Abstract
Objective
To identify distinct developmental trajectories of weight-bearing pain (WBP) and non-weight-bearing pain (NWBP) and examine the trajectory predictors in individuals with or at risk of knee osteoarthritis.
Methods
We included 971 participants from the Osteoarthritis Initiative whose baseline magnetic resonance imaging data and 9-year follow-up data on pain were available. We applied group-based trajectory modeling to identify WBP and NWBP trajectories over 9 years. Univariate and multivariate multinomial logistic regression analyses were performed to examine the predictors of identified trajectories.
Results
Three distinct WBP trajectories were identified: “no pain” (32.4%), “mild pain” (44.6%), and “moderate pain” (23%). Three distinct NWBP trajectories were identified: “no pain” (50.9%), “mild pain” (33.4%), and “moderate pain” (15.7%). In multivariate analyses, high body mass index, depression, multisite pain, radiographic knee OA, and comorbidities were associated with worse development trajectories for WBP and NWBP. Weak quadriceps strength and bone marrow lesion were only associated with worse WBP trajectories, whereas low education level was only associated with worse NWBP trajectories.
Conclusion
The developmental course of pain is heterogeneous in WBP or NWBP. Quadriceps strength and bone marrow leisure may be WBP-specific predictors, whereas education level may be a NWBP-specific predictor. The assessment of knee pain should be more accurate, which may help select appropriate therapeutic targets.
Keywords: knee osteoarthritis, pain, weight-bearing, trajectories, predictors
Introduction
Osteoarthritis (OA), the most prevalent joint disorder, is marked by pathological changes of the entire joint, including cartilage, synovium, subchondral bone, ligaments, muscles, and fat pads.1 Several risk factors for OA have been identified, including aging, female gender, obesity, history of joint injury, abnormal joint alignment, and quadriceps muscle weakness.1 OA mainly affecting the load-bearing joints, such as the knees, spine, and hips. Data have revealed that nearly 10% of men and 13% of women aged over 60 years have knee OA.2 Pain is the major symptom of knee OA and also the leading reason why patients seek medical intervention as pain management helps to enhance functional capacity and improve quality of life.3 However, adequate pain alleviation is still an unmet medical need, partly due to the lack of profound understanding of pain mechanisms.4
OA knee pain is a complex phenotype, with multiple factors contributing to its development, including structural abnormalities, neural sensitization, and psychological influences.5 Among various risk factors, MRI-detected structural damage has garnered considerable attention in recent research, bone marrow lesion (BML) and synovitis are recognized as critical factors in pain generation, while infrapatellar fat pad also contributes to pain development by secreting pro-inflammatory adipokines.6 OA knee pain can be classified into weight-bearing pain (WBP) and non-weight-bearing pain (NWBP) based on the conditions in which it occurs. WBP refers to discomfort experienced when the joint is under load, such as during standing, walking, or climbing stairs. NWBP, on the other hand, refers to pain felt when the joint is at rest, such as while sitting or lying. In recent years, accumulating clinical evidence7,8 has indicated that WBP and NWBP are mediated by different mechanisms. While mechanical nociception and BML may dominate in WBP,8 neuroplastic and biochemical mechanisms may dominate in NWBP.7 However, most existing studies are either cross-sectional or short-term longitudinal, and the differences in the long-term developmental trajectories of WBP and NWBP remain unclear, which makes early targeted pain management challenging.
There have been several attempts to identify overall pain trajectories in knee OA and their associated predictors. The most commonly identified predictors influencing pain trajectories include higher body mass index, the presence of comorbidities, psychological problems, and lower education level.9–11 Therefore, this study aimed to separately identify WBP and NWBP trajectories and examine their respective predictors, based on those identified in previous research. Additionally, this study will focus on the impact of MRI-detected structural abnormalities on the trajectories as a predictor. The goal is to identify specific prognostic markers and therapeutic targets for more precise pain-relief treatments.
