Abstract
Background:
Real-world data on the use, healthcare resource utilization (HCRU), and associated costs of antifibrotic therapies in patients with idiopathic pulmonary fibrosis (IPF) are limited.
Objectives:
To assess the prevalence of antifibrotic treatment, characteristics of patients receiving treatment, discontinuation rates, and HCRU and costs associated with treatment.
Design:
This retrospective study analyzed de-identified longitudinal and cross-sectional data, respectively, from two US claims databases: Optum’s de-identified Clinformatics® Data Mart Database (CDM; commercial claims, Medicare Advantage) and the Veterans Health Administration (VHA) database. The study periods were October 1, 2013–March 31, 2019 and October 1, 2014–September 30, 2019, respectively. Eligible individuals were adults with ⩾1 diagnosis claim for IPF.
Methods:
Antifibrotic prevalence, patient demographics, treatment discontinuation rates, and HCRU and costs were determined separately for each cohort and described using summary statistics. Bivariate comparisons were analyzed using Chi-square and Student’s t-tests for categorical and continuous variables, respectively.
Results:
Overall, 4223 and 4459 eligible patients were identified in the CDM and VHA databases, respectively. Prevalence of antifibrotic uptake was 9.2% and 29.1% and the rate of index treatment discontinuation was 47% and 66% during follow-up in the CDM and VHA cohorts, respectively. Antifibrotic-treated patients were significantly younger (p < 0.0001) with lower mean Charlson Comorbidity Index scores at baseline versus untreated patients in both cohorts. In the CDM cohort, the number of outpatient and pharmacy visits was significantly higher in treated versus untreated patients during follow-up (both p < 0.0001). A similar trend was observed for the VHA cohort. Total follow-up costs in both cohorts were significantly higher in treated versus untreated patients due to higher pharmacy costs (CDM; p < 0.0001) or higher outpatient and pharmacy costs (VHA; p < 0.0001).
Conclusion:
The low prevalence of antifibrotic usage in both cohorts, together with the high rate of antifibrotic discontinuation, and increased HCRU and costs in treated versus untreated patients, support the need for novel treatment options for IPF.
Trial registration:
Not applicable.
Keywords: idiopathic pulmonary fibrosis, antifibrotics, claims database
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive disease associated with worsening dyspnea, loss of lung function, and a poor clinical prognosis. 1 Without antifibrotic treatment, patients have a mean survival time of 4 years. 2
In October 2014, the United States Food and Drug Administration (FDA) approved two antifibrotic therapies—nintedanib and pirfenidone—for the treatment of IPF, after they were shown to slow disease progression as measured by a decline in forced vital capacity in clinical trials.3,4 While these trials were not powered to assess overall survival,5,6 real-world studies utilizing claims databases and registry data have shown that antifibrotic treatment is associated with improved clinical outcomes.7–9 As these antifibrotics were the first therapies to be approved for IPF, their availability fundamentally changed the therapeutic landscape for patients with this disease.
Nonetheless, studies have suggested that the uptake of antifibrotics in real-world clinical practice has been low and discontinuation rates high, 10 potentially due to the high side effect burden 11 and/or high out-of-pocket costs to patients. 10 To date, few real-world studies have examined the prevalence of antifibrotic treatment use and the associated healthcare resource utilization (HCRU) and costs.10,12–15 The present study evaluated the use of antifibrotic treatment in two distinct US populations: patients with private health insurance coverage and veterans receiving healthcare services through the Veterans Health Administration (VHA). The specific study objectives were to assess the prevalence of antifibrotic treatment among patients with IPF and to describe the patient characteristics, discontinuation rates, and HCRU and costs among those receiving antifibrotic treatment.
Methods
Study design
This was a retrospective cohort study using de-identified claims data from two separate databases representing distinct populations of patients with IPF: Optum’s de-identified Clinformatics® Data Mart Database (CDM) and the VHA database. CDM is derived from a database of administrative health claims for members of large commercial and Medicare Advantage health plans and includes healthcare-related data from more than 150 million individuals. 16 The VHA is the largest integrated health system in the US, serving more than 9 million veterans per year at 1312 healthcare facilities. 17 Data from the CDM and VHA databases were analyzed separately; no data were pooled.
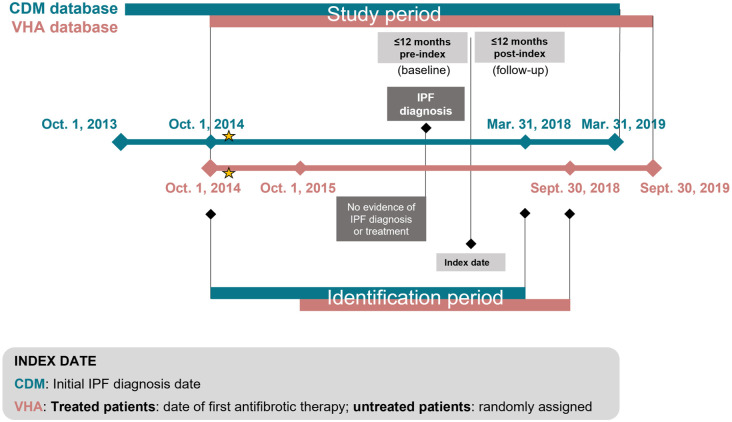
In the CDM analysis, the overall study period was from October 1, 2013 to March 31, 2019 (Figure 1). The identification period for eligible patients was October 1, 2014 to March 31, 2018 and the index date—defined as the date of the first IPF diagnosis—was within this period. For the VHA analysis, the overall study period was from October 1, 2014 to September 30, 2019. The identification period for eligible patients was from October 1, 2015 to September 30, 2018. The index date was the date of the first prescription for antifibrotic treatment, and for untreated patients, the index date was randomly selected within the same identification window to reflect the randomness of index date assignment in the treated cohort and to minimize selection bias.
