Abstract
Objectives. Post exposure prophylaxis (PEP) with the hepatitis B vaccine (HBVac) in combination with HBV immunoglobulins (HBIG) significantly minimizes the odds of vertical transmission of HBV to newborn infants. In this retrospective study, we aimed to evaluate the compliance and efficacy of PEP in a tertiary care center in Saudi Arabia. Methods. Infants were tested with HBV serological markers at 7 months of age to assess their PEP protection rate. Results. Out of 13,125 mothers who delivered in KAMC, 105 (0.8%) mothers were found to have HBsAg positive, with a prevalence of 8 per 1000 live births. All infants (n = 100) completed their PEP as per protocol before discharge from the hospital (2 days after delivery). Among infants (n = 59; 56.2%) who were tested at 7 months of age, all (100%) were found to be negative for HBV. Conclusion. PEP achieved 100% efficacy among infants who complied with the study protocol at 7 months of follow-up. The prevalence of hepatitis B among pregnant women was 8 per 1000 live births.
Keywords: hepatitis B virus, vertical transmission, immuno-prophylaxis, vaccination, prevention strategies, Saudi Arabia
Introduction
Chronic hepatitis B virus (HBV) infection is estimated to affect approximately 240 to 360 million people worldwide and almost a third of the world’s population presents with the historical evidence of hepatitis infection which ranks 15th and has a substantial morbidity and mortality, primarily via chronic infection. 1 People who are chronically infected with HBV are at risk of developing cirrhosis, hepatic decompensation, and hepatocellular carcinoma (HCC).2,3 Since the virus is detected in all body fluids such as serum, urine, breast milk, saliva, urine, tears, vaginal secretions, menstrual blood, and semen, its transmission can be via perinatal, percutaneous, close contact through open cuts and sores, sexual intercourse etc.4,5
The most common method of transmission of HBV is perinatal transmission at birth or horizontal transmission to/between young children. 6 The risk of perinatal transmission is associated with 4 important factors (i) hepatitis Be Ag status of the mother, (ii) serum HBs levels, (iii) HBV viral load, and (iv) earlier age of pregnancy.7,8 It has been demonstrated that the rate of vertical transmission of infection in children from HBeAg-positive women ranges from 70% to 90% in comparison to only 32% for HBeAg-negative women.2,9
The World Health Organization (WHO) via its Global Health Sector Strategy aims to decrease the HBV prevalence to <0.1% in children below 5 years of age.10,11 Thus, preventing the vertical transmission of the infection is critical and is based on the following 3 pillars: (i) Administration of timely HBV vaccine (within 24 hours) to a newborn and follow up with at least 3 doses to complete the primary series, regardless of the HBsAg status of the pregnant mother, (ii) effective screening of pregnant women for HBsAg and administration of hepatitis B immunoglobulin (HBIG) to infants born to HBsAg-positive and HBeAg-positive mothers, and (iii) antiviral prophylaxis in pregnant women with HBV DNA≥200,000 IU/mL.11-13
The Saudi Arabian vaccination program has added HBVac as the seventh primary immunogenic since 1989, ensuring that all newborn infants are duly vaccinated at the time of birth; however, the population aged above 30 years is still regarded as a risk group and there is a gap in HBV care which needs to be screened proactively.14-16 In Saudi Arabia, the prevalence of HBV has witnessed a dramatic decline over the last 3 decades with the establishment of the HBV immunization program, 17 and it has been reported to be 1.7% which is lower than the worldwide prevalence of 3.6%.18-20 Furthermore, owing to the universal childhood vaccination program, the prevalence of chronic HBV infection in the younger Saudi population (<30 years) is estimated to be <0.5%.20,21
The aim of this study was to assess compliance with the administration of the PEP as per the standard protocol and to report the outcomes of adherence to the recommended guidelines for PEP. The following objectives were kept in mind when carrying out this study: to evaluate the efficacy and compliance of postexposure prophylaxis (PEP) among infants and compare the efficacy of PEP to the international conversion rates.