Materials and Methods
Participants
Participants were selected from the Osteoarthritis Initiative (OAI) database, which is openly accessible at https://nda.nih.gov/oai/. The OAI is a multicenter, prospective study of knee OA sponsored by the United States National Institutes of Health.12 In total, 4796 participants with or at risk of knee OA were recruited from four clinical centers in the USA (Maryland/Johns Hopkins University, Brown University, University of Pittsburgh, and Ohio State University). Detailed inclusion and exclusion criteria have been reported elsewhere.13 The OAI study was approved by the institutional review boards of each center (Approval Number 10–00532), and all participants provided written informed consent. In this study, to construct long-term pain trajectories and explore the influence of various potential predictors, particularly MRI-detected structural abnormalities, we recruited participants whose baseline MRI Osteoarthritis Knee Scores (MOAKS) and 9-year Western Ontario and McMaster Universities Osteoarthritis (WOMAC) follow-up data were available, as detailed in Supplementary Figure 1. When both the left and right sides of the knees met the aforementioned criteria, one side was randomly selected using a random number generator.
Outcome Measurement
The OAI database used the 5-point Likert scale version of the WOMAC questionnaire to assess patients’ pain levels during activities such as walking, climbing stairs, standing, resting in bed, sitting or lying down. Each item is rated on a 0–4 Likert scale: none (0), mild (1), moderate (2), severe (3), and extreme (4). According to a previous study,14 we assessed WBP by summing the scores for the items walking, climbing stairs, and standing, while NWBP was evaluated by summing the scores for the items resting in bed, sitting or lying down. We collected 9 years of WOMAC pain follow-up data to construct pain trajectories.
Potential Predictors of Pain Outcome
There have been several attempts9–11 to identify overall pain trajectories in knee osteoarthritis (OA) and their associated predictors. The most common predictors identified are high BMI, comorbidities, psychological problems, and lower education levels—all of which have been included in our study. Additionally, based on recent research findings,1,15 we have also incorporated structural defects detected by MRI and X-ray, as well as thigh muscle strength and multisite pain. How they were measured is outlined below.
Demographics
Body weight was measured using calibrated balance beam scales without heavy clothes or jewelry; height was measured using a calibrated wall-mounted stadiometer. Age and sex were obtained using questionnaires.
Education
The highest educational level was obtained from participants, and then categorized into six levels in OAI: 0 = did not graduate high school; 1 = graduated high school; 2 = attended but did not finish college; 3 = attended and graduated college; 4 = attended but did not obtain postgraduate degree; 5 = obtained postgraduate degree. We further divided education status into two categories,16 college and above (≥3) and less than college (<3).
Depression
The Center for Epidemiologic Studies Depression Scale was used to evaluate depression, which is a 20-item measure that scores symptoms associated with depression from 0 to 60.17 We defined a total score of >15 as depression.17
Multisite Pain
Participants were asked to report any additional areas of pain besides the knees. Available sites included the back, the neck, both sides of the hips, shoulders, elbows, wrists, hands, ankles, and feet. Participants with ≥3 pain sites (excluding knees) were defined as multisite pain.18
Comorbidities
Comorbidities were assessed using a validated self-administered questionnaire19 based on the Charlson index. Participants with scores ≥1 were considered to have comorbidities.
Muscle Strength
The maximal isometric knee extensor and flexor forces were measured using “Good Strength Chair”20 with the knee positioned at a 60° angle from full extension in the OAI.
Structural Defects
MRI-related grading was based on the MOAKS.21 Subchondral bone marrow lesions (BMLs) were scored according to the percentage of the volume of BML (0, none; 1, <33%; 2, 33%–66%; and 3, >66%) in 15 anatomical locations of knee joint.8 Any location scored ≥1 was defined as BML.22 Effusion synovitis was scored by a 0–3-point scale (0 = physiological amount, 1 = small, 2 = medium, and 3 = large). A score ≥1 was defined as effusion synovitis.
