Abstract
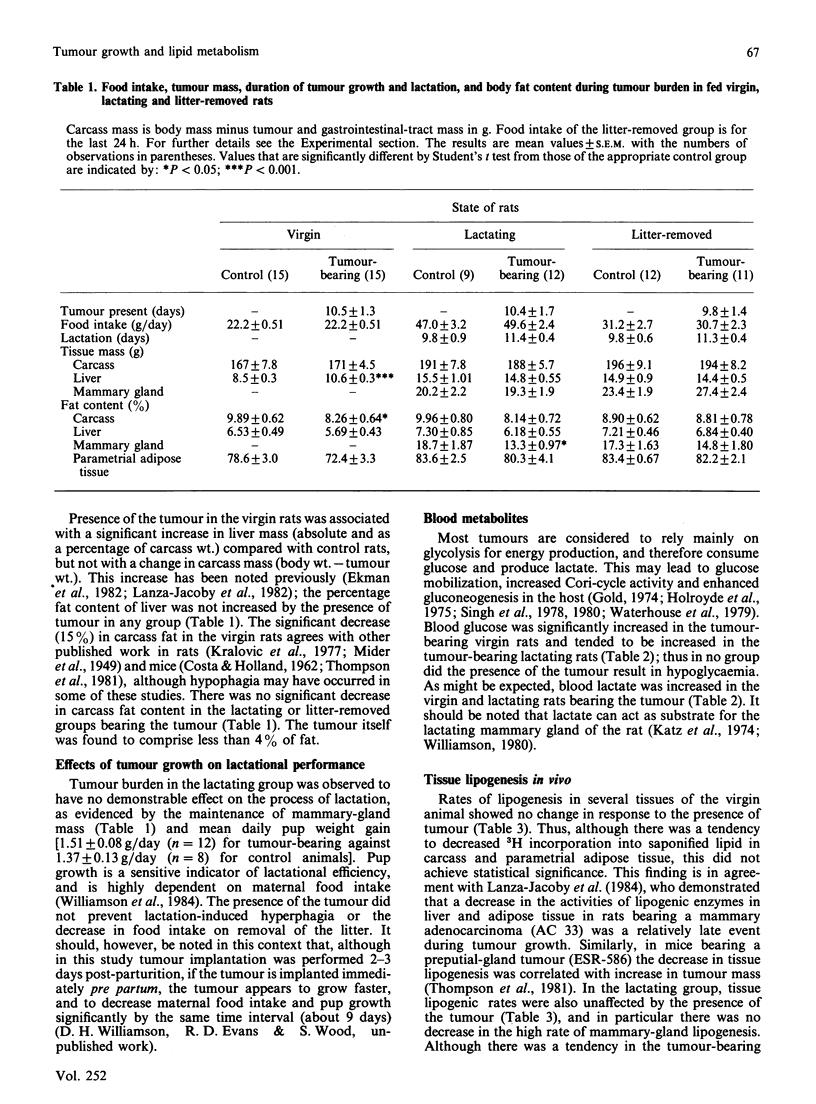
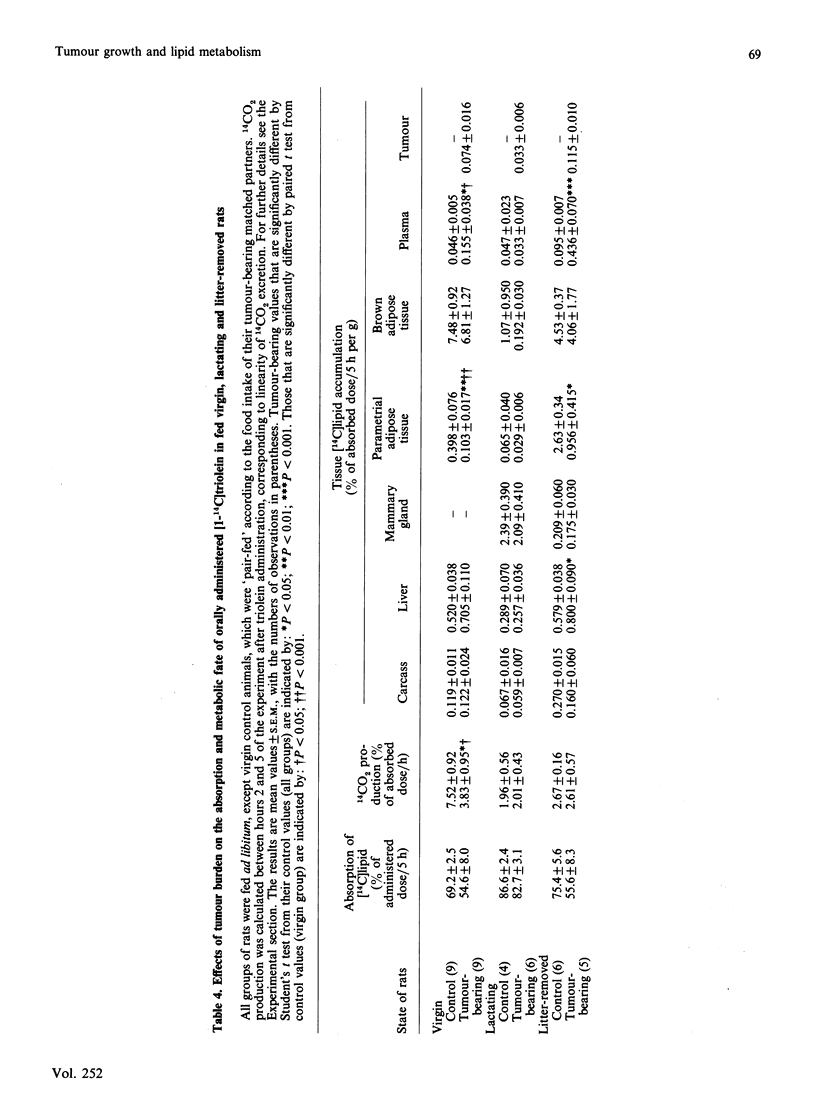
1. The effect of tumour burden on lipid metabolism was examined in virgin, lactating and litter-removed rats. 2. No differences in food intake or plasma insulin concentrations were observed between control animals and those bearing the Walker-256 carcinoma (3-5% of body wt.) in any group studied. 3. In virgin tumour-bearing animals, there was a significant increase in liver mass, blood glucose and lactate, and plasma triacylglycerol; the rate of oxidation of oral [14C]lipid to 14CO2 was diminished, and parametrial white adipose tissue accumulated less [14C]lipid compared with pair-fed controls. 4. These findings were accompanied by increased accumulation of lipid in plasma and decreased white-adipose-tissue lipoprotein lipase activity. 5. In lactating animals, tumour burden had little effect on the accompanying hyperphagia or on pup weight gain; tissue lipogenesis was unaffected, as was tissue [14C]lipid accumulation, plasma [triacylglycerol] and white-adipose-tissue and mammary-gland lipoprotein lipase activity. 6. On removal (24 h) of the litter, the presence of the tumour resulted in decreased rates of lipogenesis in the carcass, liver and white and brown adipose tissue, decreased [14C]lipid accumulation in white adipose tissue, but increased accumulation in plasma and liver, increased plasma [triacylglycerol] and decreased lipoprotein lipase activity in white adipose tissue. 7. The rate of triacylglycerol/fatty acid substrate cycling was significantly decreased in white adipose tissue of virgin and litter-removed rats bearing the tumour, but not in lactating animals. 8. These results demonstrate no functional impairment of lactation, despite the presence of tumour, and the relative resistance of the lactating mammary gland to the disturbance of lipid metabolism that occurs in white adipose tissue of non-lactating rats with tumour burden.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Robinson A. M., Girard J. R., Williamson D. H. Alterations in the rate of lipogenesis in vivo in maternal liver and adipose tissue on premature weaning of lactating rats: a possible regulatory role of prolactin. Biochem J. 1979 Jun 15;180(3):689–692. doi: 10.1042/bj1800689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Baker N., Sandborg C., Morris D., Ookhtens M. Competition for host essential and nonessential fatty acids by Ehrlich ascites carcinoma in mice. Cancer Res. 1977 Jul;37(7 Pt 1):2218–2225. [PubMed] [Google Scholar]