Figure 1.
Study design.
Stars indicate the approximate dates of FDA approval of antifibrotic treatments. Index dates were defined as follows: CDM, date of first IPF diagnosis; VHA database, date of first prescription for antifibrotic treatment (treated patients) or randomly selected within the same identification window (for untreated patients).
CDM, Optum’s de-identified Clinformatics® Data Mart Database; FDA, US Food and Drug Administration; IPF, idiopathic pulmonary fibrosis; VHA, Veterans Health Administration.
Eligible patients were adults with at least one diagnosis claim for IPF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]: 516.31; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM]: J84.112) during the identification period, and continuous medical and pharmacy coverage for 12 months before (defined as the baseline period) and after the index date, unless the patient died within 12 months post index. Exclusion criteria included any IPF diagnosis or prescription for antifibrotic treatment (i.e., nintedanib or pirfenidone) within 12 months before the initial IPF diagnosis date, as well as diagnosis of any other form of interstitial lung disease on or after the last IPF diagnosis date. Within each database, eligible patients were divided into two cohorts; the treated cohort included patients who had at least one pharmacy claim for nintedanib or pirfenidone on or after the index date assigned, while the untreated cohort included those who had no pharmacy claims for nintedanib or pirfenidone during the study period (Figure 1).
Study outcomes
For CDM, the antifibrotic treatment prevalence was calculated as the percentage of patients who initiated antifibrotic therapy within 12 months after their initial IPF diagnosis, which was further stratified by the year of initial IPF diagnosis. For the VHA dataset, the antifibrotic treatment prevalence was estimated as the proportion of patients with the use of nintedanib or pirfenidone in each year among all patients with IPF diagnoses; the antifibrotic treatment was reported as a trend. Patient demographics (age, sex, race, or geographic region) and clinical characteristics, including the Charlson Comorbidity Index (CCI) score, individual comorbidities, smoking status, and diagnostic testing, were examined in both databases on the index date or during the baseline period. The use of other therapies that are relevant to the treatment of IPF was examined both before and after the index date.
Rates of the following treatment patterns were evaluated among the treated cohort for both databases: discontinuation, defined as the absence of any additional index drug claim within 45 days after the run-out date of the index drug (the last day of the days’ supply), or evidence of a non-index antifibrotic treatment before or within 45 days of the run-out date of the index drug; true discontinuation, defined as discontinuation of the index treatment and no claim associated with other non-index or index treatment later in the entire follow-up period; re-initiation, defined as restarting the index or a non-index antifibrotic treatment 45 days after the run-out date of the index drug; and switching, defined as having evidence of a non-index antifibrotic treatment before the run-out date of the index drug or within 45 days after discontinuation of the index treatment. The proportion of days covered (PDC) was defined as the number of days in the study period covered by a medication prescription divided by the total number of days in the study period.
All-cause HCRU and costs were calculated in both databases for the baseline and follow-up periods on a per-patient, per-month (PPPM) basis to account for variable follow-up. Costs were inflated to 2019 US dollars using the annual Consumer Price Index medical expenditure category. 18
Statistical analysis
Descriptive summary statistics were calculated for each cohort. Bivariate comparisons were performed using Chi-square tests for dichotomous and polychotomous variables and Student’s t-tests for continuous variables.
Reporting guidelines
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement 19 (Supplemental Material).
Results
Patient selection and prevalence of antifibrotic therapy
Overall, 4223 patients in CDM and 4459 patients in the VHA database were identified as meeting all study criteria (Supplemental Figure 1). Rates of antifibrotic treatment usage were 10% (n = 405) for the CDM cohort and 19% (n = 850) for the VHA cohort. In the CDM cohort, the prevalence of antifibrotic therapy was 9.2% in 2018 (Figure 2(a)). In the VHA cohort, antifibrotic treatment prevalence was 29.1% in 2019 (Figure 2(b)). While antifibrotic treatment rates increased over the study period, they remained low in both cohorts (Figure 2).
Figure 2.
