Abstract
Background:
The autonomic nervous system (ANS) is affected by several factors, including major nutrients. However, their effects on the ANS remains unclear. Most studies had several limitations. They focused on humans, therefore they had difficulties excluding factors other than the nutrients. Their observation periods were too short (<4 hours) to align with typical absorption times of carbohydrates versus fats. They assessed the effects compared with the pre-prandial state rather than comparisons between different nutrient types.
Objective:
We aimed to investigate the effects of carbohydrates and fats on the ANS.
Method:
We employed a rat model to exclude the effects of external stimuli, used sufficient observation period, and compared the ANS parameters among animals fed 3 different diets. The rats were divided into carbohydrate-rich-diet (carb group) and fat-rich-diet (fat group) groups. We investigated the effects of carbohydrates and fats on the ANS by measuring heart rate variability parameters in rats. Electrodes and electrocardiography (ECG) transmitters were implanted in 14 Wistar rats maintained on a standard diet for 2 days followed by the experimental diets for 2 days. ECG readings were continuously recorded for 4 days. The R waves, function of the R-R interval, and time were calculated. A fast Fourier transform was used to obtain the power spectrum of the fluctuation (low frequency [LF: 0.2-0.75 Hz]; high frequency [HF: 0.75-3.0 Hz]; LF/HF ratio).
Result:
Compared with the standard-diet group, the carb group showed significantly increased HF activity, while the fat group showed a significantly increased LF/HF ratio.
Conclusion:
The results reveal a link between macronutrients and ANS activity.
Keywords: Autonomic nervous system, heart rate variability, nutrients, rat
Introduction
The autonomic nervous system (ANS) is the neural network that maintains the homeostasis of the human body, avoiding changes owing to environmental and internal factors by controlling the response of visceral organs. The ANS is divided into the sympathetic and parasympathetic systems. These divisions of the ANS work together with the innervation of the effector organs, resulting in 1 system being responsible for constriction and the other for relaxation of the effector smooth muscle fibers. Disturbances in the ANS can lead to illnesses/disorders. Moreover, increased parasympathetic activity or decreased sympathetic activity results in improved cardiovascular survival, 1 signifying the important effect of the ANS on quality of life and life expectancy in humans.
The ANS can be modified by exercise, 2 psychological state, 3 diet, 4 drugs, 5 and several other environmental factors. Food is an important element affecting the ANS. Mediterranean diet, 6 fish-based dishes that are rich in omega 3 fats, 7 and yogurt enriched with α-lactalbumin 8 are associated with enhancement of the parasympathetic system and have beneficial health effects. Some foods cause sympathetic activation that negatively affects human health. 4 Major nutrients, such as proteins, carbohydrates, and fats, also modify the ANS. In one study, a diet rich in carbohydrates induced increased parasympathetic activity and reduced sympathetic activity during exercise compared with the corresponding outcomes after providing a low-carbohydrate diet. 9 In another study, intake of trans fats resulted in poor parasympathetic activity. 10 However, reports on the effects of the major nutrients are limited, and the effects of major nutrients on the ANS remain unclear.
Most previous studies examining the association between the major nutrients and the ANS had several limitations. They were mostly human studies, in which confounding factors obscured the nutrients’ effect on the ANS. Moreover, they were classified into short-term (shorter than 1 week) and long-term (more than 1 week) studies. Among the short-term studies, the observation periods were <4 hours, except for that in the study by Lima–Silva et al where it was 48 hours. 9 However, it takes approximately 3 hours for gastric emptying after an ordinary meal, 11 carbohydrates are absorbed and maintained in stable condition in 3 to 4 hours after intake, 12 and fats require at least 6 hours to be digested. 13 Therefore, the observation periods may have been too short to sufficiently discriminate the effects of major nutrients, such as carbohydrate versus fats on the ANS. Furthermore, most previous studies have compared ANS activity in pre-prandial and postprandial states to investigate the effects of the nutrients on the ANS; the comparison was not between the effect of different types of nutrients on ANS activity.
This study addressed these limitations by using a rat model to exclude the impacts of confounding factors intervening in human studies. The measurements were conducted for 2 days in the control and experimental periods, which was deemed sufficient for nutrient digestion and absorption. The comparison between diets rich in carbohydrates or fats and the standard diet were made; the comparison was not performed with the state before the experimental diets were provided.
The ANS has been evaluated using cardiovascular reflexes, heart rate variability (HRV), catecholamine measurements, sudomotor function, and microneurography 14 ; among these, HRV is considered the most promising surrogate marker of ANS function. 4 In this study, rats were implanted with electrodes and a battery, and HRV was measured after 1 week.