- Bendtzen K., Mandrup-Poulsen T., Nerup J., Nielsen J. H., Dinarello C. A., Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986 Jun 20;232(4757):1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- COSTA G., HOLLAND J. F. Effects of Krebs-2 carcinoma on the lipide metabolism of male Swiss mice. Cancer Res. 1962 Oct;22:1081–1083. [PubMed] [Google Scholar]
- Costa G., Lyles K., Ullrich L. Effects of human and experimental cancer on the conversion of 14C tripalmitin to 14CO2. Cancer. 1976 Sep;38(3):1259–1265. doi: 10.1002/1097-0142(197609)38:3<1259::aid-cncr2820380328>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Cryer A., Riley S. E., Williams E. R., Robinson D. S. Effect of nutritional status on rat adipose tissue, muscle and post-heparin plasma clearing factor lipase activities: their relationship to triglyceride fatty acid uptake by fat-cells and to plasma insulin concentrations. Clin Sci Mol Med. 1976 Mar;50(3):213–221. doi: 10.1042/cs0500213. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and the pathogenesis of the acute-phase response. N Engl J Med. 1984 Nov 29;311(22):1413–1418. doi: 10.1056/NEJM198411293112205. [DOI] [PubMed] [Google Scholar]
- Eggstein M., Kreutz F. H. Eine neue Bestimmung der Neutralfette im Blutserum und Gewebe. I. Prinzip, Durchführung und Besprechung der Methode. Klin Wochenschr. 1966 Mar 1;44(5):262–267. doi: 10.1007/BF01747716. [DOI] [PubMed] [Google Scholar]
- Ekman L., Karlberg I., Edström S., Lindmark L., Scherstén T., Lundholm K. Metabolic alterations in liver, skeletal muscle, and fat tissue in response to different tumor burdens in growing sarcoma-bearing rats. J Surg Res. 1982 Jul;33(1):23–31. doi: 10.1016/0022-4804(82)90005-1. [DOI] [PubMed] [Google Scholar]
- Gold J. Cancer cachexia and gluconeogenesis. Ann N Y Acad Sci. 1974;230:103–110. doi: 10.1111/j.1749-6632.1974.tb14440.x. [DOI] [PubMed] [Google Scholar]
- Hansell D. T., Davies J. W., Shenkin A., Burns H. J. The oxidation of body fuel stores in cancer patients. Ann Surg. 1986 Dec;204(6):637–642. doi: 10.1097/00000658-198612000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson P., Newsholme E. A., Williamson D. H. Effects of lactation and removal of pups on the rate of triacyglycerol/fatty acid substrate cycling in white adipose tissue of the rat. Biochem J. 1987 Apr 1;243(1):267–271. doi: 10.1042/bj2430267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld A., Greengard O. The dedifferentiated pattern of enzymes in livers of tumor-bearing rats. Cancer Res. 1972 Sep;32(9):1826–1832. doi: 10.2172/4649739. [DOI] [PubMed] [Google Scholar]
- Holroyde C. P., Gabuzda T. G., Putnam R. C., Paul P., Reichard G. A. Altered glucose metabolism in metastatic carcinoma. Cancer Res. 1975 Dec;35(12):3710–3714. [PubMed] [Google Scholar]