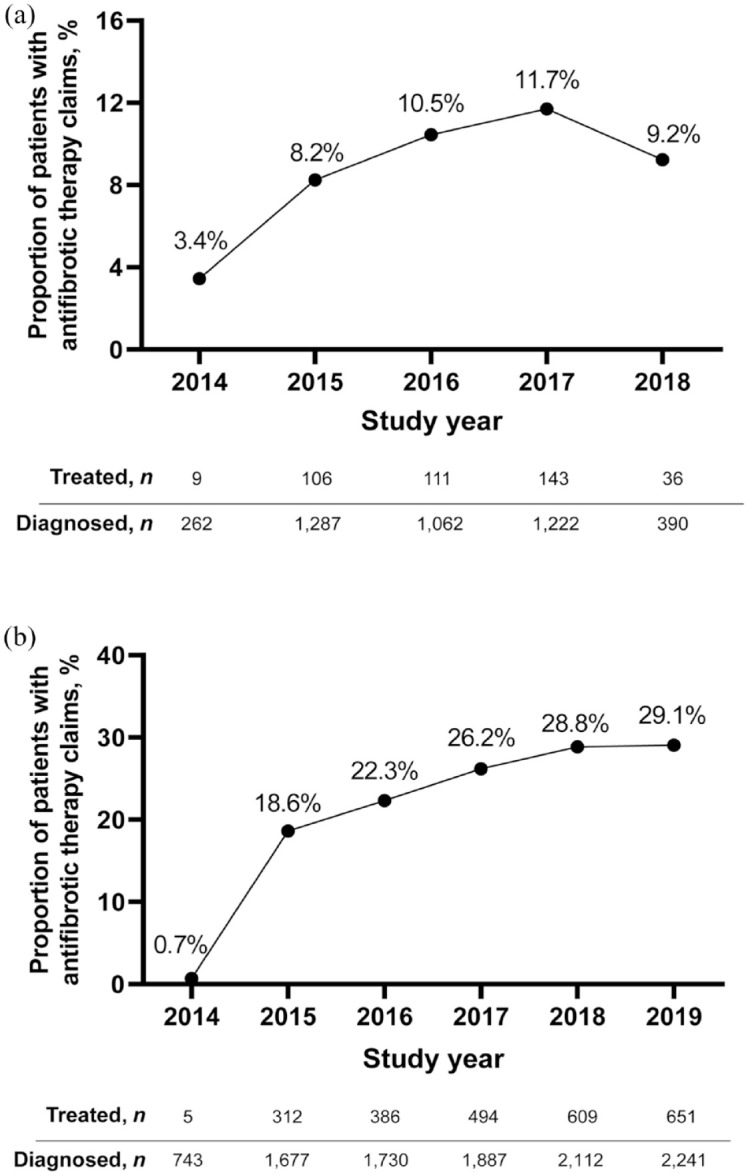
Prevalence of antifibrotic therapy use in patients with IPF. (a) CDM and (b) VHA.
CDM, Optum’s de-identified Clinformatics® Data Mart Database; IPF, idiopathic pulmonary fibrosis; VHA, Veterans Health Administration.
Baseline patient characteristics
As shown in Table 1, most patients were over 65 years old (CDM, 87%; VHA, 90%) and male (CDM, 51%; VHA, 98%). The mean age of patients treated with antifibrotic therapy versus those who were untreated was 73 versus 75 years (p < 0.0001) for the CDM cohort and 73 versus 80 years (p < 0.0001) for the VHA cohort. Mean CCI scores for treated versus untreated patients were 1.8 versus 2.5 (p < 0.0001) in the CDM cohort and 2.1 versus 2.3 (p = 0.0167) in the VHA cohort. In the CDM cohort, a significantly higher proportion of treated versus untreated patients had comorbid gastroesophageal reflux disease (GERD; 40% vs 31%; p < 0.0001) and obstructive sleep apnea (14% vs 10%; p = 0.0157). In the VHA cohort, there was a significantly higher proportion of treated versus untreated patients with comorbidities of GERD (37% vs 26%; p < 0.0001), obstructive sleep apnea (26% vs 16%; p < 0.0001), obesity (18% vs 12%; p < 0.0001), and diabetes (39% vs 34%; p = 0.0068).
Table 1.
Baseline patient demographic and clinical characteristics.
| Characteristics | CDM | VHA | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 4223) |
Treated (n = 405) |
Untreated (n = 3818) |
p Value | Overall (n = 4459) | Treated (n = 850) | Untreated (n = 3609) | p Value | |
| Age, mean (SD), years | 75.1 (10.2) | 72.9 (7.6) | 75.4 (10.4) | <0.0001 | 78.4 (15.7) | 73.4 (9.6) | 79.5 (17.1) | <0.001 |
| Age group, n (%) | ||||||||
| 18–34 years | 18 (0.4) | 0 | 18 (0.5) | 0.0789 | <12 b | 0 | <12 b | 0.3315 |
| 35–54 years | 169 (4.0) | 8 (2.0) | 161 (4.2) | 69 (1.6) | <12 b | 63 (1.8) | 0.0271 | |
| 55–64 years | 375 (8.9) | 37 (9.1) | 338 (8.9) | 385 (8.6) | 74 (8.7) | 311 (8.6) | 0.9341 | |
| ⩾65 years | 3661 (86.7) | 360 (88.9) | 3301 (86.5) | 4001 (89.7) | 770 (90.6) | 3231 (89.5) | 0.3588 | |
| Sex, n (%) | ||||||||
| Male | 2168 (51.3) | 266 (65.7) | 1902 (49.8) | <0.0001 | 4364 (97.9) | 842 (99.1) | 3522 (97.6) | 0.0076 |
| Female | 2055 (48.7) | 139 (34.3) | 1916 (50.2) | 95 (2.1) | 8 (0.9) | 87 (2.4) | ||
| Race, n (%) | ||||||||
| White | 2943 (69.7) | 283 (69.9) | 2660 (69.7) | 0.8428 | 3736 (83.8) | 737 (86.7) | 2999 (83.1) | 0.0102 |
| Black | 339 (8.0) | 35 (8.6) | 304 (8.0) | 317 (7.1) | 53 (6.2) | 264 (7.3) | 0.2704 | |
| Other | 941 (22.3) | 87 (21.5) | 854 (22.4) | 110 (2.5) | 15 (1.8) | 95 (2.6) | 0.1424 | |
| Unknown | 0 | 0 | 0 | 296 (6.6) | 45 (5.3) | 251 (7.0) | 0.0802 | |
| CCI score, mean (SD) | 2.5 (2.2) | 1.8 (1.6) | 2.5 (2.2) | <0.0001 | 2.2 (2.2) | 2.1 (1.9) | 2.3 (2.3) | 0.0167 |
| Comorbidities, n (%) | ||||||||
| COPD | 1403 (33.2) | 118 (29.1) | 1285 (33.7) | 0.0663 | 1585 (35.6) | 311 (36.6) | 1274 (35.3) | 0.4805 |
| Bacterial pneumonia | 273 (6.5) | 20 (4.9) | 253 (6.6) | 0.1889 | 158 (3.5) | 27 (3.2) | 131 (3.6) | 0.5201 |
| Lung cancer | 135 (3.2) | <5 a | 131 (3.4) | 0.0079 | 118 (2.7) | <12 b | 110 (3.1) | <0.0006 |
