (84.6) | 1 (3.9) | 3 (11.5) | 26 (100) |
| Cefuroxime/Ceftriaxone | 26 (100) | 0 | 0 | |
| Trimethoprim sulfa | 18 (69.2) | 0 | 8 (39.8) | |
| Rifampicin | 26 (100) | 0 | 0 | |
| Staphylococcus aureus | ||||
| Oxacillin | 13 (54.2) | 0 | 11 (45.8) | 24(100) |
| Clindamycin | 24 (100) | 0 | 0 | |
Figure 1.
Streptococcus pneumoniae serotypes, HOMI 2016-2022.
Among the 98 cases of confirmed S. pneumoniae infection, 97 (99%) had complete vaccination data and 82 (84.5%) had a complete vaccination schedule with three doses of PCV10. Among the 32 patients confirmed to have H. influenzae infection, 28 (87.5%) had complete vaccination data, of whom 17 (60.7%) had a complete vaccination schedule with three doses of the pentavalent vaccine. The relationships of the isolated serotypes with the vaccination status are presented in Table 3. It is observed that six of seven (85.7%) patients with serotypes included in the PCV10 and 39 of 47 (83%) with Spn19A had received three PCV10 doses, and three of five (60%) patients with H. influenzae type b had three doses of the pentavalent vaccine.
Table 3.
Confirmed bacterial pneumonia (BP) cases for Streptococcus pneumoniae and Haemophilus influenzae and vaccine status - HOMI 2016-2022.
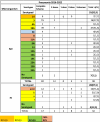 |
PCV, pneumococcal conjugate vaccine.
Antibiotic susceptibility data were obtained for 91 (92.9%) of the S. pneumoniae isolates, 45 (95.7%) of the Spn19A isolates, and 26 (81.3%) of the H. influenzae isolates. Table 4 shows the proportions of antibiotic-resistant strains. A higher proportion of resistance was observed for Spn19A than for the other serotypes.
S. aureus was the third most common microorganism and was responsible for 24 (12.6%) of the confirmed pneumonia cases; 45.8% were methicillin resistant, and all the isolates were susceptible to clindamycin.
Discussion
This research presents the results of sentinel BP surveillance carried out from 2016 to 2022 in children under 5 years of age who were treated at HOMI, Hospital pediátrico de la Misericordia. This surveillance is essential for understanding the epidemiological patterns of pneumonia and determining the prevalence of bacterial agents and their serotypes during the study period.
The proportion of suspected cases requiring hospitalization has decreased over time, from 15.6% in 2016 to 7.4% in 2022, which is related to the implementation of measures to prevent acute respiratory infection, including vaccination against S. pneumoniae, H. influenzae, influenza, and SARS-CoV-2. Other studies have shown the positive impact of these vaccines [1,8,9].
The number of suspected and probable cases decreased in 2020 and 2021 but increased in 2022, similar to observations in other countries [10,11]. This pattern is related to the measures implemented during the COVID-19 pandemic, which decreased the incidence of viral and bacterial infections [10,11]. Studies carried out in Israel have shown that the incidence of respiratory syncytial virus infection, pneumococcal pneumonia, and invasive pneumococcal disease decreased during the pandemic but that the nasopharyngeal carriage of S. pneumoniae did not decrease, highlighting the importance of respiratory viruses in the genesis of pneumococcal pneumonia [11]. In the present study, a seasonal pattern was observed, which was associated with the months with the highest rainfall (March-June); these data are very similar to those reported in Bogotá from 2015 to 2023 [12].
The proportion of suspected cases that met the criteria of probable cases was 43.7%. This percentage is higher than that reported in a previous study carried out in Bogotá by the Saludcoop group, in which it was 35.1% and included children aged 0-36 months [13]. This percentage is lower than that reported in the results of sentinel surveillance in Bogotá in 2016 [14] and similar to that reported in Bogotá from 2016 to 2020 [15]. The PAHO sentinel surveillance protocol estimates that 40% of suspected cases meet the criteria for probable cases.