- Jones R. G., Ilic V., Williamson D. H. Regulation of lactating-rat mammary-gland lipogenesis by insulin and glucagon in vivo. The role and site of action of insulin in the transition to the starved state. Biochem J. 1984 Oct 15;223(2):345–351. doi: 10.1042/bj2230345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Van de Velde R. L. Lipogenesis by acini from mammary gland of lactating rats. J Biol Chem. 1974 Nov 25;249(22):7348–7357. [PubMed] [Google Scholar]
- Kawakami M., Murase T., Ogawa H., Ishibashi S., Mori N., Takaku F., Shibata S. Human recombinant TNF suppresses lipoprotein lipase activity and stimulates lipolysis in 3T3-L1 cells. J Biochem. 1987 Feb;101(2):331–338. doi: 10.1093/oxfordjournals.jbchem.a121917. [DOI] [PubMed] [Google Scholar]
- Kralovic R. C., Zepp F. A., Cenedella R. J. Studies of the mechanism of carcass fat depletion in experimental cancer. Eur J Cancer. 1977 Oct;13(10):1071–1079. doi: 10.1016/0014-2964(77)90003-2. [DOI] [PubMed] [Google Scholar]
- Lanza-Jacoby S., Lansey S. C., Miller E. E., Cleary M. P. Sequential changes in the activities of lipoprotein lipase and lipogenic enzymes during tumor growth in rats. Cancer Res. 1984 Nov;44(11):5062–5067. [PubMed] [Google Scholar]
- Lanza-Jacoby S., Miller E. E., Rosato F. E. Changes in the activities of lipoprotein lipase and the lipogenic enzymes in tumor-bearing rats. Lipids. 1982 Dec;17(12):944–949. doi: 10.1007/BF02534590. [DOI] [PubMed] [Google Scholar]
- Munday M. R., Williamson D. H. Diurnal variations in food intake and in lipogenesis in mammary gland and liver of lactating rats. Biochem J. 1983 Jul 15;214(1):183–187. doi: 10.1042/bj2140183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson-Ehle P., Schotz M. C. A stable, radioactive substrate emulsion for assay of lipoprotein lipase. J Lipid Res. 1976 Sep;17(5):536–541. [PubMed] [Google Scholar]
- Oller do Nascimento C. M., Williamson D. H. Evidence for conservation of dietary lipid in the rat during lactation and the immediate period after removal of the litter. Decreased oxidation of oral [1-14C]triolein. Biochem J. 1986 Oct 1;239(1):233–236. doi: 10.1042/bj2390233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookhtens M., Montisano D., Lyon I., Baker N. Inhibition of fatty acid incorporation into adipose tissue triglycerides in Ehrlich ascites tumor-bearing mice. Cancer Res. 1986 Feb;46(2):633–638. [PubMed] [Google Scholar]
- Price S. R., Mizel S. B., Pekala P. H. Regulation of lipoprotein lipase synthesis and 3T3-L1 adipocyte metabolism by recombinant interleukin 1. Biochim Biophys Acta. 1986 Dec 19;889(3):374–381. doi: 10.1016/0167-4889(86)90201-6. [DOI] [PubMed] [Google Scholar]