| Cardiovascular conditions | 2114 (50.1) | 177 (43.7) | 1937 (50.7) | 0.0071 | 1891 (42.4) | 371 (43.7) | 1520 (42.1) | 0.4167 |
| GERD | 1343 (31.8) | 163 (40.3) | 1180 (30.9) | 0.0001 | 1247 (28.0) | 316 (37.2) | 931 (25.8) | <0.0001 |
| Obstructive sleep apnea | 428 (10.1) | 55 (13.6) | 373 (9.8) | 0.0157 | 807 (18.1) | 218 (25.7) | 589 (16.3) | <0.0001 |
| Obesity | 602 (14.3) | 66 (16.3) | 536 (14.0) | 0.2166 | 586 (13.1) | 155 (18.2) | 431 (11.9) | <0.0001 |
| Depression | 619 (14.7) | 41 (10.1) | 578 (15.1) | 0.0067 | 62 (1.4) | 17 (2.0) | 45 (1.3) | 0.0916 |
| Diabetes | 1504 (35.6) | 150 (37.0) | 1354 (35.5) | 0.5295 | 1569 (35.2) | 333 (39.2) | 1236 (34.3) | 0.0068 |
| Anxiety | 682 (16.2) | 58 (14.3) | 624 (16.3) | 0.2929 | 412 (9.2) | 90 (10.6) | 322 (8.9) | 0.1313 |
| Pulmonary hypertension | 545 (12.9) | 50 (12.4) | 495 (13.0) | 0.7238 | 323 (7.2) | 73 (8.6) | 250 (6.9) | 0.0928 |
| Diagnostic tests | ||||||||
| HRCT | 1910 (45.2) | 298 (73.6) | 1612 (42.2) | <0.0001 | 1956 (43.9) | 599 (70.5) | 1357 (37.6) | <0.0001 |
| Lung biopsy | 89 (2.1) | 16 (4.0) | 73 (1.9) | 0.0066 | <12 b | <12 b | <12 b | 0.0044 |
| Smoking, yes, n (%) | 1323 (31.3) | 127 (31.4) | 1196 (31.3) | 0.9892 | 727 (16.3) | 185 (21.8) | 542 (15.0) | <0.0001 |
CDM and bVHA patient privacy specifications prohibit the disclosure of exact patient numbers when n < 5 and n < 12, respectively.
Bold values indicate significance. Differences were considered significant at p ≤ 0.05.
CCI, Charlson Comorbidity Index; CDM, Optum’s de-identified Clinformatics® Data Mart Database; COPD, chronic obstructive pulmonary disease; GERD, gastroesophageal reflux disease; HRCT, high-resolution computed tomography; SD, standard deviation; VHA, Veterans Health Administration.
In the CDM cohort, a significantly higher proportion of treated versus untreated patients were taking proton pump inhibitors (41% vs 32%; p = 0.0002) and corticosteroids (58% vs 53%; p = 0.0479) during baseline. Similarly, for the VHA cohort, a significantly higher proportion of treated patients were taking proton pump inhibitors (57% vs 40%; p < 0.0001) and corticosteroids (49% vs 42%; p = 0.0002) during baseline compared with untreated patients.
Baseline HCRU and costs
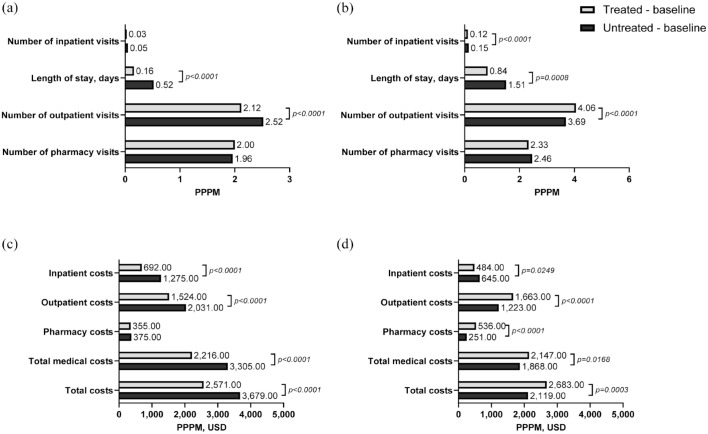
In the CDM cohort, the number of inpatient visits PPPM during baseline was similar in treated and untreated patients; however, treated patients had a significantly shorter length of inpatient stay PPPM during baseline (0.16 vs 0.52 day; p < 0.0001) compared with untreated patients (Figure 3(a)). In addition, the number of outpatient visits was significantly lower in treated versus untreated patients during baseline (2.12 vs 2.52; p < 0.0001). In the VHA cohort, treated patients had a significantly lower number of inpatient visits during baseline (0.12 vs 0.15; p < 0.0001) and significantly shorter inpatient stays (0.84 vs 1.51 days; p = 0.0008) than did untreated patients (Figure 3(b)). The number of outpatient visits during baseline (4.06 vs 3.69; p < 0.0001) was significantly higher in treated versus untreated patients.