Radiographic grading was based on the Kellgren–Lawrence (KL) grading system,23 which has been widely used in knee OA. Radiographic knee OA (ROA) was defined as KL ≥ 2.24
Statistical Analysis
Group-based trajectory modeling (GBTM) is a type of finite mixture model that uses maximum likelihood estimation to analyze longitudinal repeated measures data and identifies distinct trajectories.25 We used censored normal model, which is appropriate for scales with a predefined range,26 to identify groups following similar procedures of pain evolvement. To determine the optimal number of trajectories, we iteratively tested 3–5 trajectories and varied the shapes (ie, linear, quadratic, and cubic) of potential trajectories. We selected the optimal model based on a combination of model parsimony, interpretability, and fit indices. We used the Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) to assess model fit, with smaller AIC and BIC values representing a better model fit.27 Furthermore, we evaluated the model fit using mean posterior probabilities (PPs) of allocating individuals to each trajectory, with the closer the mean PP value to 1, the better the model. It was suggested that the mean PP of each trajectory should not be lower than 0.7.25 Model interpretability indicated that each subgroup had enough participants for further statistical analyses; here, we sought models in which each group covered at least 10% of all participants according to a previous study.22
A three-stage approach was used to determine the association between predictors and the likelihood of certain participant being included in a specific trajectory. First, we identified the optimal model using the aforementioned evaluation criteria. Then, we applied univariate multinomial logistic regression to determine the relationship between potential predictors and pain trajectories, with the “no pain” trajectory regarded as the reference group. Finally, we applied multivariate multinomial logistic regression with mutual adjustment to further examine the independent effects of each predictor on pain trajectories.28 To eliminate the potential influence of individuals without OA on the results, we conducted a sensitivity analysis by excluding participants without pain or OA, then reconstructed the pain trajectories and identified the predictors.
Baseline characteristics were compared across groups using the chi-square test, analysis of variance, or nonparametric tests, where appropriate. Statistical Package for the Social Sciences (version 24.0; IBM Corp., Armonk, NY, USA) was used for data cleaning and performing regression analyses. SAS 9.4 (SAS Institute, Cary NC) was used for building the GBTM.
Results
Participants
The flowchart (Supplementary Figure 1) summarizes the participant inclusion process. Out of 4796 OAI participants, 1332 had complete baseline data for BML and Effusion-synovitis scores using MOAKS. From these, 971 participants with complete WOMAC data at both baseline and the 9-year follow-up were finally included. The baseline characteristics of the participants are presented in Table 1 while the distribution of pain among the participants is presented in Supplementary Table 1. Of all participants, 39.3% were male with a mean age of 61 years and BMI of 29.2 kg/m2. 39% of the participants experienced neither WBP nor NWBP, 30.5% reported only WBP, 2.7% had only NWBP, and 27.8% experienced both types of pain. Moreover, 8.5% were diagnosed with depression, 49.5% had multisite pain, and 62.8% had college education or higher. The mean maximal isometric knee extensor and flexor forces were 344 N and 144 N, respectively. Furthermore, 52% were diagnosed with ROA, 55.1% and 80.8% had effusion synovitis and BML, respectively. All variables did not have missing data.
Table 1.
Characteristics of the Three Weight-Bearing Pain Trajectory Groups
| Characteristics | Total Cohort | WBP | P | ||
|---|---|---|---|---|---|
| No Pain | Mild Pain | Moderate Pain | |||
| Age (years), mean(SD) | 61.02 (8.46) | 61.67 (8.66) | 60.68 (8.34) | 60.74 (8.38) | 0.249 |
| BMI (kg/m2), mean (SD) | 29.24 (4.82) | 28.02 (4.45) | 29.32 (4.72) | 30.84 (5.04) | <0.01 |
| Male, n (%) | 382 (39.3%) | 133 (42.2%) | 166 (38.3%) | 83 (37.2%) | 0.43 |
| Depression, n (%) | 83 (8.5%) | 16 (5.1%) | 34 (7.9%) | 33 (14.8%) | <0.01 |
| Multisite pain, n (%) | 481(49.5%) | 103 (32.7%) | 235 (54.3%) | 143 (64.1) | <0.01 |
| College education, n (%) | 610 (62.8%) | 209 (66.3%) | 293 (67.7%) | 108 (48.4%) | <0.01 |
| Presence of any comorbidity, n (%) | 214 (22%) | 53 (16.8%) | 95 (21.9) | 66 (29.6%) | <0.01 |
| Quadriceps (N), mean (SD) | 344.48 (123.64) | 364.33 (119.55) | 344.12 (128.26) | 317.13 (115.15) | <0.01 |
| Hamstrings (N), mean (SD) | 144.24 (64.99) | 153.07 (64.68) | 144.1 (65) | 132.03 (63.68) | <0.01 |
| ROA, n (%) | 505 (52.0%) | 102 (32.4%) | 224 (51.7%) | 179 (80.3%) | <0.01 |
| Effusion, n (%) | 535 (55.1%) | 144 (45.7%) | 240 (55.4%) | 151 (67.7%) | <0.01 |
| BML, n (%) | 785 (80.8) | 215 (68.3%) | 364 (84.1%) | 206 (92.4%) | <0.01 |
Note: Bold denotes statistically significant results.