- Price S. R., Olivecrona T., Pekala P. H. Regulation of lipoprotein lipase synthesis by recombinant tumor necrosis factor--the primary regulatory role of the hormone in 3T3-L1 adipocytes. Arch Biochem Biophys. 1986 Dec;251(2):738–746. doi: 10.1016/0003-9861(86)90384-x. [DOI] [PubMed] [Google Scholar]
- Price S. R., Olivecrona T., Pekala P. H. Regulation of lipoprotein lipase synthesis in 3T3-L1 adipocytes by cachectin. Further proof for identity with tumour necrosis factor. Biochem J. 1986 Dec 1;240(2):601–604. doi: 10.1042/bj2400601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. M., Girard J. R., Williamson D. H. Evidence for a role of insulin in the regulation of lipogenesis in lactating rat mammary gland. Measurements of lipogenesis in vivo and plasma hormone concentrations in response to starvation and refeeding. Biochem J. 1978 Oct 15;176(1):343–346. doi: 10.1042/bj1760343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semb H., Peterson J., Tavernier J., Olivecrona T. Multiple effects of tumor necrosis factor on lipoprotein lipase in vivo. J Biol Chem. 1987 Jun 15;262(17):8390–8394. [PubMed] [Google Scholar]
- Singh J., Grigor M. R., Thompson M. P. Glucose homeostasis in rats bearing a transplantable sarcoma. Cancer Res. 1980 May;40(5):1699–1706. [PubMed] [Google Scholar]
- Singh J., Grigor M. R., Thompson M. P. Glucose tolerance and hormonal changes in rats bearing a transplantable sarcoma. Int J Biochem. 1981;13(10):1095–1100. doi: 10.1016/0020-711x(81)90172-5. [DOI] [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. P., Koons J. E., Tan E. T., Grigor M. R. Modified lipoprotein lipase activities, rates of lipogenesis, and lipolysis as factors leading to lipid depletion in C57BL mice bearing the preputial gland tumor, ESR-586. Cancer Res. 1981 Aug;41(8):3228–3232. [PubMed] [Google Scholar]
- Vernon R. G., Flint D. J. Control of fatty acid synthesis in lactation. Proc Nutr Soc. 1983 Jun;42(2):315–331. doi: 10.1079/pns19830035. [DOI] [PubMed] [Google Scholar]
- Waterhouse C., Jeanpretre N., Keilson J. Gluconeogenesis from alanine in patients with progressive malignant disease. Cancer Res. 1979 Jun;39(6 Pt 1):1968–1972. [PubMed] [Google Scholar]
- Waterhouse C., Kemperman J. H. Carbohydrate metabolism in subjects with cancer. Cancer Res. 1971 Sep;31(9):1273–1278. [PubMed] [Google Scholar]
- Wilde C. J., Kuhn N. J. Lactose synthesis in the rat, and the effects of litter size and malnutrition. Biochem J. 1979 Aug 15;182(2):287–294. doi: 10.1042/bj1820287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Munday M. R., Jones R. G. Biochemical basis of dietary influences on the synthesis of the macronutrients of rat milk. Fed Proc. 1984 Jun;43(9):2443–2447. [PubMed] [Google Scholar]