In total, 68% of the patients presented with respiratory distress, which, together with hypoxemia, is considered a sensitive clinical sign for the diagnosis of pneumonia [14,16].
The sentinel surveillance protocol of the WHO/PAHO is based on the results of chest radiography [5,6], which has shown variable sensitivity (93% in some studies [17] and 34.3% in others) [18]. It has also been estimated that the negative predictive value for infection by S. pneumoniae from a normal chest X-ray is 86.3% [19]. In the present study, consolidation was the most frequent finding, which is consistent with findings reported in other studies [13]. An increase in cases of complicated pneumonia was observed, similar to that reported in other studies [16,20]. The proportion of PICU admissions (35.6%) was greater than that reported in the 2016 sentinel surveillance report (28.2%) [14] and that reported by Jain (21%) [21] and lower than that reported by Del Rosal (44%) and Gutiérrez (51.6%), possibly because these studies described the behavior of confirmed cases of S. pneumoniae [16,22], unlike the present study which included probable and confirmed cases. The lethality rate was higher among confirmed cases (6.3%) than among probable cases (1.8%). The lethality rate of probable cases was higher than that reported by Jain et al. (<1%) [21]. The lethality of patients from whom S. pneumoniae was isolated (8.1%) was greater than that reported by Rojas et al. (3.1%) [23] and lower than that reported in other studies (8.3-8.7%) [16,24]. Spn11A (100%), Spn6C (25%), and Spn3 (18.7%) presented the highest lethality rates by serotype, and it is important to highlight that there was only one case of Spn11A. The lethality from pneumococcus in this study was greater than that from H. influenzae (3.1%) and other microorganisms (5%), possibly because of the virulence factors of the microorganism. The high proportion of admissions to the PICU and the higher fatality rate observed in this study may be related to the fact that it was conducted in a high-complexity pediatric hospital where patients with more severe infections are referred. This may also be related to the delay in seeking medical care by the family (median of 4 days, IQR 3-7) and the prevalence of Spn19A associated with multidrug resistance and Spn 3 associated with complicated pneumonia and worse outcomes [16].
The percentage of positive blood cultures in pneumonia patients is low because, in most cases, the microorganism spreads contiguously from the upper respiratory tract. It is estimated that only 10-20% of cases occur due to hematogenous dissemination [2,17,21]. The percentage of positive blood cultures (6.2%) was similar to that reported by Lakhani et al. (6.1%) and Jain et al. (8%) [17,21] and higher than that reported by Benavides (1.5%) [13], probably related to the implementation of incubation methods, such as the CO2 chamber and the use of automated methods for detection. The percentage of positive cultures was lower in patients who received antibiotics before consultation (5% vs 7%). These findings demonstrate that previous antibiotic administration influences blood culture results.
S. pneumoniae and H. influenzae were the most common microorganisms, similar to the findings of other studies [1,25]. Viruses are currently considered to be the most frequent cause of pneumonia, and the relationship between them and invasive bacterial disease has been demonstrated [2,11]. Therefore, incorporating this search into sentinel surveillance protocols is recommended.
The most frequent serotype of S. pneumoniae was 19A, followed by Spn3, Spn14, and Spn6C, a finding similar to that reported in the 2016 sentinel surveillance report [14]. According to the 2016-2020 report [15], in the national surveillance system [26], in other studies carried out in Colombia [16] and in other countries of the region [27], a phenomenon was observed, especially in the countries after the use of PCV7 and PCV10. Most of the patients had a complete vaccination scheme, and no cross-protection against Spn19A was observed. No isolates of Spn22F or Spn33F included in PCV15 were detected, and one isolate of Spn8 and one of Spn11A included in pneumococcal conjugate vaccine of 20 serotypes (PCV20) were detected. The proportion of serotypes included in PCV10 was 7.7%, in PCV13 was 78%, in PCV15 was 78%, and in PCV20 was 80.2%. If cross-protection of PCV13, PCV15, and PCV20 against Spn6C is assumed, this percentage increases by 4.4%.