While the effects of fats on ANS activity have been previously reported in experimental animals, 15 our study was one of the few to examine the effects of carbohydrates on ANS activity in animals. The rats were fed a diet rich in carbohydrates or fats, and their HRV parameters were examined and compared with their HRV while on a standard diet.
Materials and Methods
This study was approved by the animal experiment committee (approval number: 211 in 2019) of Kyorin University and was conducted according to the guidelines for animal experiments at Kyorin University.
Fourteen 4-week-old male Wistar rats weighing 200 to 300 g were included in this study. The animals were housed in cages in a lightproof chamber at a temperature of 25 ± 1°C in 12/12 hours light/dark cycle. Rats were exposed to light between 6 AM and 6 PM, and they remained in the dark between 6 PM and 6 AM. All rats were provided standard rat pellets (MF; Oriental Yeast Co, Tokyo, Japan). Then, the experimental pellets (carbohydrate-rich or fat-rich pellets) were provided for 2 days at approximately 4 PM. The rats ate the pellets ad libitum, which mirrors a natural feeding condition. Water was also supplied ad libitum. Cages were cleaned at approximately 10 AM.
Transmitter Implantation
The rats were anesthetized using pentobarbital sodium (35 mg/kg) for sedation and butorphanol tartrate (0.5 μg/kg) for analgesia. For electrocardiography (ECG), a telemetric transmitter (TA10ETA-F20 or TA10EA-F20; Data Sciences, St. Paul, MN, USA) was implanted in the subcutaneous space of the dorsal chest. Paired bipolar-wire electrodes were submerged in the subcutaneous space over the trapezium. The ECG recordings were obtained 1 week after the transmitter was implanted.
ECG Recordings
Each rat was placed on a signal-receiving board (CTR-86; Data Sciences) within its cage. The ECG was recorded for 24 hours using an ECG processor (Softron Ltd., Tokyo, Japan). The signals were sampled every 1 minute, and the data were stored on a hard disk.
HRV Analysis
The ECG data obtained using the ECG processor were analyzed using power spectral analysis. 16 The computer program detected R waves, calculated the R-R interval, and formulated the function of the R-R interval and time; this function was transformed into a continuous function using a spline curve. From this time series, datasets of 512 points were resampled at 100 ms. Therefore, the data obtained during 51.2 seconds were accumulated every 24 seconds, resulting in approximately 1800 data points over 12 h (Study 1) and 150 data points over a 1 hour period (Study 2). These data were averaged. The Hamming window was applied to each dataset to minimize spectral leakage. A fast Fourier transform was used to obtain the power spectrum of the fluctuation. Squared magnitudes and the products of the computed discrete Fourier transforms were averaged to obtain spectral estimates. Low-frequency (LF) and high-frequency (HF) bands were defined as 0.2 to 0.75 Hz and 0.75 to 3.0 Hz, respectively. 16 The ratio of LF-to-HF (LF/HF) power was also calculated.
Protocol
At 1 week after the ECG leads and the transmitter were implanted, the ECG recordings were obtained for 2 days, during which time the rats were fed a standard rat diet (MF, the standard pellet for rats: Oriental Yeast Co.; Table 1). The rats were then randomly divided into 2 groups (the carb and fat groups) with an equal body weight distribution. The rats in the carb group (n = 7) were provided carbohydrate-rich pellets (AIN-93G: Oriental Yeast Co.; Table 1), and the rats in the fat group (n = 7) were provided fat-rich pellets (HFD60: Oriental Yeast Co.; Table 1). ECG data were recorded for 2 days, during which time the rats were fed the experimental diets.
Table 1.
Constituents of pellets.
| HFD60 | AIN-93G | MF | |
|---|---|---|---|
| (Standard pellet) | |||
| Total calories (kcal/100 g) | 493 | 400 | 359 |
| Protein (calorie %) | 22 | 20 | 23 |
| Fat (calorie %) | 33 | 7 | 5 |
| Saturated fatty acid (g/100 g pellet) | 14.1 | 3 | 2 |
| Carbohydrate (calorie %) | 26 | 64 | 55 |
| Polysaccharide (g/100 g pellet ) | 32 | 54 | 43 |
| Fiber (calorie %) | 6 | 3 | 3 |
| Miscellaneous (calorie %) | 13 | 6 | 4 |
Abbreviations: AIN93G, high-carbohydrate pellet; HFD, high-fat pellet.