Methods
Study Design
This was a retrospective cohort study designed and performed at King Abdulaziz Medical City, Jeddah and was carried out between June 2016 and May 2020 (4 years).
Ethical Approval
The study was approved by the Institutional Review Board (IRB; RJ20-044-J) committee of the University. The chart review of the electronic medical records from the patient care and hospital information system (BestCare) was done. Written informed consent from participating subjects and or their guardians was taken as per Helsinki principles.
Sampling
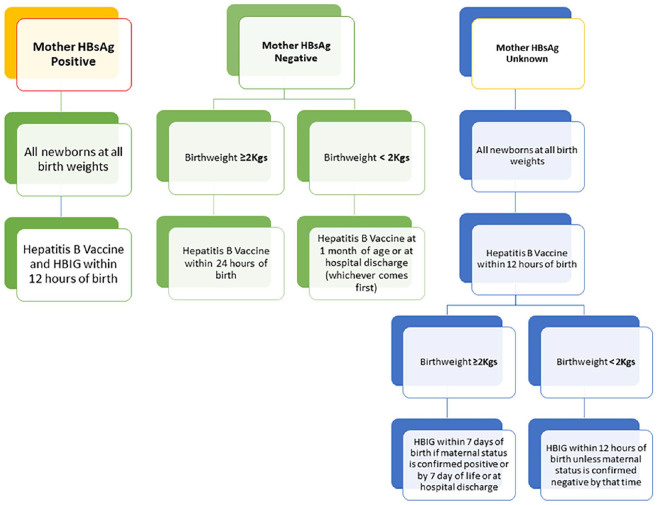
All pregnant mothers who were HBsAg positive at the time of delivering their newborn infants at KAMC, Jeddah, were included in the analysis. All live newborn infants whose mothers were HBsAg positive and subsequently planned to receive the hepatitis B vaccine (HBVac) and hepatitis B immunoglobulin (HBIG) within 12 hours of birth were included in this study. The newborn infants who failed to complete the vaccination protocol at 2, 4, and 6 months as per standard vaccination guidelines were excluded from the study. Serologic testing of babies, using chemiluminescent micro particle immunoassay (ABBOTT Alinity, UK), was carried out at 7 months using HBsAg and anti-HBs titers after the completion of 3 doses of vaccine. The sensitivity of the test is 100% as compared to PCR. The standard protocol followed in the Pediatric Department is based on the American Academy of Pediatrics (AAP) and Centers for Disease Control and Prevention vaccination guidelines (Figure 1).
Figure 1.
American Academy of Pediatrics (AAP) vaccination protocol used for the vaccination of pediatric patients.
Data Analysis
Statistical analysis was performed using SPSS software, version 27.0, for Windows (SPSS, Inc., Chicago, IL, USA). Categorical variables were presented as frequencies and percentages. Patients’ demographics and characteristics were analyzed using frequency histograms to ascertain distribution and outliers. Measure of central tendency for example, mean ± standard deviation was used. Co-morbidities were studied as possible risk factors using univariate analysis.