A high proportion of pneumococcus strains resistant to PEN, CRO, and macrolides were detected. Colombian surveillance has shown that S. pneumoniae nonsusceptibility rates to β-lactams increased during 2005-2023, associated with serotypes such as 19A [28]; PEN resistance increased from 11.53% to 30.18%, CRO resistance increased from 8.04% to 24.25%, and macrolide resistance increased from 5.24% to 47.8%. Similar findings were reported in this study. The increase in isolates of serotype 19A nonsusceptible to β-lactams may be due to the imprudent use of antibiotics or the spread of multiresistant 19A clones (ST320, ST276, and ST1118), among others [29], which is similar to what has been reported in other studies in Colombia and Brazil [14,26,27,30]. Based on these findings, in July 2022, Colombia changed the recommended vaccine to PCV13 in a 2 + 1 scheme for the cohort of children born after May 1, 2022. Considering that 41.7% of patients with pneumonia are older than 2 years and that Spn19A predominates, the implementation of a catch-up strategy for boys and girls aged 2 to 5 years should be evaluated because this strategy has proven to be successful in Taiwan [31].
The most frequent type of H. influenzae was non-typeable H. influenzae, at 62.5%, similar to that reported in other studies [32]. Resistance to ampicillin (11.5%) was lower than that reported in the national surveillance report from 2015 to 2019 (33%) and resistance to trimethoprim sulfa was similar. No isolates resistant to chloramphenicol, cefuroxime, or CRO were found [33]. The systematic administration of a vaccine against H. influenzae type b has decreased its incidence; however, some cases were reported in patients who had received three doses of the vaccine, which is why, since 2023, Colombia has implemented a booster dose with the pentavalent vaccine at 18 months. The ratio of resistance to ampicillin is low; therefore, ampicillin, ampicillin/sulbactam, or amoxicillin/clavulanate should be used to treat infections caused by this organism.
S. aureus is an emerging microorganism in pneumonia; in the present study, it was responsible for 12.6% of pneumonia cases, a higher percentage than that reported by Frush et al. [34] (1%). This microorganism is associated with more severe conditions and is, therefore, suspected to be involved in cases of complicated pneumonia. Resistance to methicillin was similar to that reported in other studies; the high susceptibility to clindamycin allows its use in cases where this microorganism is suspected [35].
The findings of this study have clinical implications. For patients with BP, empirical treatment with amoxicillin at 90 mg/kg/day, crystalline PEN at 300,000 IU/kg/day, or ampicillin at 200 mg/kg/day is recommended as first-line treatment. This approach adequately covers S. pneumoniae isolates that are sensitive (MIC ≤2) (48,4%) and those with intermediate sensitivity (MIC 4) (38,5%, 86.9% of isolates in total). Amoxicillin or ampicillin affects sensitive (MIC ≤1) H. influenzae (84.6%). In cases of suspected S. aureus infection (complicated pneumonia), the addition of clindamycin is recommended. An increase in the resistance of Streptococcus pneumoniae is associated with the emergence of Spn19A multidrug-resistant strains. The switch from PCV10 to PCV13 vaccination may reduce resistance by protecting against this serotype; however, given the current increase in resistance to PEN and CRO, the use of cloranfenicol, linezolid, or ceftaroline should be considered for patients who do not respond adequately to initial treatment or who have PEN and third-generation cephalosporin-resistant pneumococcus isolates.