Figure 3.
All-cause HCRU and costs during the baseline period.a (a) HCRU, CDM. (b) HCRU, VHA. (c) Costs, CDM. (d) Costs, VHA.
aDefined as the 12 months prior to the index date.
CDM, Optum’s de-identified Clinformatics® Data Mart Database; HCRU, healthcare resource utilization; PPPM, per patient per month; USD, US dollars; VHA, Veterans Health Administration.
Total baseline costs in the CDM cohort were significantly lower in treated versus untreated patients ($2571 vs $3679; p < 0.0001), which was driven by significantly lower inpatient and outpatient costs (both p < 0.0001; Figure 3(c)). Total baseline costs in the VHA cohort were significantly higher in treated versus untreated patients ($2683 vs $2119; p = 0.0003). Outpatient and pharmacy costs were both significantly higher in treated patients (p < 0.0001), but inpatient costs were significantly lower in treated versus untreated patients ($484 vs $645; p = 0.0249) (Figure 3(d)).
Antifibrotic treatment patterns
During the follow-up period, 47% of treated patients in the CDM cohort discontinued their index antifibrotic medication; 32% were true discontinuers, 11% had evidence of a treatment restart, and 4% had a treatment switch (Table 2). The CDM cohort had a mean PDC of 56%, and 29% of patients had ⩾80% PDC. In the VHA cohort, index treatment discontinuation occurred in 66% of patients; 23% had true discontinuation, 39% had a treatment restart, and 5% had a treatment switch. For the VHA cohort, the mean PDC was 56%, and 32% had ⩾80% PDC.
Table 2.
Antifibrotic treatment discontinuations and PDC.
| Treatment pattern measurements | CDM (n = 405) | VHA (n = 850) |
|---|---|---|
| Discontinuation, a n (%) | 191 (47.2) | 565 (66.5) |
| True discontinuation, b n (%) | 131 (32.3) | 195 (22.9) |
| Re-initiation, c n (%) | 43 (10.6) | 330 (38.8) |
| Switch, d n (%) | 17 (4.2) | 40 (4.7) |
| PDC, e mean (SD) | 0.56 (0.30) | 0.56 (0.41) |
| PDC ⩾80%, n (%) | 118 (29.1) | 269 (31.7) |
Absence of any additional index drug claim within 45 days after the last day of the supply or evidence of a non-index antifibrotic before or within 45 days of the run-out date.
Discontinuation of index treatment without additional claims associated with the index or non-index antifibrotic treatment later in the follow-up period.
Discontinuation of index treatment followed by a restart of index or non-index antifibrotic treatment after a 45-day treatment gap.
Evidence of a non-index treatment before the run-out date of the index drug or within 45 days after discontinuation of index treatment.
Number of nonoverlapping days in the follow-up period covered by the index prescription divided by the total number of days in the follow-up period.
CDM, Optum’s de-identified Clinformatics® Data Mart Database; PDC, proportion of days covered; SD, standard deviation; VHA, Veterans Health Administration.
Follow-up HCRU and costs
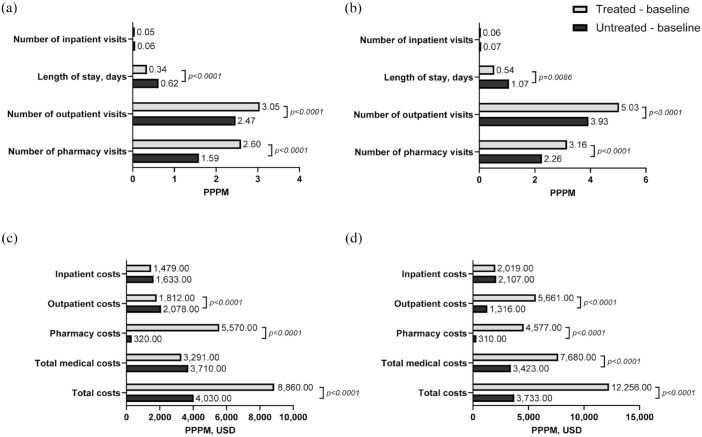
For the CDM cohort, the number of inpatient visits to PPPM during the follow-up period was similar in treated and untreated patients. However, when considering the average length of inpatient stays PPPM across all patients in the cohort (including those without hospitalizations), treated patients had significantly shorter stays compared with untreated patients (0.34 vs 0.62 days, respectively; p < 0.0001) (Figure 4(a)). The number of outpatient visits and pharmacy visits was significantly higher in treated versus untreated patients during follow-up (outpatient: 3.05 vs 2.47; p < 0.0001; pharmacy: 2.60 vs 1.59; p < 0.0001). A similar trend was observed for the VHA cohort (Figure 4(b)).
Figure 4.
All-cause HCRU and costs during follow-up.a (a) Follow-up HCRU, CDM. (b) Follow-up HCRU, VHA. (c) Follow-up Costs, CDM. (d) Follow-up Costs, VHA.
aDefined as the 12 months post-index date.
CDM, Optum’s de-identified Clinformatics® Data Mart Database; HCRU, healthcare resource utilization; PPPM, per patient per month; USD, US dollars; VHA, Veterans Health Administration.