Results
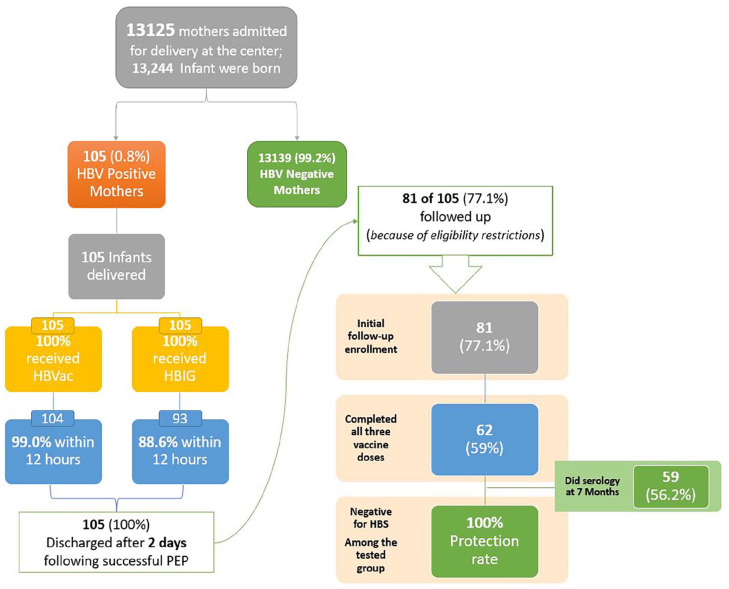
In this observational study, 13215 mothers were admitted to our center for scheduled delivery, of whom 105 tested positive for HBV. 13,244 infants were born during this observational period. 105 neonates were born to mothers with HBsAg positive status, with an incidence rate of 8 per 1000 live births. Thus, the HBsAg-positive rate of pregnant women was 1% (105/13244).
Among the HBsAg-positive mothers, 18 (17.1%) were <30 years of age, 49 (46.7%) had no previously diagnosed co-morbidities, and the majority (n = 73.3%) of them were detected to be HBV positive during their antenatal care (Table 1). Additionally, 6 (5.7%) mothers were found to be positive for hepatitis B E antigen (HBeAg). Furthermore, 45 (42.8%) of the neonates were females, 98 (93.3%) had a birth weight of ≥2 kg, and 68.6% were born full term (gestational age ≥38) (Tables 2 and 3). All included neonates received HBVac and HBIG post-delivery. However, 99.1% of newborns received the first HBVac and 88.6% received HBIG within 12 hours of delivery (Figure 2).
Table 1.
Characteristics of Mothers with HBV Infection vis a vis with Their HB Antigen and Antibody Status.
| n = 105 | % | |
|---|---|---|
| Maternal age | ||
| <30 Years | 18 | 17.1 |
| ≥30 Years | 87 | 82.9 |
| Mode of delivery | ||
| Vaginal delivery | 66 | 62.9 |
| Caesarian delivery | 39 | 37.1 |
| Maternal comorbidities | ||
| GDM | 20 | 19.0 |
| Gestational HTN | 1 | 0.9 |
| Others | 35 | 33.3 |
| None | 49 | 46.7 |
| Timing of screening and diagnosis | ||
| Antenatal | 77 | 73.3 |
| First trimester | 12 | 11.4 |
| Second trimester | 7 | 6.7 |
| Third trimester | 4 | 3.8 |
| At the time of delivery | 5 | 4.8 |
| Maternal HBeAg status | ||
| Positive | 6 | 5.7 |
| Negative | 91 | 86.7 |
| Unknown | 8 | 7.6 |
Table 2.
Characteristics of Neonates Born to HBV Positive Mothers and Their HB Antigen and Antibody Status.
| Gender | ||
| Male | 60 | 57.1 |
| Female | 45 | 42.8 |
| Gestational age | ||
| ≤38 Weeks | 33 | 31.4 |
| 38 Weeks | 72 | 68.6 |
| Birth weight | ||
| <2 kg | 7 | 6.7 |
| ≥2 kg | 98 | 93.3 |
| Mode of delivery | ||
| Vaginal delivery | 66 | 62.9 |
| Caesarian delivery | 39 | 37.1 |
Table 3.