Data Analysis
Study 1: 12-h-average study
Heart rate (HR) and HRV data, including HF, LF, and the LF/HF ratio, were divided into 2 categories: 6 PM to 6 AM, that is, the dark period, and 6 AM to 6 PM, that is, the light period. The data obtained during each period while the rats were fed standard pellets (control period) and those obtained during the experimental period were averaged. The HR and HRV data collected during the dark period on each day while the rats were fed the experimental pellets (1800 data points for each type of pellet) were compared with those collected during the dark period on the 2 days while the rats were fed the standard pellets (3600 data points). The data collected during the light period were compared in the same manner.
Study 2: One-hour-average study
The HR and HRV data collected during the 1-hour study period on day 1 of the experimental period were compared with the corresponding data collected during the 2 days of the control period.
Statistical Analysis
The HR and HRV (HF, LF, and LF/HF ratio) data were considered to follow the standard distribution per the central limit theorem. A one-sample t-test was used to compare the HR, HF, LF, and LF/HF ratio data collected during the 12-hour study period with the experimental pellets, with those collected during the two 12-hour study periods with the standard pellets (Study 1). The HR and HRV data collected during the 1-hour study period were compared between each experimental-diet group and the standard-diet group using the one-sample t-test (Study 2). The HR, HF, LF, and LF/HF ratio data of each experimental-diet group and the standard-diet group were compared using a two-sample t-test. The baseline body weights of the rats in each experimental group are expressed as medians and interquartile ranges and compared using the Mann–Whitney U test. Statistical significance was set at p < .05. All statistical analyses were performed using the StatFlex software, version 5 (Artec Ltd., Osaka, Japan).
Results
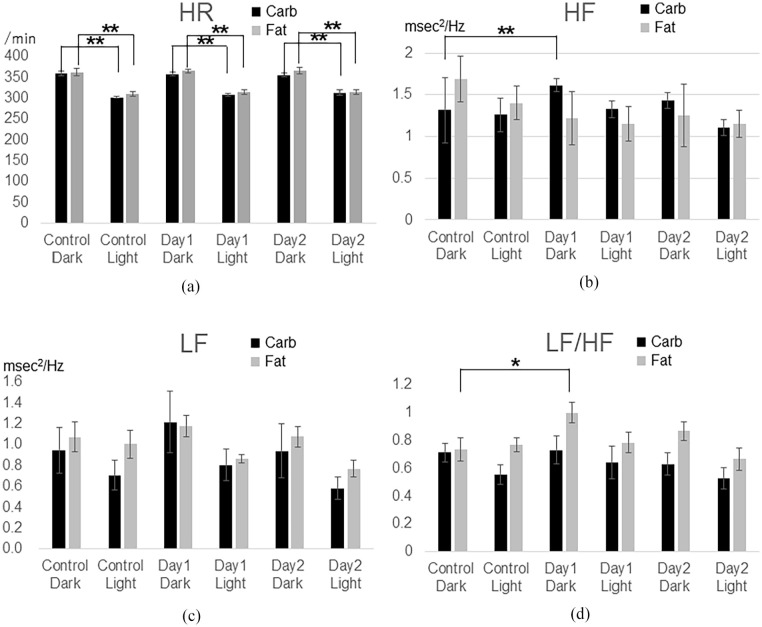
No arrhythmias were observed on the ECG recordings of any of the rats throughout the study. In Study 1, the HRs were not significantly different between the control and experimental periods on any of the days (Figure 1). However, the HRs were significantly higher during the dark periods than during the light periods for each group (control, P < .01; carb group, P < .01; fat group, P < .01). The HF power was significantly higher during the dark period on the first day when the rats were fed carbohydrate-rich pellets than when the rats were fed standard pellets (P < .01; Figure 1). The HF power values were not significantly different between the dark and light periods in any of the diet groups. The LF power was not significantly different among the 3 diet periods (standard, carbohydrate, and fat) or between the dark and light periods in any of the diet periods (Figure 1). The LF/HF ratio during the dark period on the first day was significantly higher in the fat group than in the standard group (P < .05; Figure 1). The HR and HRV were not significantly different between the carb and fat groups during the control period.
Figure 1.
Carbohydrate and fat intake effects on heart rate (HR) and HR variability parameters: 12-h-average analysis.
(a) The HRs of the rats in the standard-diet period are not significantly different from those in the experimental period during the dark or light periods. However, the HRs are significantly higher during the dark period than during the light period on days one and two for all types of diet (P < .01).
(b) The high frequency (HF) power is significantly higher in the carb-diet period than in the standard-diet period during the dark period on day one (P < .05) but not significantly different between the dark and light periods in all diet periods.
(c) The low frequency (LF) power is not significantly different between the standard- and experimental-diet periods and between the dark and light periods.
(d) The LF/HF ratio is significantly higher in the fat-diet period than in the standard-diet period during the dark period on day one (P < .05).