Total follow-up costs for the CDM cohort were significantly higher in treated versus untreated patients ($8860 vs $4030; p < 0.0001), which was driven by significantly higher pharmacy costs ($5570 vs $320; p < 0.0001) (Figure 4(c)). Outpatient costs during follow-up were significantly lower in treated versus untreated patients ($1812 vs $2078; p < 0.0001). For the VHA cohort, total follow-up costs were significantly higher in treated versus untreated patients ($12,256 vs $3733) due to significantly higher outpatient and pharmacy costs in treated patients (p < 0.0001) (Figure 4(d)).
Discussion
In this study, the real-world prevalence and patterns of antifibrotic treatment for IPF, as well as patient characteristics, HCRU, and costs associated with antifibrotic treatment, were investigated among US patients with commercial health insurance or Medicare Advantage, and among veterans receiving healthcare through the VHA. Overall, antifibrotic treatment rates were low in both populations, and treatment discontinuation was relatively high. HCRU and costs were generally higher in patients receiving antifibrotic treatment due to higher pharmacy costs alone (CDM) or a combination of higher outpatient and pharmacy costs (VHA).
The antifibrotic treatment rates observed in the study (10%, CDM cohort; 19%, VHA cohort) were considerably lower than those reported in other European and US studies conducted over similar periods (e.g., 46% in a 2016 chart review of European patients 20 ; 70% in the US-based Idiopathic Pulmonary Fibrosis–PRospective Outcomes (IPF-PRO) Registry 21 ). This variation may be, in part, due to differences in the selected patient populations, healthcare systems, and study population size. For example, the European chart review included patients under the care of respiratory physicians, 20 while the IPF-PRO registry study included consenting patients diagnosed with IPF at tertiary centers in the US, with follow-up every 6 months. 22 The IPF-PRO registry study was also conducted in a smaller population than this study (n = 782), which may have resulted in a more selective sample. Therefore, these studies included patients who were under specialist care, which may not be representative of the experiences of patients with IPF in the general population. Similar administrative claims studies in patients with private insurance or Medicare Advantage, providing coverage of the general population, or VHA enrollees, comprising predominantly older males (consistent with the known demographic of IPF), revealed antifibrotic treatment rates of 26% 10 and 17%, 15 respectively, among patients with IPF. Differences in the study methodology and selection criteria are likely to account for the different antifibrotic treatment rates reported in ours and those previous studies. Taken together, these results demonstrate that in different real-world IPF populations, although antifibrotic use increased after FDA approval, a sizable proportion of patients with IPF do not receive any antifibrotic treatment.
Our analysis also showed that in the minority of patients who received antifibrotic treatment, as many as 47% to 66% discontinued treatment, and only about 30% had ⩾80% PDC, a measure of treatment adherence. This compares with an antifibrotic treatment discontinuation/switching rate of around 40% and a PDC ⩾80% in 50% of patients in a similar US-based claim study. 12 Discontinuation rates were lower among patients in the Pulmonary Fibrosis Foundation (PFF) Patient Registry (11% each for pirfenidone and nintedanib); however, the longitudinal nature of the registry’s data collection allowed for discernment of the most common reason for treatment discontinuation, which was side effect burden. 13 The reasons for discontinuation in the present study are not clear; however, the lower discontinuation rates in the PFF study may, in part, be explained by patients being under more specialist care, such that any issues with treatment can be more carefully monitored and managed. 13 The most common adverse events seen with antifibrotic treatment in IPF clinical trials were gastrointestinal events with both antifibrotic treatments and skin-related events with pirfenidone only23–26; similar ongoing side effects may be less acceptable in real-world settings where timely healthcare access is limited or more costly. Given the poor prognosis associated with IPF and the lack of alternate treatment options, the high real-world antifibrotic discontinuation rates highlight the urgent need for new IPF treatments.
In comparing the characteristics of patients with IPF who received antifibrotic treatment relative to those who did not, this analysis showed that treated patients were generally younger, with lower mean CCI scores and fewer comorbidities at baseline. These results are consistent with those from a similar US-based claim study 10 and similar to PFF Patient Registry data, which showed negative correlations between antifibrotic use and age, although baseline comorbidities were not largely different between treated and untreated patients. 13 As this study was based on claims data, no information on disease severity and/or lung function was available in the databases. Therefore, it is not known how disease severity differed between treated and untreated patients and whether this may have impacted treatment with antifibrotics.
Different patterns of HCRU and costs were also observed when comparing treated with untreated patients. In the CDM cohort, antifibrotic treatment was associated with significantly more outpatient and pharmacy visits and higher total costs. However, the increase in total costs was almost entirely attributable to pharmacy costs, as treated and untreated patients had similar total medical costs and outpatient costs were significantly higher in untreated patients. In the VHA cohort, treated versus untreated patients had a significant increase in outpatient and pharmacy visits and all cost categories except for inpatient costs. The costs of IPF clinical management as determined in this analysis do not reflect the direct costs to the patients themselves. The impact of economic factors and direct costs to patients may also influence rates of antifibrotic treatment usage across different healthcare systems across the world.