Descriptive Data of the Administration of Hepatitis Vaccine and Immunoglobulins to Neonates.
| First dose of hepatitis B vaccine given | ||
| No | 0 | 0 |
| Yes | 105 | 100.0 |
| Time the neonate received the vaccine (hours after birth) | ||
| ≤12 Hours | 104 | 99.0 |
| >12 Hours | 1 | 0.9 |
| HBIG given | ||
| Yes | 105 | 100 |
| No | 0 | 0 |
| Time the neonate received HBIG (hours after birth) | ||
| ≤12 Hours | 93 | 88.6 |
| >12 Hours | 12 | 11.4 |
| HBIG route of administration | ||
| IV | 6 | 5.7 |
| IM | 99 | 94.3 |
| Neonates who completed the vaccine series (81) | ||
| Completed | 62 | 77 |
| Unknown | 19 | 23 |
Figure 2.
Workflow of the study, of the 105 infants born to HBsAg-Positive mothers, only 81 (77.1%) were followed up among which only 62 (77%) completed all three vaccines within the stipulated time.
After application of the chosen inclusion criteria, of the 81 neonates (77.1%) eligible for follow-up, 62 (77%) completed the full vaccination series. A 7-month serological test of 59/62 (95%) who completed the vaccine series was negative for HBsAg with a 100% protection rate.
Discussion
In this study, we found that the HBsAg-positive rate of pregnant women was 0.8%, 77.1% of the infants complied with the initial follow-up and 59% of the infants complied with the vaccination series of all 3 doses within the stipulated time of 7 months and were all negative for HBsAg, achieving a 100% protection rate. These results were different from the study that reported a failure of immunoprophylaxis in their population. 22
Furthermore, we found that 6 of the HBsAg-positive mothers were also HBeAg positive; however, none of their newborn infants tested positive for either HBs or HBe antigen, and hence we did not find any mother-to-infant transmission, which contrasts with another study whereby 10 infants born to HBeAg-positive mothers were found to be chronically infected. 23 Two recent studies identified the increased residual risk of mother to infant transmission of HBV despite the full HBV.24,25 The majority (60.9%) of newborn infants in this study were delivered vaginally, which is regarded as one of the risk factors for vertical transmission of HBV infection. The rates were in concordance with other reported ones.7,26
Additionally, we found that the majority (82.9%) of the HBs AG-positive mothers were aged above 30 years, which is the key risk factor for HBV infection (16). These same results have already been reported by different studies across the globe.7,22,27,28 Moreover, the majority of the newborn infants (93.3%) had ≥2 kg of birthweight, which was also reported by other studies, but some others reported the birth weight of newborn infants >3 kg in their populations.7,27,28-30
In this study, we found that the effectiveness of the HBVac and HBI in the prevention of the vertical transmission of disease was conferring 100% prophylaxis. In Saudi Arabia, immunoprophylaxis is provided to all newborn infants born to HBsAg-positive mothers within a maximum of 12 hours from birth, as per the American Academy of Paediatrics (AAP) and Centres for Disease Control and Prevention (CDC) protocol. Indeed, the prevention of vertical transmission of HBV infection is critically dependent upon full adherence to recommended protocols,16,17 and because of this, the prevalence of chronic HBV is reported to be lower in the Saudi population within the age range of 30 years. 16
Study Limitations
As this retrospective study was carried out in only 1 center in the country’s western region, the findings and conclusions may be limited in scope for generalization to an entire population. Furthermore, the sample size was not calculated for this retrospective study as we included all HBsAg positive pregnant mothers, which may be deemed as another limitation. Additionally, the study involved a relatively small sample size, many of which had missing data, and almost 23% lost follow-up to receive the vaccine series at 2, 4, and 6 months and serological testing at 7 months (27%). Additionally, the reasons for dropping out of the follow up was not explored because of the inherent design of the study.
Conclusion
In our study, the prevalence of hepatitis B among pregnant women was 8 per 1000 live births. Immunoprophylaxis of 100% was achieved in newborn infants at 7 months of follow-up. However, compliance remained a key issue for the completion of the vaccination series and serology testing. Immunoprophylaxis can be improved by encouraging the vaccination of newborn infants born to HBsAg-positive mothers within a maximum of 4 to 12 hours from birth. All pregnant women who are over the age of 30 should be proactively screened for HBV infection prenatally to reduce the burden of diseases and increase the effectiveness of the PEP in HBSAg-positive mothers, and vaccination programmes should be directed towards the high-risk populations as well.