The HR and HRV parameters (HF, LF, LF/HF) are averaged for 2 days before starting the experimental diets. The HR and HRV parameters (HF, LF, LF/HF) during 12 hours of darkness and 12 hours of light on day one and day two are presented here.
Carb: carbohydrate, LF/HF: ratio of LF to HF, *P < .05, **P < .01
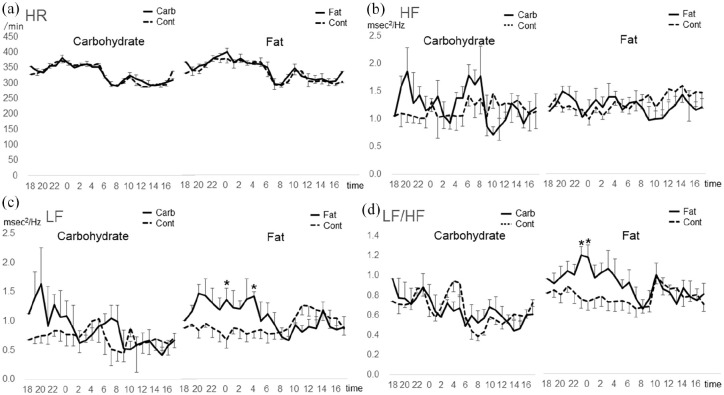
In Study 2, the HF power tended to be, but not significantly, higher in the carbohydrate-diet period than in the standard-diet period (Figure 2). The LF power was significantly higher between 0 AM and 01 PM (P < .05) and between 4 AM and 5 AM (P < .05) in the fat-diet period than in the standard-diet period (Figure 2). The LF/HF ratio between 11 PM and 1 AM was significantly higher in the fat-diet period than in the standard-diet period (P < .05).
Figure 2.
Effects of intake of carbohydrates and fats on heart rate (HR) and HR variability parameters: 1-hour-average analysis.
(a) The HR is not significantly different among the standard and experimental diets.
(b) The high frequency (HF) power has a higher tendency in the carb-diet period than in the standard-diet period during the dark period but is not significantly different between any of the experimental- and standard-diet periods.
(c) The low frequency (LF) power is significantly higher between 00:00 and 01:00 and between 04:00 and 05:00 in the fat-diet period than in the standard-diet period (P < .05).
(d) The LF/HF ratio is significantly higher between 23:00 and 01:00 in the fat-diet period than in the standard-diet period (P < .05).
Carb: carbohydrate, LF/HF: ratio of LF to HF, *P < .05
The weights of the rats fed experimental (median, 287 g; interquartile range, 238-295 g) and standard (median, 266 g; interquartile range, 247-285 g) pellets were not significantly different.
Discussion
In this study, the effects of intake of carbohydrates and fats on HRV parameters were examined using a rat model to determine the effects of these major nutrients on the ANS. The HF power significantly increased in the carb group, indicating increased parasympathetic activity. In contrast, the LF/HF ratio significantly increased in the fat group, indicating increased sympathetic activity.
Most previous studies on the short-term effects (<1 week) of the intake of carbohydrates and fats on the ANS were conducted in the 1980s to mid-2010s, used HRV or plasma norepinephrine concentrations as a measure of the effects on the ANS, and compared pre-prandial and postprandial values to determine the effects of different foods.9,15,17-25 These representative studies (10 studies focusing on carbohydrates and six on fats) have been arranged in Table 2, demonstrating that sympathetic activity was increased and parasympathetic activity decreased after the ingestion of carbohydrates; after the ingestion of fats, half of the studies showed the same result but the other half showed no change.
Table 2.
Previous representative studies regarding the short-term effects of the intake of carbohydrates and fats on the ANS.