The present study provides clinical, epidemiological, and microbiological information on BP patterns in children under 5 years of age at a sentinel hospital in Colombia. The strengths of this study are the coordinated action of different institutions (HOMI, Fundación Hospital pediátrico de la Misericordia, Bogotá Health Secretariat, Instituto Nacional de Salud, Ministry of Health and PAHO), the prospective collection of information, which was maintained during the pandemic, the high percentage of serotyping, and the characteristics of the sentinel hospital, which is a level IV pediatric hospital, with infrastructure and epidemiological support to ensure the quality of the data. The sentinel surveillance data were used by the Ministry of Health to support the decision to switch to PCV13 and include the booster dose of H. influenzae through the administration of the pentavalent vaccine at 18 months. One of the limitations of the study is the exclusion of children over 5 years old. Previous studies have shown that 14% of invasive pneumococcal pneumonia cases occur in children of that age [16,24]. However, the study was conducted following the PAHO protocol, which targets children under 5 years of age. This allows better comparability with other countries and regions that conduct similar epidemiological surveillance. Another limitation of this study is that because it was carried out at a level IV referral hospital, the data may not be extrapolated to other populations. There may be bias due to errors in data collection, which was estimated at 7.8%. To minimize this bias, supervision and control are used in the processes of data collection, entry, and consolidation. In cases where a deviation is found, primary sources are consulted, including medical records, vaccination cards, and laboratory results, to obtain the correct data. There may be bias due to the performance of the diagnostic methods used. The descriptive data analysis may be limited for some inferential analyses. Selection biases may occur because, owing to the level of complexity of the institution wherein patients with severe pneumonia are treated, there may also be biases derived from radiological interpretation. To reduce interobserver variability, workshops were held with radiologists and the criteria defined by the WHO for the report. The limitations of this study include the lack of inclusion of viral infection data within the protocol and the loss of some of the microorganisms due to contamination, dead strains, or failure to send the samples to the reference laboratory. The sending process has been standardized, and constant communication is maintained between the hospital laboratory, the study monitor, and the reference laboratories, verifying the viability of the samples sent before they are discarded.
Conclusion
The measures implemented, including vaccination against S. pneumoniae, H. influenzae type b, influenza, and other respiratory pathogens, have decreased the morbidity and mortality due to acute respiratory infection (ARI) in children under 5 years of age; however, BP continues to be a frequent cause of consultation, hospitalization, and mortality in this age group, and S. pneumoniae continues to be the most frequent agent, with an increase in resistance to PENs, CRO, and macrolides and greater lethality than that caused by other microorganisms. The exchange of serotypes observed with the implementation of pneumococcal conjugate vaccines and the absence of vaccines that cover all serotypes make it necessary to have surveillance systems that allow the detection of these changes and the implementation of vaccines that include emerging serotypes. A sentinel surveillance strategy is useful for achieving this goal. Furthermore, it is necessary to conduct nasopharyngeal carriage studies and longitudinal studies to track the long-term effects of vaccination. Viral diagnostics should be included in the sentinel surveillance protocol, and molecular studies should be conducted to determine the clones associated with antimicrobial resistance.
Declarations of competing interest
Germán Camacho-Moreno has received support for participation in congresses and conference payments from Pfizer, Adium, Biomerieux, Sanofi and Merck Sharp and Dohme (MSD); has participated in the MSD advisory council; and has received support from Pfizer and MSD for other research. The other authors declare that they do not have conflicts of interest.
Acknowledgments
Funding
The present study was financed with resources from the following participating institutions: Secretaria Distrital de Salud, HOMI, Instituto Nacional de Salud, Pan American Health Organization, and the Ministry of Health.
Ethical approval statement
This was a risk-free study because the patients did not undergo any intervention or tests other than those indicated for the disease. This study was approved by the ethics and research committee of the HOMI, Fundación Hospital pediátrico de la Misericordia (CEI 2015, 2015).
Author contributions
Drs. Germán Camacho Moreno, Carolina Duarte, Maria del Pilar Perdomo, Luz Yanet Maldonado, Jaime Moreno, Adriana Bautista, conceptualized and designed the study, designed the data collection instruments, analyzed the information, drafted the initial manuscript, and critically reviewed and revised the manuscript. Drs. Carolina Duarte, Luz Yanet Maldonado, Jaime Moreno, Daniela Jerez, Olga Sanabria, Adriana Bautista, processed the laboratory samples. Drs. Germán Camacho Moreno, Carolina Duarte, Maria del Pilar Perdomo, Luz Yanet Maldonado, Jacqueline Palacios, Jaid Constanza Rojas, Jaime Moreno, Daniela Jerez, María Cristina Duarte, Evelyn Degraff, Olga Sanabria, Eliana Sabogal, Adriana Bautista, Yenny Elizalde, Karen Jimenez coordinated and supervised data collection, collected data, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2024.100449.