Acknowledgments
We are very grateful to the patient’s family for their enthusiasm and participation in this study. We also would like to acknowledge generous support of King Khalid Hospital, MNGHA for our research activities. We would like to express their deep gratitude towards all the residents and nurses of King Khalid Hospital, MNGHA, who helped in the data collection of this study.
Footnotes
Author Contributions: MAQ and SSA: idea, proposal, manuscript writing/literature search. HAN, HM, AM, MA Hindi, AA, MA Harbi, MH, AA, MA, and SA: data retrieval and entry. MAQ and SSA: data analysis result writing. MAQ and SSA: revisions and referencing. MAQ and SSA: literature survey, manuscript writing and review.
Availability of Data and Materials: Access to the raw data is available on request from the corresponding authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: The Institutional Review Board (IRB) of King Abdullah International Medical Research Centre, Jeddah (KAIMRC-J) (RJ20-044-J) approved the study protocol.
ORCID iD: Syed Sameer Aga  https://orcid.org/0000-0002-8186-1149
https://orcid.org/0000-0002-8186-1149
References
- 1. MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med. 2015;5(5):a021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burns GS, Thompson AJ. Viral hepatitis B: clinical and epidemiological characteristics. Cold Spring Harb Perspect Med. 2014;4(12):a024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-2128. doi: 10.1016/S0140-6736(12)61728-0 Erratum in: Lancet. 2013;381(9867):628. AlMazroa, Mohammad A; Memish, Ziad A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng Y, Lu Y, Ye Q, et al. Should chronic hepatitis B mothers breastfeed? A meta-analysis. BMC Public Health. 2011;11:502. doi: 10.1186/1471-2458-11-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong F, Pai R, Van Schalkwyk J, Yoshida EM. Hepatitis B in pregnancy: a concise review of neonatal vertical transmission and antiviral prophylaxis. Ann Hepatol. 2014;13(2):187-195. [PubMed] [Google Scholar]
- 6. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662. [DOI] [PubMed] [Google Scholar]
- 7. Kadwar AI, Mustafa MM, Shreef KI, Ziuo FY. Effectiveness of prophylaxis for hepatitis B in infants born to mothers serologically positive in Benghazi Medical center. Libyan J Sci Technol. 2021;13(2):76-81. [Google Scholar]
- 8. Davies J, Littlejohn M, Locarnini SA, et al. The molecular epidemiology of hepatitis B in the indigenous people of Northern Australia. J Gastroenterol Hepatol. 2013;28:1234-1241. [DOI] [PubMed] [Google Scholar]
- 9. Akhter S, Talukder MQ, Bhuiyan N, et al. Hepatitis B virus infection in pregnant mothers and its transmission to infants. Indian J Pediatr. 1992;59(4):411-415. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization. Interim guidance for country validation of viral hepatitis elimination. 2021:1-96. Accessed May 25, 2023. https://www.who.int/publications/i/item/9789240028395
- 11. World Health Organization. Prevention of mother-to-child transmission of hepatitis B virus: Guidelines on antiviral prophylaxis in pregnancy. 2020:1-58. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK561127/pdf/Bookshelf_NBK561127.pdf [PubMed]
- 12. World Health Organization. Hepatitis B. Accessed October 17, 2023. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b#:~:text=WHO%20estimates%20that%20296%20million,carcinoma%20(primary%20liver%20cancer).