This study has several limitations that should be considered in the context of the results. As is the case for any administrative claims study, it is possible that the dataset could have been affected by missing or incorrectly inputted data. The use of an IPF diagnosis code for a given patient could have indicated probable, rather than confirmed, disease. Within the databases, no clear indication was present to verify that the medications were taken as prescribed. In addition, neither the exact doses of index treatment nor whether dose reductions occurred prior to discontinuation were collected as part of this analysis; thus, it cannot be inferred if a dose reduction may have lessened the need for switching. Finally, the databases do not provide information on why some patients were treated and others were not. Thus, the low rate of antifibrotic usage may reflect the patients presenting with mild IPF, for which antifibrotics may not have been considered necessary, or with advanced IPF, for which antifibrotics may not have been considered beneficial.
Conclusion
In conclusion, this analysis of real-world cohorts of patients with IPF under general care in the US demonstrates that despite the availability of approved antifibrotic treatments for this population, an ongoing unmet need exists for additional disease management options. The results from the longitudinal CDM analysis suggest there remains a substantial delay or barrier in initiating IPF treatment, while the results of the cross-sectional VHA analysis also suggest that the uptake of antifibrotic therapies has increased since their approval. In both study populations, the proportion of patients with IPF using antifibrotics was low compared with data from registry studies, a finding that is consistent across IPF patient populations treated in a variety of healthcare systems. One potential reason for low antifibrotic usage may be the known side effects of nintedanib and pirfenidone, which is supported by the high rates of antifibrotic treatment discontinuation and low rates of adherence to antifibrotic treatment observed in this analysis, despite the lack of approved treatment alternatives. HCRU and costs for patients with IPF were generally high, and higher in those treated with antifibrotics compared with those not treated with antifibrotics. Taken together, these findings provide further evidence of the need for novel IPF treatment options in addition to the currently approved treatments.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666241280704 for Real-world antifibrotic treatment patterns in patients with idiopathic pulmonary fibrosis: retrospective analyses of two large healthcare administrative databases in the United States by Ying Qiu, Julia Zhu, Pooja Chopra, Brandon Elpers, Christopher Dieyi, Clare Byrne, Jackson Tang, Ye Wang, Kousalya Govindaraj and Aryeh Fischer in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-2-tar-10.1177_17534666241280704 for Real-world antifibrotic treatment patterns in patients with idiopathic pulmonary fibrosis: retrospective analyses of two large healthcare administrative databases in the United States by Ying Qiu, Julia Zhu, Pooja Chopra, Brandon Elpers, Christopher Dieyi, Clare Byrne, Jackson Tang, Ye Wang, Kousalya Govindaraj and Aryeh Fischer in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors would like to thank Yuexi Wang and Adesuwa Ogbomo, previously of STATinMED, for study conceptualization and data interpretation and Rajesh Mallampati and Risho Singh, previously of STATinMED, and Ching An Wang of Bristol Myers Squibb for data analysis. Medical writing support was provided by Medical Expressions (Chicago, IL, USA) and was funded by Bristol Myers Squibb.
Footnotes
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ying Qiu, Bristol Myers Squibb, Princeton, NJ, USA.
Julia Zhu, Bristol Myers Squibb, Princeton, NJ, USA.
Pooja Chopra, Bristol Myers Squibb, 3401 Princeton Pike, Lawrenceville Township, NJ 08648, USA.
Brandon Elpers, Bristol Myers Squibb, Princeton, NJ, USA.
Christopher Dieyi, STATinMED Research, Plano, TX, USA.
Clare Byrne, STATinMED Research, Plano, TX, USA.
Jackson Tang, STATinMED Research, Plano, TX, USA.
Ye Wang, Bristol Myers Squibb, Princeton, NJ, USA.
Kousalya Govindaraj, MuSigma, Bengaluru, India.
Aryeh Fischer, Bristol Myers Squibb, Princeton, NJ, USA.
Declarations
Ethics approval and consent to participate: As this was a retrospective study, no consent was required. All data in the study were deidentified and therefore ethics approval was not required; the study was entirely compliant with HIPAA.
Consent for publication: Not applicable.
Author contributions: Ying Qiu: Conceptualization; Data interpretation; Writing – review & editing.
Julia Zhu: Conceptualization; Data interpretation; Writing – review & editing.
Pooja Chopra: Conceptualization; Data interpretation; Writing – review & editing.
Brandon Elpers: Conceptualization; Data interpretation; Writing – review & editing.
Christopher Dieyi: Conceptualization; Data interpretation; Formal analysis; Writing – review & editing.
Clare Byrne: Conceptualization; Data interpretation; Writing – review & editing.
Jackson Tang: Conceptualization; Data interpretation; Writing – review & editing.
Ye Wang: Formal analysis; Data interpretation; Writing – review & editing.
Kousalya Govindaraj: Formal analysis; Data interpretation; Writing – review & editing.
Aryeh Fischer: Conceptualization; Data interpretation; Writing – review & editing.
Funding: The study was funded by Bristol Myers Squibb.
Y.Q., P.C., B.E., Y.W., and A.F. are employees of Bristol Myers Squibb. Y.Q., B.E., and A.F. hold shares in Bristol Myers Squibb. J.Z. was employed by and owned shares in Bristol Myers Squibb at the time of the study. C.B., C.D., and J.T. are employees of STATinMED, which provided paid consultancy to Bristol Myers Squibb. K.G. and Y.W. declare no conflicts of interest.
Availability of data and materials: The data used were licensed from Optum and Veterans Health Administration and are not publicly available.
