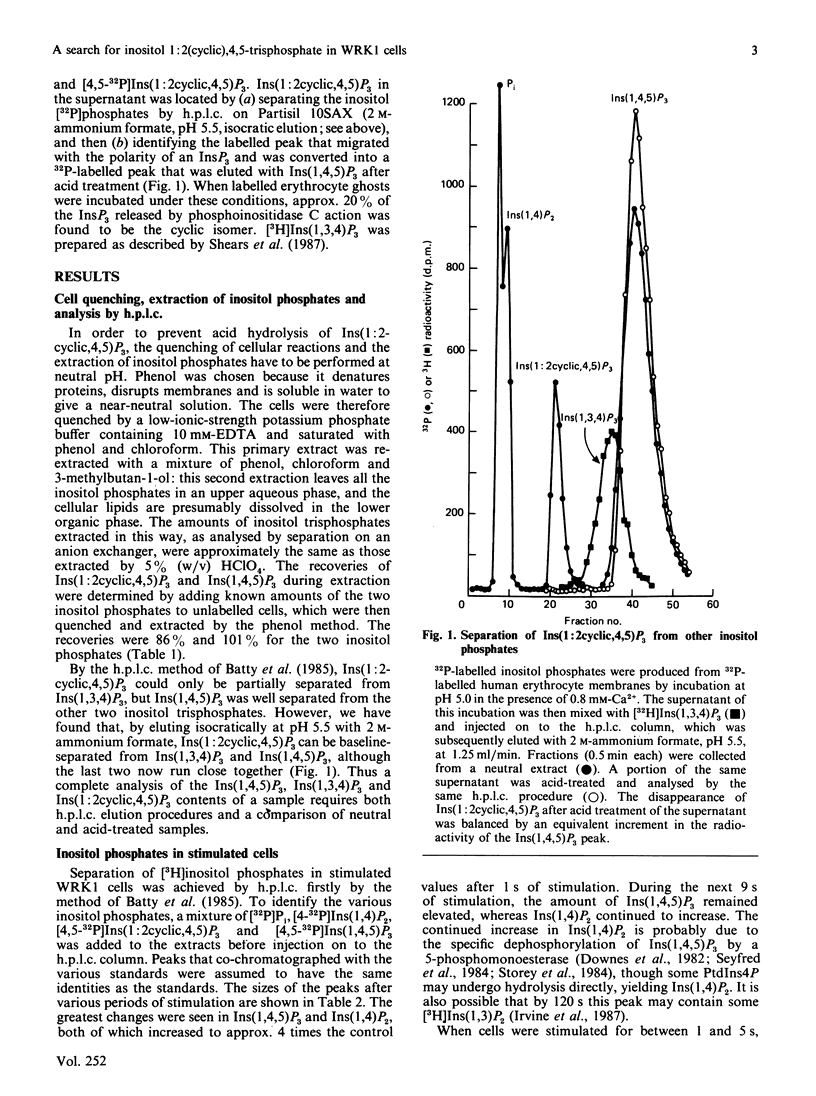
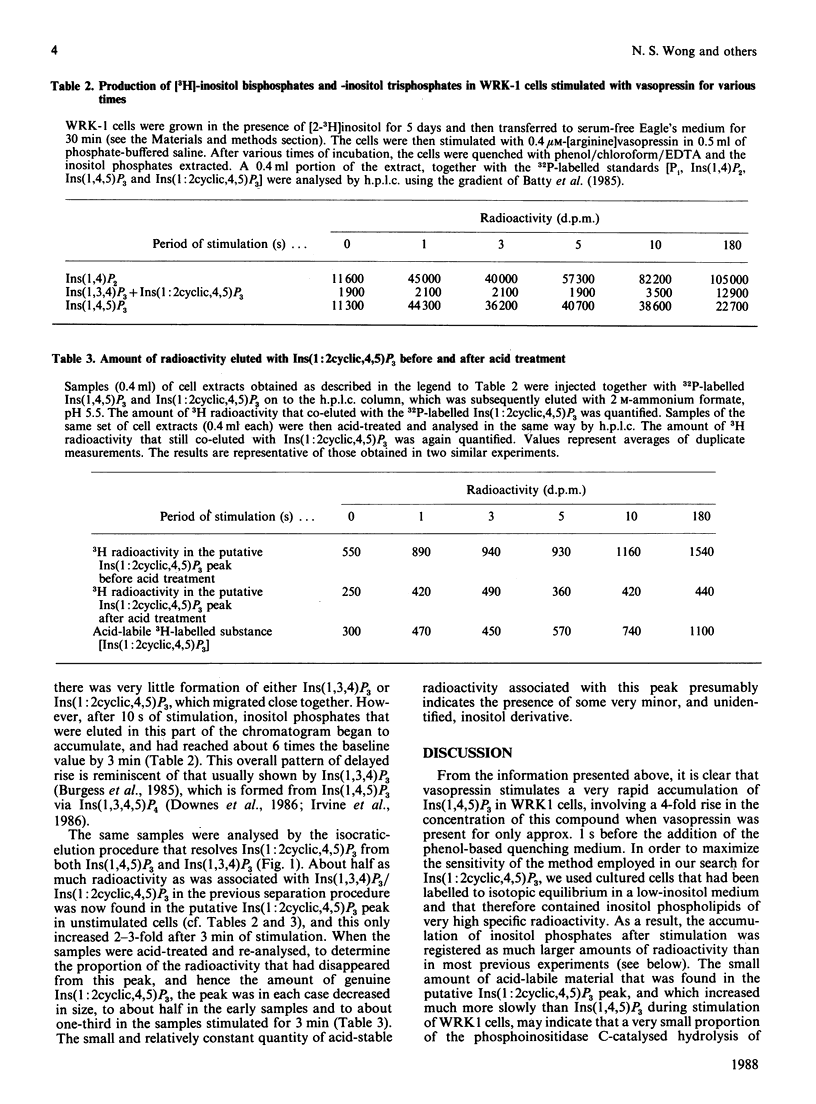
Abstract
1. A method has been devised for quenching cell incubations with an aqueous phenol/chloroform/EDTA mixture of neutral pH, to allow the analysis of acid-labile cell components. 2. Using this method, we have searched for the appearance of Ins(1:2cyclic,4,5)P3 [inositol 1:2(cyclic),4,5-trisphosphate] in WRK1 mammary tumour cells that were labelled to high specific radioactivity with [3H]inositol and then stimulated with 0.4 microM-vasopressin. 3. Vasopressin caused a very rapid accumulation of Ins(1,4,5)P3 (inositol 1,4,5-trisphosphate), followed by a slower decline towards the original concentration. An acid-labile and inositol-labelled compound with the chromatographic properties of Ins(1:2cyclic,4,5)P3 was present in unstimulated cells at less than 5% of the elevated concentration of Ins(1,4,5)P3. Its concentration rose 2-3-fold during stimulation for 3 min, at which time its concentration was about 5% of the elevated concentration of Ins(1,4,5)P3. 4. We conclude that Ins(1,4,5)P3 is the major product of phosphoinositidase C-catalysed phosphatidylinositol 4,5-bisphosphate hydrolysis in vasopressin-stimulated WRK1 cells. Ins(1:2cyclic,4,5)P3 is unlikely to be an important intracellular messenger in these cells, at least during the first few minutes of stimulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T. M., Wilson D. B., Bross T. E., Majerus P. W. Isolation and characterization of the inositol cyclic phosphate products of phosphoinositide cleavage by phospholipase C. Metabolism in cell-free extracts. J Biol Chem. 1986 Jan 5;261(1):122–126. [PubMed] [Google Scholar]
- Dawson R. M., Freinkel N., Jungalwala F. B., Clarke N. The enzymic formation of myoinositol 1:2-cyclic phosphate from phosphatidylinositol. Biochem J. 1971 May;122(4):605–607. doi: 10.1042/bj1220605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. F., Hokin L. E. Inositol 1,2-cyclic 4,5-trisphosphate concentration relative to inositol 1,4,5-trisphosphate in pancreatic minilobules on stimulation with carbamylcholine in the absence of lithium. Possible role as a second messenger in long- but not short-term responses. J Biol Chem. 1987 Oct 15;262(29):13892–13895. [PubMed] [Google Scholar]