Appendix. Supplementary materials
References
- 1.Wahl B, O'Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health. 2018;6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer Sauteur PM. Childhood community-acquired pneumonia. Eur J Pediatr. 2024;183:1129–1136. doi: 10.1007/s00431-023-05366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrasquilla G, Porras-Ramírez A, Martinez S, DeAntonio R, Devadiga R, Talarico C, et al. Trends in all-cause pneumonia and otitis media in children aged <2 years following pneumococcal conjugate vaccine introduction in Colombia. Hum Vaccin Immunother. 2021;17:1173–1180. doi: 10.1080/2165515.2020.180599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litt D, Slack MPE, Nakamura T, Gray S, Seaton S, Fagan EJ, et al. Evaluation of the World Health Organization global invasive bacterial vaccine-preventable disease (IB-VPD) surveillance network's laboratory external quality assessment programme, 2014–2019. J Med Microbiol. 2023;72 doi: 10.1099/jmm.0.001644. [DOI] [PubMed] [Google Scholar]
- 5.Vigilancia de las neumonías y meningitis bacterianas en menores de 5 años. Organización Panamericana de la Salud, 2020. 10.37774/9789275321881. [DOI]
- 6.Ellis SM, Flower C. World Health Organization; Geneva: 2006. The WHO manual of diagnostic imaging: radiographic anatomy and interpretation of the chest and the pulmonary system. [Google Scholar]
- 7.Lewis James S II. Clinical and Laboratory Standards Institute; Berwyn: 2022. M100 performance standards for antimicrobial S.I. [Google Scholar]
- 8.López EL, Glatstein E, Ezcurra GC, Iacono M, Teplitz E, Garnero AV, et al. Rapid decrease in rates of hospitalization resulting from invasive pneumococcal disease and community-acquired pneumonia in children aged <60 months after 13-valent Pneumococcal Conjugate Vaccine introduction in Argentina. J Pediatric Infect Dis Soc. 2018;7:30–35. doi: 10.1093/jpids/piw089. [DOI] [PubMed] [Google Scholar]
- 9.Messiah SE, Talebi Y., Swartz MD, Sabharwal R, Han H, Bergqvist E, et al. Long-term immune response to SARS-CoV-2 infection and vaccination in children and adolescents. Pediatr Res. 2024;96:525–534. doi: 10.1038/s41390-023-02857-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw D, Abad R, Amin-Chowdhury Z, Bautista A, Bennett D, Broughton K, et al. Trends in invasive bacterial diseases during the first 2 years of the COVID-19 pandemic: analyses of prospective surveillance data from 30 countries and territories in the IRIS Consortium. Lancet Digit Health. 2023;5:e582–e593. doi: 10.1016/S2589-7500(23)00108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan R, Van Der Beek BA, Ben-Shimol S, Greenberg D, Shemer-Avni Y, Weinberger DM, et al. The COVID-19 pandemic as an opportunity for unravelling the causative association between respiratory viruses and pneumococcus-associated disease in young children: a prospective study. EBiomedicine. 2023;90 doi: 10.1016/j.ebiom.2023.104493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Secretaria de Salud de Bogota. Canal endemico de Infeccion respiratoria aguda en Bogotá, https://saludata.saludcapital.gov.co/osb/indicadores/canal-endemico-ira/; n.d. [accessed 17 February 2024].