| Author | Year | Participants | Time (h) | Number of sample size | Control | Results | Prot calorie % | Fat calorie % | Carb calorie % | |
|---|---|---|---|---|---|---|---|---|---|---|
| Carb | ||||||||||
| 1 | Cao L 17 | 2016 | Older M versus F | 3 | 14 versus 21 | Pre-prandial | M: vasc LF↑ | 13 | 9 | 78 |
| F: cardiac HF↓ | ||||||||||
| 2 | KuwaharaK 20 | 2011 | Young HL M | 2 | 8 | Pre-prandial | LF/HF↑ HF↓ | 15 | 25 | 60 |
| 3 | Lima-Silva AE 9 | 2010 | HL | 48 | 12 | Pre-prandial, fasting | 16 | 31 | 53 a | |
| Low carb versus | low Carb: LF/HF↑ HF↓ | 40 | 28 b | |||||||
| high carb | 11 | 17 | 73 c | |||||||
| 4 | Kanaley JA 19 | 2007 | Mid-age F | 1 | 42 | Pre-prandial | Total↑ | 0 | 0 | 100 d |
| 5 | Tentolouris N 24 | 2003 | Lean versus obese F | 3 | 15 versus 15 | Pre-prandial | Lean: LF/HF↑ HF↓ | 4 | 0 | 95 |
| 6 | Lu CL 21 | 1999 | HL | 1 | 9 | Pre-prandial | LF/HF↑ HF↓ | 13 | 51 | 36 e |
| 7 | Paolisso G 22 | 1997 | Young HL | 2 | 17 | Water versus glucose | LF/HF↑ | 0 | 0 | 100 d |
| 8 | HeseltineD 18 | 1990 | HL | 2 | 9 | Pre-prandial | Young: Nor↑ | 21 | 7 | 72 |
| 9 | PotterJF 23 | 1989 | HL | 2 | 7 | Pre-prandial | Nor↑ | 21 | 7 | 72 |
| 10 | Welle S 25 | 1981 | M | 4 | 7 | Pre-prandial | Nor↑ | 0 | 0 | 100 d |
| Fat | ||||||||||
| 2 | Kuwahara K | 2011 | Young HL M | 2 | 8 | Pre-prandial | LF/HF↑ HF↓ | 9 | 73 | 18 |
| 11 | Shaltout HA | 2005 | M rat | 3 | 7 versus 9 | Fasting versus intralipid | HF↓ | 100 f | ||
| 5 | Tentolouris N | 2003 | Lean versus Obese F | 3 | 15 versus 15 | Pre-prandial | Obese: LF/HF↑ | 8 | 88 | 4 |
| 8 | Heseltine D | 1990 | HL | 2 | 9 | Pre-prandial | - | 25 | 66 | 19 |
| 9 | Potter JF | 1989 | HL | 2 | 7 | Pre-prandial | - | 25 | 66 | 19 |
| 10 | Welle S | 1981 | M | 4 | 7 | Pre-prandial | - | 0 | 100 g | 0 |
Abbreviations: ANS, autonomic nervous system; Carb, carbohydrate; F, female; HF, high frequency; HL, healthy person; LF, low frequency; M, male; Mid-age, middle aged; Prot, protein; Time, duration of measurement; total, total power; vasc, vascular; a, control carbohydrate diet; b, low carbohydrate diet; c, high carbohydrate diet; d, oral glucose administration; e, low-calorie meal; f, lipid solution; -, no change; g, corn oil emulsion.
However, most of these studies had several limitations, such as insufficient elimination of confounding factors other than the food inherent in human studies, shorter observation periods of less than 4 hours in 15 studies out of 16 studies in terms of the nutrient’s absorption and metabolism, and the comparison between the pre-prandial and postprandial ANS regardless of comparison with the ANS in the control diet.
This study used a rat model to exclude the effects of external stimuli on the ANS, such as social events, 26 emotion,3,27 and audiovisual information, 28 ruling out the effects of other factors as much as possible. The activity of the ANS in the rats was measured in an unconstrained manner after they had fed on the experimental pellets. This study is one of the few animal studies on the effects of essential nutrients on ANS activity. 15 Regarding the short observation period, confirmed as shown in Figure 2d, the LF/HF, an indicator of sympathetic nervous system activity, significantly increased with fat-rich pellets at 5 and 6 hours after ingestion. In our study the effects of the nutrients on the ANS were evaluated for 2 days in the control and experimental periods, which was deemed sufficient for nutrient digestion and absorption. Thereafter, the ANS parameters of the rats fed a diet rich in carbohydrates or fats were compared with those of the rats when fed a standard diet, not before the diet was provided. Only Lima–Silva et al reported a study 9 comparing HRV after consumption of different amounts of carbohydrates. According to their study, a controlled-carbohydrate diet resulted in higher parasympathetic and lower sympathetic HRV scores than the corresponding scores after providing a low-carbohydrate diet. A recent study stated that the postprandial elevation of the blood glucose level was associated with worsening HRV according to blood insulin concentrations after single glucose ingestion. 29 This may explain the postprandial ANS change of lower parasympathetic and higher sympathetic HRV after carbohydrate ingestion compared with that in the pre-prandial state, as argued by the most previous studies mentioned above.9,15,17-25 Although the effects of carbohydrates on the ANS have not been determined, 4 it has been reported that carbohydrates are required to maintain psychological stability through increased intracerebral serotonin levels. 30 Additionally, the report by He et al. was not directly related to the carbohydrate effect on the ANS, but diets containing limited amounts of carbohydrates have been reported to increase senescence in rats and decrease survival via the deranged Enterobacteria. 31 These studies may suggest that ingesting adequate amounts of carbohydrates may have beneficial effects on overall health and survival, including a balanced ANS state. In addition, Lutfi and Elhakeem reported that a rise of blood glucose concentration was proportional to higher parasympathetic and lower sympathetic ANS activity using HRV in patients with fasting blood glucose less than diabetic range, 32 strongly supporting our results of mildly elevated carbohydrate composition (64%) favorable for ANS different from the most previous studies with high glucose composition of 73% to 95%.9,17,24 In our study, the standard pellet contained 55% of carbohydrate. However, excessive glucose among the carbohydrates was harmful for HRV,33,34 meaning that the influence of carbohydrates on HRV may be determined based on the glucose composition, carbohydrate volume, and other factors. Further studies are needed to confirm our results.