Abbreviations: WBP Weight-bearing pain; BMI Body mass index; ROA Radiographic knee osteoarthritis; BML Bone marrow lesion.
Trajectory Modeling
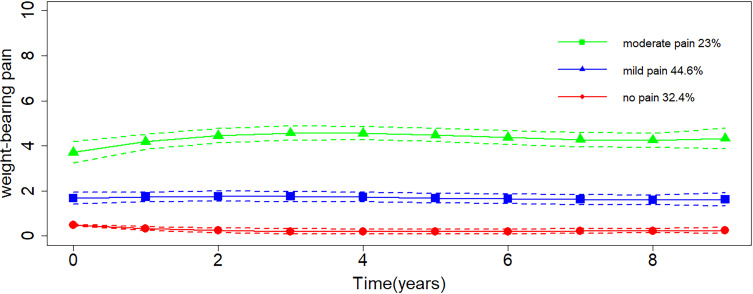
Figure 1 presents the trajectory of WBP, with the y-axis showing the level of WBP and the x-axis representing the follow-up time. Three trajectories were identified (Figure 1), and they were labeled as “no pain” (32.4%), “mild pain” (44.6%), and “moderate pain” (23%). All trajectories remained stable over time. Compared with other models, the three-trajectory model had smaller AIC and BIC values (Supplementary Table 2), and all trajectories had high mean PP (no pain, 0.94; mild pain, 0.93; moderate pain, 0.93), indicating a good model fit. Each group had a sufficient number of participants indicating a good clinical interpretability.
Figure 1.
Trajectories of weight-bearing pain severity over 9 years. Higher scores indicate more severe pain.
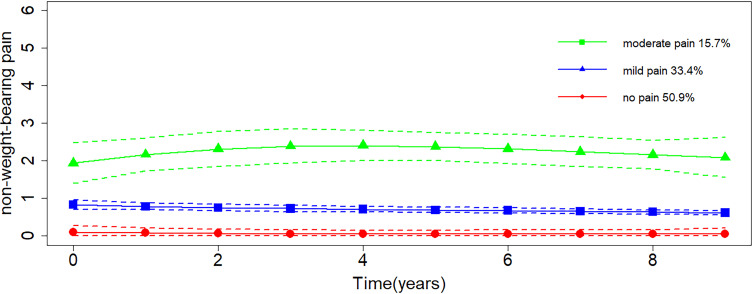
For NWBP, three trajectories were identified (Figure 2), and they were labeled as “no pain” (50.9%), “mild pain” (33.4%), and “moderate pain” (15.7%). None of the trajectories showed obvious fluctuations over time. The AIC and BIC values of the model and the mean PP of each trajectory (Supplementary Table 3) indicated a good model fit. For each trajectory, adequate sample size indicated a good clinical interpretability. The distribution of participants across WBP and NWBP trajectory groups was presented in Supplementary Table 4, 28.9% of participants followed the no-pain trajectories for both WBP and NWBP, while 13.1% followed the moderate pain trajectories for both.
Figure 2.
Trajectories of non-weight-bearing pain severity over 9 years. Higher scores indicate more severe pain.
Characteristics of the Participants of Each Trajectory
The baseline characteristics of participants of each WBP and NWBP trajectories are presented in Tables 1 and 2, respectively. Most of the characteristics showed significant difference among trajectories of WBP and NWBP, including BMI, quadriceps strength, hamstrings strength, depression, multisite pain, college education, ROA, effusion synovitis, and BML. Significant differences among trajectories further indicated that grouping was reasonable.
Table 2.