- 13.Benavides JA, Ovalle OO, Salvador GR, Gray S, Isaacman D, Rodgers GL. Population-based surveillance for invasive pneumococcal disease and pneumonia in infants and young children in Bogotá, Colombia. Vaccine. 2012;30:5886–5892. doi: 10.1016/j.vaccine.2012.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Camacho-Moreno G, Duarte C, García D, Calderón V, Maldonado LY, Castellar L, et al. Sentinel surveillance for bacterial pneumonia and meningitis in children under the age of 5 in a tertiary pediatric hospital in Colombia - 2016. Biomédica. 2021;41:62–75. doi: 10.7705/biomedica.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camacho Moreno G, Duarte C, Palacios J, Calvo LA, Talavera I, Castañeda JM, et al. Sentinel surveillance of bacterial pneumonia in children under 5 years treated in HOMI - Fundación Hospital Pediatrico la Misericordia in Bogotá, Colombia 2016-2020. Open Forum Infect Dis. 2021;8:S665. doi: 10.1093/ofid/ofab466.1340. [DOI] [Google Scholar]
- 16.Gutiérrez-Tobar IF, Londoño-Ruiz JP, Mariño-Drews C, Beltrán-Higuera S, Camacho-Moreno G, Leal-Castro AL, et al. Epidemiological characteristics and serotype distribution of culture-confirmed pediatric pneumococcal pneumonia before and after PCV 10 introduction, a multicenter study in Bogota, Colombia, 2008–2019. Vaccine. 2022;40:2875–2883. doi: 10.1016/j.vaccine.2022.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Lakhani D, Muley P. The association of positive chest radiograph and laboratory parameters with community acquired pneumonia in children. J Clin Diagn Res. 2013;7:1629–1631. doi: 10.7860/JCDR/2013/5132.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingerter SL, Bachur RG, Monuteaux MC, Neuman MI. Application of the World Health Organization criteria to predict radiographic pneumonia in a US-based pediatric emergency department. Pediatr Infect Dis J. 2012;31:561–564. doi: 10.1097/INF.0b013e31824da716. [DOI] [PubMed] [Google Scholar]
- 19.Andrade DC, Borges IC, Vilas-Boas AL, Fontoura MS, Araújo-Neto CA, Andrade SC, et al. Infection by Streptococcus pneumoniae in children with or without radiologically confirmed pneumonia. J Pediatr (Rio J) 2018;94:23–30. doi: 10.1016/j.jped.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Madhi F, Levy C, Morin L, Minodier P, Dubos F, Zenkhri F, et al. Change in bacterial causes of community-acquired parapneumonic effusion and pleural empyema in children 6 years after 13-valent pneumococcal conjugate vaccine implementation. J Pediatric Infect Dis Soc. 2019;8:474–477. doi: 10.1093/jpids/piy103. [DOI] [PubMed] [Google Scholar]
- 21.Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372:835–845. doi: 10.1056/NEJMoa1405870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Rosal T, Caminoa MB, González-Guerrero A, Falces-Romero I, Romero-Gómez MP, Baquero-Artigao F, et al. Outcome of severe bacterial pneumonia in the era of pneumococcal vaccination. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.576519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas JP, Leal AL, Patiño J, Montañez A, Camacho G, Beltrán S, et al. Characterization of patients who died of invasive pneumococcal disease in the child population of Bogota, Colombia. Rev Chil Pediatr. 2016;87:48–52. doi: 10.1016/j.rchipe.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Camacho-Moreno G, Leal AL, Patiño-Niño J, Vasquez-Hoyos P, Gutiérrez I, Beltrán S, et al. Serotype distribution, clinical characteristics, and antimicrobial resistance of pediatric invasive pneumococcal disease in Colombia during PCV10 mass vaccination (2017–2022) Front Med (Lausanne) 2024;11 doi: 10.3389/fmed.2024.1380125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Von Mollendorf C, Berger D, Gwee A, Duke T, Graham SM, Russell FM, et al. Aetiology of childhood pneumonia in low- and middle-income countries in the era of vaccination: a systematic review. J Glob Health. 2022;12:10009. doi: 10.7189/jogh.12.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Instituto Nacional de Salud. Informe de Vigilancia por laboratorio de Streptococcus pneumoniae en Colombia, 2016–2021, file:///C:/Users/USER/Dropbox/Mi%20PC%20(DESKTOP-56NQROC)/Downloads/vigilancia-por-laboratorio-de-streptococcus-pneumoniae-en-colombia-2016-2021.pdf; 2021 [accessed 13 June 2022].