- Downes C. P., Hawkins P. T., Irvine R. F. Inositol 1,3,4,5-tetrakisphosphate and not phosphatidylinositol 3,4-bisphosphate is the probable precursor of inositol 1,3,4-trisphosphate in agonist-stimulated parotid gland. Biochem J. 1986 Sep 1;238(2):501–506. doi: 10.1042/bj2380501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P. T., Berrie C. P., Morris A. J., Downes C. P. Inositol 1,2-cyclic 4,5-trisphosphate is not a product of muscarinic receptor-stimulated phosphatidylinositol 4,5-bisphosphate hydrolysis in rat parotid glands. Biochem J. 1987 Apr 1;243(1):211–218. doi: 10.1042/bj2430211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol-4,5-bisphosphate phosphodiesterase and phosphomonoesterase activities of rat brain. Some properties and possible control mechanisms. Biochem J. 1984 Feb 15;218(1):177–185. doi: 10.1042/bj2180177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Lander D. J., Heslop J. P., Berridge M. J. Inositol(3,4)bisphosphate and inositol(1,3)bisphosphate in GH4 cells--evidence for complex breakdown of inositol(1,3,4)trisphosphate. Biochem Biophys Res Commun. 1987 Feb 27;143(1):353–359. doi: 10.1016/0006-291x(87)90672-3. [DOI] [PubMed] [Google Scholar]
- Ishii H., Connolly T. M., Bross T. E., Majerus P. W. Inositol cyclic triphosphate [inositol 1,2-(cyclic)-4,5-triphosphate] is formed upon thrombin stimulation of human platelets. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6397–6401. doi: 10.1073/pnas.83.17.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G., Michell R. H. A membrane-bound activity catalysing phosphatidylinositol breakdown to 1,2-diacylglycerol, D-myoinositol 1:2-cyclic phosphate an D-myoinositol 1-phosphate. Properties and subcellular distribution in rat cerebral cortex. Biochem J. 1973 Mar;131(3):433–442. doi: 10.1042/bj1310433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Formation and actions of calcium-mobilizing messenger, inositol 1,4,5-trisphosphate. Am J Physiol. 1987 Feb;252(2 Pt 1):G149–G157. doi: 10.1152/ajpgi.1987.252.2.G149. [DOI] [PubMed] [Google Scholar]