Regarding fats, Rono 35 reported an animal study with a sufficient observation period, comparing pellets including 3 types of fat oil. According to this study, the effect of fats on HRV varied by fat type; specifically, the best results on HRV were observed with sunflower oil and the worst with palm oil, essentially depending on the saturated fat content (10.4 g/100 g pellet in sunflower oil, and 19.3 g/100 g pellet in palm oil). In our study, the experimental fat pellets (HFD-60) contained 14.1 g of saturated fat per 100 g pellet by our calculation, while our standard pellet contained only 5 g of fat. These 2 studies demonstrate that food containing more saturated fat may aggravate HRV, leading to activation of the sympathetic nervous system, implying that the fat effect on the sympathetic nervous system may vary based on fat composition. The effects of fats on sympathetic activation with behavioral aggressiveness were reportedly altered based on the fat composition. 36
The feeding behavior of rats is different from that of humans, as rats feed several times during the dark period.37,38 Rats are typically active during dark periods and respond to external light.39 In this study, the HRs were higher during the dark period than during the light period in all diet periods, while the LF/HF ratio, which represents sympathetic activity, was higher during the dark period than during the light period for most of the control and experimental periods. These results are consistent with the diurnal changes in the ANS in rats.16 The HF was not significantly different between the dark and light periods, probably because the rats could not sufficiently rest owing to the presence of human activities, such as feeding or cleaning of the cages during the light periods. In this study, the rats were provided experimental pellets before the beginning of the dark period on day one. The standard, carbohydrate-rich, or fat-rich pellets were provided ad libitum to detect the maximum effect of the pellets. Our intention was not to conduct a direct quantitative comparison of the effects of the 2 major nutrients on ANS activity. Instead, our focus was on qualitative assessment, evaluating how each nutrient affects sympathetic or parasympathetic activity compared with the standard pellets.
Limitations of the Study
First, the baseline HRV values varied among the rats. This may be attributed to differences in operative stress that arose when the electrical leads, telemetric transmitter, and battery were implanted or because of natural differences. There was a 1-week recovery period between lead implantation and the study period to reduce the effects of implantation. However, all rats showed similar functional changes in ANS activity after feeding on the experimental pellets, although the extent of changes differed. Second, the changes in the ANS were less pronounced on day two than on day one. Rats tend to be cautious regarding new foods, 40 which may have resulted in search-eating behaviors. Rats also tend to eat less when they are provided a preferred food in a previous eating period. 38 After ingesting the carbohydrate-rich pellets, the parasympathetic activity increased, indicating that the rats were satisfied with this diet. Therefore, the rats may have ingested more carbohydrate-rich pellets on day 1 than on day two. This presumption should have been confirmed by measuring daily consumption of the pellets. Third, it has been reported that glucose level is changed after ingesting high-fat diet. 41 However, this study could not evaluate the autonomic changes through altered glucose metabolism caused by high-fat diet. Further studies in various fat types will be necessary in this context.
Conclusion
In rats, an appropriate carbohydrate-rich diet increased parasympathetic activity and a fat-rich diet increased sympathetic activity in a short-time observation period of 2 days. Further experimental and clinical studies are required in the future to validate our findings, especially with changing macronutrient’s subtypes and volume. Interestingly, the results revealed a link between macronutrients and ANS activity, offering insights into the physiological impact of dietary habits, such as reported serotonin secretion stimulated by carbohydrates leading to psychological stability, and aggressiveness induced by sympathetic accentuation, possibly related to fat consumption as mentioned before. These insights can guide the studies specific for each nutrient and adequate observation periods to shape future dietary recommendations.
Abbreviations
ANS, autonomic nervous system
ECG, electrocardiography
HF, high frequency
HR, heart rate
HRV, heart rate variability
LF, low frequency
Acknowledgments
The authors are deeply grateful for English language editing by Editage.