- 27.Agudelo CI, Castañeda-Orjuela C, Brandileone MCC, Echániz-Aviles G, Almeida SC, Carnalla-Barajas MN, et al. The direct effect of Pneumococcal Conjugate Vaccines on invasive pneumococcal disease in children in the Latin American and Caribbean region (SIREVA 2006–17): a multicentre, retrospective observational study. Lancet Infect Dis. 2021;21:405–417. doi: 10.1016/S1473-3099(20)30489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grupo de Microbiología, Instituto Nacional de Salud. Vigilancia por laboratorio de S. pneumoniae 2005–2023, https://app.powerbi.com/view?r=eyJrIjoiYjI0MDM2YjctODdhNS00YjA1LTgxYzUtNmQ1ZmJkNmIxM2MwIiwidCI6ImE2MmQ2YzdiLTlmNTktNDQ2OS05MzU5LTM1MzcxNDc1OTRiYiIsImMiOjR9; 2023 [accessed 29 August 2024].
- 29.Ramos V, Parra EL, Duarte C, Moreno J. Characterization of Streptococcus pneumoniae invasive serotype 19A isolates recovered in Colombia. Vaccine. 2014;32:755–758. doi: 10.1016/j.vaccine.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Brandileone M-CC, Almeida SCG, Bokermann S, Minamisava R, Berezin EN, Harrison LH, et al. Dynamics of antimicrobial resistance of Streptococcus pneumoniae following PCV10 introduction in Brazil: nationwide surveillance from 2007 to 2019. Vaccine. 2021;39:3207–3215. doi: 10.1016/j.vaccine.2021.02.063. [DOI] [PubMed] [Google Scholar]
- 31.Lu C-Y, Chiang C-S, Chiu C-H, Wang ET, Chen YY, Yao SM, et al. Successful control of Streptococcus pneumoniae 19A replacement with a catch-up primary vaccination program in Taiwan. Clin Infect Dis. 2019;69:1581–1587. doi: 10.1093/cid/ciy1127. [DOI] [PubMed] [Google Scholar]
- 32.León ME, Kawabata A, Nagai M, Rojas L, Chamorro G, Zárate N, et al. Epidemiologic study of Haemophilus influenzae causing invasive and non-invasive disease in Paraguay (1999–2017) Enferm Infecc Microbiol Clin (Engl Ed) 2021;39:59–64. doi: 10.1016/j.eimc.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Sanabria O, Bautista A, Duarte C, Instituto Nacional de Salud. Vigilancia por laboratorio de aislamientos colombianos de Haemophilus influenzae 2015–2019, https://app.powerbi.com/view?r=eyJrIjoiMDIzYzMyYWEtMWExZC00YWZiLTgwM2UtYWE0NDUzYTdhMTgzIiwidCI6ImE2MmQ2YzdiLTlmNTktNDQ2OS05MzU5LTM1MzcxNDc1OTRiYiIsImMiOjR9; n.d. [accessed 10 February 2024].
- 34.Frush JM, Zhu Y, Edwards KM, Grijalva CG, Thomsen IP, Self WH, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13:848–852. doi: 10.12788/jhm.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutierrez-Tobar I, Carvajal C, Vasquez-Hoyos P, Díaz-Díaz A, Londono Ruiz JP, Andrade J, et al. Epidemiological and microbiological characteristics of S. aureus pediatric infections in Colombia 2018–2021, a national multicenter study (Staphylored Colombia) Front Pediatr. 2024;12 doi: 10.3389/fped.2024.1386310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.