- 13. Abaalkhail FA, Al-Hamoudi WK, Khathlan A, et al. SASLT practice guidelines for the management of Hepatitis B virus—an update. Saudi J Gastroenterol. 2021;27(3):115-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al-Faleh FZ, Ayoola EA, Al-Jeffry M, et al. Integration of hepatitis B vaccine into the expanded program on immunization: the Saudi Arabian experience. Ann Saudi Med. 1993;13(3):231-236. [DOI] [PubMed] [Google Scholar]
- 15. Abdo AA, Sanai FM, Al-Faleh FZ. Epidemiology of viral hepatitis in Saudi Arabia: are we off the hook? Saudi J Gastroenterol. 2012;18(6):349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aljumah AA, Babatin M, Hashim A, et al. Hepatitis B care pathway in Saudi Arabia: current situation, gaps and actions. Saudi J Gastroenterol. 2019;25(2):73-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdo AA, Sanai FM. Viral hepatitis in Saudi Arabia. An unfinished story. Saudi Med J. 2015;36(7):785-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546-1555. [DOI] [PubMed] [Google Scholar]
- 19. Sanai F, Alkhatry M, Alzanbagi A, Kumar S. Hepatitis B virus infection in Saudi Arabia and the UAE: public health challenges and their remedial measures. J Infect Public Health. 2023;16(9):1410-1417. [DOI] [PubMed] [Google Scholar]
- 20. Sanai FM, Alghamdi M, Dugan E, et al. A tool to measure the economic impact of Hepatitis B elimination: a case study in Saudi Arabia. J Infect Public Health. 2020;13(11):1715-1723. [DOI] [PubMed] [Google Scholar]
- 21. Alswaidi FM, Memish ZA, Al Hakeem RF, Atlam SA. Saudi Arabian expatriate worker fitness-screening programme: a review of 14 years of data. EMHJ-East Mediterr Health J. 2013;19(7):664-670. [PubMed] [Google Scholar]
- 22. Zhang L, Gui X, Wang B, et al. A study of immunoprophylaxis failure and risk factors of hepatitis B virus mother-to-infant transmission. Eur J Pediatr. 2014;173(9):1161-1168. [DOI] [PubMed] [Google Scholar]
- 23. Wen WH, Chang MH, Zhao LL, et al. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol. 2013;59(1):24-30. [DOI] [PubMed] [Google Scholar]
- 24. Shimakawa Y, Veillon P, Birguel J, et al. Residual risk of mother-to-child transmission of hepatitis B virus infection despite timely birth-dose vaccination in Cameroon (ANRS 12303): a single-centre, longitudinal observational study. Lancet Glob Health. 2022;10(4):e521-e529. [DOI] [PubMed] [Google Scholar]
- 25. Ko K, Kim R, Nagashima S, et al. Residual risk of mother-to-child transmission of HBV despite timely Hepatitis B vaccination: a major challenge to eliminate hepatitis B infection in Cambodia. BMC Infect Dis. 2023;23(1):1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Zhu Q, Zhang X. Effect of delivery mode on maternal-infant transmission of hepatitis B virus by immunoprophylaxis. Chin Med J. 2002;115(10):1510-1512. [PubMed] [Google Scholar]
- 27. Zahra A, Zahra AL, Saideh S, Sedighe M, Neda A. Efficacy of post-exposure prophylaxis in infants born to HBsAg positive mothers in Iran IIt Authentic. Iran J Pediatr. 2016;26(3):e5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schillie S, Walker T, Veselsky S, et al. Outcomes of infants born to women infected with hepatitis B. Pediatrics. 2015;135(5):e1141-e1147. [DOI] [PubMed] [Google Scholar]
- 29. Wang C, Wang C, Jia ZF, et al. Protective effect of an improved immunization practice of mother-to-infant transmission of hepatitis B virus and risk factors associated with immunoprophylaxis failure. Medicine (Baltimore). 2016;95(34):e4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Song YM, Sung J, Yang S, et al. Factors associated with immunoprophylaxis failure against vertical transmission of hepatitis B virus. Eur J Pediatr. 2007;166(8):813-818. [DOI] [PubMed] [Google Scholar]