Footnotes
Author Contributions: HK offered immense support to the experiments by providing the experimental materials and overseeing the techniques in the methodology and resources. HK also collected the data and carried out statistical analyses. TS was involved in the data analysis, conceptualization, data curation, investigation, methodology procedures, project administration, resource allocation, running the software, supervision, data validation, visualization, and drafting of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate: This study was approved by the animal experiment committee (approval number: 211 in 2019) of Kyorin University and was conducted according to the guidelines for animal experiments at Kyorin University.
Consent for Publication: Not applicable.
ORCID iD: Toru Satoh  https://orcid.org/0000-0002-6498-1966
https://orcid.org/0000-0002-6498-1966
References
- 1. Lauer MS. Autonomic function and prognosis. Cleve Clin J Med. 2009;76:S18-S22. [DOI] [PubMed] [Google Scholar]
- 2. Besnier F, Labrunée M, Pathak A, et al. Exercise training-induced modification in autonomic nervous system: an update for cardiac patients. Ann Phys Rehabil Med. 2017;60:27-35. [DOI] [PubMed] [Google Scholar]
- 3. Kreibig SD. Autonomic nervous system activity in emotion: a review. Biol Psychol. 2010;84:394-421. [DOI] [PubMed] [Google Scholar]
- 4. Young HA, Benton D. Heart-rate variability: a biomarker to study the influence of nutrition on physiological and psychological health? Behav Pharmacol. 2018;29:140-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker DE. Basic and clinical pharmacology of autonomic drugs. Anesth Prog. Winter. 2012;59:159-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dai J, Lampert R, Wilson PW, et al. Mediterranean dietary pattern is associated with improved cardiac autonomic function among middle-aged men: a twin study. Circ Cardiovasc Qual Outcomes. 2010;3:366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xin W, Wei W, Li XY. Short-term effects of fish-oil supplementation on heart rate variability in humans: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2013;97:926-935. [DOI] [PubMed] [Google Scholar]
- 8. Jaatinen N, Korpela R, Poussa T, et al. Effects of daily intake of yoghurt enriched with bioactive components on chronic stress responses: a double-blinded randomized controlled trial. Int J Food Sci Nutr. 2014;65:507-514. [DOI] [PubMed] [Google Scholar]
- 9. Lima-Silva AE, Bertuzzi R, Dalquano E, et al. Influence of high- and low-carbohydrate diet following glycogen-depleting exercise on heart rate variability and plasma catecholamines. Appl Physiol Nutr Metab. 2010;35:541-547. [DOI] [PubMed] [Google Scholar]
- 10. Soares-Miranda L, Stein PK, Imamura F, et al. Trans-fatty acid consumption and heart rate variability in 2 separate cohorts of older and younger adults. Circ Arrhythm Electrophysiol. 2012;5:728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camilleri M, Colemont LJ, Phillips SF, et al. Human gastric emptying and colonic filling of solids characterized by a new method. Am J Physiol. Aug. 1989;257:G284-G290. [DOI] [PubMed] [Google Scholar]
- 12. Shin Y, Park S, Choue R. Comparison of time course changes in blood glucose, insulin and lipids between high carbohydrate and high fat meals in healthy young women. Nutr Res Pract. 2009;3:128-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunningham KM, Daly J, Horowitz M, Read NW. Gastrointestinal adaptation to diets of differing fat composition in human volunteers. Gut. 1991;32:483-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zygmunt A, Stanczyk J. Methods of evaluation of autonomic nervous system function. Arch Med Sci. 2010;6:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaltout HA, Abdel-Rahman AA. Mechanism of fatty acids induced suppression of cardiovascular reflexes in rats. J Pharmacol Exp Ther. 2005;314:1328-1337. [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto M, Kuwahara M, Tsubone H, Sugano S. Diurnal variation of autonomic nervous activity in the rat: investigation by power spectral analysis of heart rate variability. J Electrocardiol. 1999;32:167-171. [PubMed] [Google Scholar]
- 17. Cao L, Graham SL, Pilowsky PM. Carbohydrate ingestion induces sex-specific cardiac vagal inhibition, but not vascular sympathetic modulation, in healthy older women. Am J Physiol Regul Integr Comp Physiol. 2016;311:R49-R56. [DOI] [PubMed] [Google Scholar]
- 18. Heseltine D, Potter JF, Hartley G, MacDonald IA, James OFW. Blood pressure, heart rate and neuroendocrine responses to a high carbohydrate and a high fat meal in healthy young subjects. Clin Sci (Lond). 1990;79:517-522. [DOI] [PubMed] [Google Scholar]