- Sekar M. C., Dixon J. F., Hokin L. E. The formation of inositol 1,2-cyclic 4,5-trisphosphate and inositol 1,2-cyclic 4-bisphosphate on stimulation of mouse pancreatic minilobules with carbamylcholine. J Biol Chem. 1987 Jan 5;262(1):340–344. [PubMed] [Google Scholar]
- Seyfred M. A., Farrell L. E., Wells W. W. Characterization of D-myo-inositol 1,4,5-trisphosphate phosphatase in rat liver plasma membranes. J Biol Chem. 1984 Nov 10;259(21):13204–13208. [PubMed] [Google Scholar]
- Shears S. B., Parry J. B., Tang E. K., Irvine R. F., Michell R. H., Kirk C. J. Metabolism of D-myo-inositol 1,3,4,5-tetrakisphosphate by rat liver, including the synthesis of a novel isomer of myo-inositol tetrakisphosphate. Biochem J. 1987 Aug 15;246(1):139–147. doi: 10.1042/bj2460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. C., Rosing J., Mol A. The phosphorylation potential generated by respiring mitochondria. Biochim Biophys Acta. 1973 Apr 5;292(3):534–553. doi: 10.1016/0005-2728(73)90003-0. [DOI] [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Wang P., Toyoshima S., Osawa T. Partial purification and characterization of membrane-bound and cytosolic phosphatidylinositol-specific phospholipases C from murine thymocytes. J Biochem. 1986 Oct;100(4):1015–1022. doi: 10.1093/oxfordjournals.jbchem.a121780. [DOI] [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Hofmann S. L., Majerus P. W. Hydrolysis of polyphosphoinositides by purified sheep seminal vesicle phospholipase C enzymes. J Biol Chem. 1984 Oct 10;259(19):11718–11724. [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Sherman W. R., Berger R. A., Majerus P. W. Inositol cyclic phosphates are produced by cleavage of phosphatidylphosphoinositols (polyphosphoinositides) with purified sheep seminal vesicle phospholipase C enzymes. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4013–4017. doi: 10.1073/pnas.82.12.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Connolly T. M., Bross T. E., Majerus P. W., Sherman W. R., Tyler A. N., Rubin L. J., Brown J. E. Isolation and characterization of the inositol cyclic phosphate products of polyphosphoinositide cleavage by phospholipase C. Physiological effects in permeabilized platelets and Limulus photoreceptor cells. J Biol Chem. 1985 Nov 5;260(25):13496–13501. [PubMed] [Google Scholar]