ORCID iD: Pooja Chopra  https://orcid.org/0009-0005-6221-691X
https://orcid.org/0009-0005-6221-691X
References
- 1. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khor YH, Ng Y, Barnes H, et al. Prognosis of idiopathic pulmonary fibrosis without anti-fibrotic therapy: a systematic review. Eur Respir Rev 2020; 29: 190158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 4. King TE, Jr., Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. [DOI] [PubMed] [Google Scholar]
- 5. Fisher M, Nathan SD, Hill C, et al. Predicting life expectancy for pirfenidone in idiopathic pulmonary fibrosis. J Manag Care Spec Pharm 2017; 23: S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lancaster L, Crestani B, Hernandez P, et al. Safety and survival data in patients with idiopathic pulmonary fibrosis treated with nintedanib: pooled data from six clinical trials. BMJ Open Respir Res 2019; 6: e000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dempsey TM, Sangaralingham LR, Yao X, et al. Clinical effectiveness of antifibrotic medications for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2019; 200: 168–174. [DOI] [PubMed] [Google Scholar]
- 8. Mooney J, Reddy SR, Chang E, et al. Antifibrotic therapies reduce mortality and hospitalization among Medicare beneficiaries with idiopathic pulmonary fibrosis. J Manag Care Spec Pharm 2021; 27: 1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Andrade JA, Neely ML, Hellkamp AS, et al. Effect of antifibrotic therapy on survival in patients with idiopathic pulmonary fibrosis. Clin Ther 2023; 45: 306–315. [DOI] [PubMed] [Google Scholar]
- 10. Dempsey TM, Payne S, Sangaralingham L, et al. Adoption of the antifibrotic medications pirfenidone and nintedanib for patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc 2021; 18: 1121–1128. [DOI] [PubMed] [Google Scholar]
- 11. Somogyi V, Chaudhuri N, Torrisi SE, et al. The therapy of idiopathic pulmonary fibrosis: what is next? Eur Respir Rev 2019; 28: 190021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corral M, DeYoung K, Kong AM. Treatment patterns, healthcare resource utilization, and costs among patients with idiopathic pulmonary fibrosis treated with antifibrotic medications in US-based commercial and Medicare Supplemental claims databases: a retrospective cohort study. BMC Pulm Med 2020; 20: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holtze CH, Freiheit EA, Limb SL, et al. Patient and site characteristics associated with pirfenidone and nintedanib use in the United States; an analysis of idiopathic pulmonary fibrosis patients enrolled in the Pulmonary Fibrosis Foundation Patient Registry. Respir Res 2020; 21: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lancaster L, Bonella F, Inoue Y, et al. Idiopathic pulmonary fibrosis: physician and patient perspectives on the pathway to care from symptom recognition to diagnosis and disease burden. Respirology 2022; 27: 66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaul B, Lee JS, Petersen LA, et al. Disparities in antifibrotic medication utilization among veterans with idiopathic pulmonary fibrosis. Chest 2023; 164: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Optum. Real world health care experiences, https://www.optum.com/content/dam/optum/resources/productSheets/5302_Data_Assets_Chart_Sheet_ISPOR.pdf (2015, accessed 23 November 2022).
- 17. US Department of Veterans Affairs. Veterans Health Administration: about VHA, https://www.va.gov/health/aboutvha.asp (accessed 23 November 2022).
- 18. US Bureau of Labor Statistics. Consumer Price Index, https://www.bls.gov/cpi (accessed 12 December 2022).
- 19. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 20. Maher TM, Molina-Molina M, Russell AM, et al. Unmet needs in the treatment of idiopathic pulmonary fibrosis-insights from patient chart review in five European countries. BMC Pulm Med 2017; 17: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Salisbury ML, Conoscenti CS, Culver DA, et al. Antifibrotic drug use in patients with idiopathic pulmonary fibrosis. Data from the IPF-PRO registry. Ann Am Thorac Soc 2020; 17: 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Brien EC, Durheim MT, Gamerman V, et al. Rationale for and design of the Idiopathic Pulmonary Fibrosis-PRospective Outcomes (IPF-PRO) registry. BMJ Open Respir Res 2016; 3: e000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Noth I, Oelberg D, Kaul M, et al. Safety and tolerability of nintedanib in patients with idiopathic pulmonary fibrosis in the USA. Eur Respir J 2018; 52: 1702106. [DOI] [PubMed] [Google Scholar]
- 24. Lancaster L, Albera C, Bradford WZ, et al. Safety of pirfenidone in patients with idiopathic pulmonary fibrosis: integrated analysis of cumulative data from 5 clinical trials. BMJ Open Respir Res 2016; 3: e000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ESBRIET® (pirfenidone) Capsules and Film-Coated Tablets Prescribing Information. South San Francisco, CA: Genentech USA, Inc., 2023. [Google Scholar]
- 26. OFEV® (nintedanib capsules) Prescribing Information. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc., 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666241280704 for Real-world antifibrotic treatment patterns in patients with idiopathic pulmonary fibrosis: retrospective analyses of two large healthcare administrative databases in the United States by Ying Qiu, Julia Zhu, Pooja Chopra, Brandon Elpers, Christopher Dieyi, Clare Byrne, Jackson Tang, Ye Wang, Kousalya Govindaraj and Aryeh Fischer in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-2-tar-10.1177_17534666241280704 for Real-world antifibrotic treatment patterns in patients with idiopathic pulmonary fibrosis: retrospective analyses of two large healthcare administrative databases in the United States by Ying Qiu, Julia Zhu, Pooja Chopra, Brandon Elpers, Christopher Dieyi, Clare Byrne, Jackson Tang, Ye Wang, Kousalya Govindaraj and Aryeh Fischer in Therapeutic Advances in Respiratory Disease