- 19. Kanaley JA, Baynard T, Franklin RM, et al. The effects of a glucose load and sympathetic challenge on autonomic function in obese women with and without type 2 diabetes mellitus. Metabolism. 2007;56:778-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuwahara K, Okita Y, Kouda K, Nakamura H. Effects of modern eating patterns on the cardiac autonomic nervous system in young Japanese males. J Physiol Anthropol. 2011;30:223-231. [DOI] [PubMed] [Google Scholar]
- 21. Lu CL, Zou X, Orr WC, Chen JD. Postprandial changes of sympathovagal balance measured by heart rate variability. Dig Dis Sci. 1999;44:857-861. [DOI] [PubMed] [Google Scholar]
- 22. Paolisso G, Manzella D, Ferrara N, et al. Glucose ingestion affects cardiac ANS in healthy subjects with different amounts of body fat. Am J Physiol. 1997;273:E471-E478. [DOI] [PubMed] [Google Scholar]
- 23. Potter JF, Heseltine D, Hartley G, et al. Effects of meal composition on the postprandial blood pressure, catecholamine and insulin changes in elderly subjects. Clin Sci. 1989;77:265-272. [DOI] [PubMed] [Google Scholar]
- 24. Tentolouris N, Tsigos C, Perea D, et al. Differential effects of high-fat and high-carbohydrate isoenergetic meals on cardiac autonomic nervous system activity in lean and obese women. Metabolism. 2003;52:1426-1432. [DOI] [PubMed] [Google Scholar]
- 25. Welle S, Lilavivat U, Campbell RG. Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism. 1981;30:953-958. [DOI] [PubMed] [Google Scholar]
- 26. Fagan SE, Zhang W, Gao Y. Social adversity and antisocial behavior: mediating effects of autonomic nervous system activity. J Abnorm Child Psychol. 2017;45:1553-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chalmers JA, Quintana DS, Abbott MJ, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry. 2014;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McConnell PA, Froeliger B, Garland EL, Ives JC, Sforzo GA. Auditory driving of the autonomic nervous system: listening to theta-frequency binaural beats post-exercise increases parasympathetic activation and sympathetic withdrawal. Front Psychol. 2014;5:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eckstein ML, Brockfeld A, Haupt S, et al. Acute changes in heart rate variability to glucose and fructose supplementation in healthy individuals: a double-blind randomized crossover placebo-controlled trial. Biology. 2022;11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wurtman RJ, Wurtman JJ. Brain serotonin, carbohydrate-craving, obesity and depression. Obes Res. 1995;3 Suppl 4:477s-480s. [DOI] [PubMed] [Google Scholar]
- 31. He C, Wu Q, Hayashi N, et al. Carbohydrate-restricted diet alters the gut microbiota, promotes senescence and shortens the life span in senescence-accelerated prone mice. J Nutr Biochem. 2020;78:108326. [DOI] [PubMed] [Google Scholar]
- 32. Lutfi MF, Elhakeem RF. Effect of fasting blood glucose level on heart rate variability of healthy young adults. PLoS One. 2016;11:e0159820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scott EM, Greenwood JP, Vacca G, et al. Carbohydrate ingestion, with transient endogenous insulinaemia, produces both sympathetic activation and vasodilatation in normal humans. Clin Sci (Lond). 2002;102:523-529. [PubMed] [Google Scholar]
- 34. Fagius J. Sympathetic nerve activity in metabolic control—some basic concepts. Acta Physiol Scand. 2003;177:337-343. [DOI] [PubMed] [Google Scholar]
- 35. Rono D. Effect of variable high fat diets on heart rate variability and selected modifiable cardiovascular risk factors. University of Nairobi; 2016. http://erepository.uonbi.ac.ke/handle/11295/97440 [Google Scholar]
- 36. Haagensen AM, Sørensen DB, Sandøe P, et al. High fat, low carbohydrate diet limit fear and aggression in Göttingen minipigs. PLoS One. 2014;9:e93821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zorrilla EP, Inoue K, Fekete EM, et al. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450-R1467. [DOI] [PubMed] [Google Scholar]
- 38. Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111:128-141. [DOI] [PubMed] [Google Scholar]
- 39. Drickamer LC, Springer LM. Methodological aspects of the interval trapping method with comments on nocturnal activity patterns in house mice living in outdoor enclosures. Behav Processes. 1998;43:171-181. [DOI] [PubMed] [Google Scholar]
- 40. Cowan PE. Neophobia and neophilia: new-object and new-place reactions of three Rattus species. J Comp Physiol Psychol. 1977;91:63-71. [Google Scholar]
- 41. Lichtenstein AH, Schwab US. Relationship of dietary fat to glucose metabolism. Atherosclerosis. 2000;150:227-243. [DOI] [PubMed] [Google Scholar]