Characteristics of the Three Non-Weight-Bearing Pain Trajectory Groups
| Characteristics | Total Cohort | NWBP | P | ||
|---|---|---|---|---|---|
| No Pain | Mild Pain | Moderate Pain | |||
| Age(years), mean (SD) | 61.02 (8.46) | 62.42 (8.53) | 60.98 (8.22) | 59.79(8.70) | 0.116 |
| BMI(kg/m2), mean (SD) | 29.24 (4.82) | 28.40 (4.58) | 29.67 (4.89) | 31.10 (4.81) | <0.01 |
| Male, n (%) | 382 (39.3%) | 215 (43.4%) | 116 (35.8%) | 51 (33.6%) | 0.026 |
| Depression, n(%) | 83 (8.5%) | 25 (5.1%) | 34 (10.5%) | 24 (15.8%) | <0.01 |
| Multisite pain, n(%) | 481 (49.5%) | 193 (39.0%) | 184 (56.8%) | 104 (68.4%) | <0.01 |
| College education, n (%) | 610 (62.8%) | 337 (68.1%) | 201 (62%) | 72 (47.4%) | <0.01 |
| Presence of any comorbidity, (%) | 214 (22%) | 92 (18.6%) | 71 (21.9%) | 51 (33.6%) | <0.01 |
| Quadriceps (N), mean (SD) | 344.48 (123.64) | 334.57 (121.82) | 334.57 (128.84) | 318.02 (111.91) | <0.01 |
| Hamstrings (N), mean (SD) | 144.24 (64.99) | 152.37 (66.36) | 137.41 (63.29) | 132.33 (60.79) | <0.01 |
| ROA, n (%) | 505 (52.0%) | 185 (37.4%) | 199 (61.4%) | 121 (79.6%) | <0.01 |
| Effusion, n (%) | 535 (55.1%) | 238 (48.1%) | 198 (61.1%) | 99 (65.1%) | <0.01 |
| BML, n (%) | 785 (80.8) | 379 (76.6) | 271 (83.6) | 135 (88.8) | <0.01 |
Note: Bold denotes statistically significant results.
Abbreviations: NWBP, Non-weight-bearing pain; BMI, Body mass index; ROA, Radiographic knee osteoarthritis; BML, Bone marrow lesion.
Univariate Logistic Regression
Results of univariate regression analyses are presented in Table 3. For WBP, BMI, BML, multisite pain, effusion synovitis, and ROA were positively correlated with mild and moderate pain trajectories, whereas quadriceps strength was negatively associated with them. Education level and hamstring strength were negatively correlated with the moderate pain trajectory, whereas depression and comorbidities were positively associated with it. For NWBP, BMI, BML, depression, multisite pain, effusion synovitis, and ROA were positively correlated with mild and moderate pain trajectories, whereas the male sex, quadriceps strength, and hamstring strength were negatively associated with them. Education level and age were negatively correlated with the moderate pain trajectory, whereas comorbidities were positively associated with it.
Table 3.
Unadjusted Associations of Predictors with the WBP and NWBP Trajectory Groups
| Factors | RR of WBP (95% CI)* | RR of NWBP (95% CI)* | ||
|---|---|---|---|---|
| Mild vs No | Moderate vs No | Mild vs No | Moderate vs No | |
| age | 0.986 (0.969-1.003) | 0.987 (0.967-1.007) | 0.994 (0.978-1.011) | 0.977 (0.956-0.999) |
| BMI | 1.064 (1.03-1.099) | 1.134 (1.092-1.178) | 1.06 (1.029-1.093) | 1.124 (1.082-1.168) |
| quadriceps | 0.999 (0.998-1) | 0.997 (0.995-0.998) | 0.998 (0.997-1) | 0.997 (0.996-0.999) |
| hamstrings | 0.998 (0.996-1) | 0.995 (0.992-0.998) | 0.996 (0.994,0.999) | 0.995 (0.992-0.998) |
| Sex (male) | 0.851 (0.633-1.144) | 0.811 (0.571-1.153) | 0.726 (0.544-0.969) | 0.658 (0.449,0.962) |
| With BML | 2.454 (1.729-3.482) | 5.636 (3.257-9.755) | 1.565 (1.091-2.244) | 2.431 (1.408-4.194) |
| depression | 1.592 (0.863-2.939) | 3.246 (1.739-6.059) | 2.204 (1.289-3.77) | 3.525 (1.948-6.38) |
| Multisite pain | 2.443 (1.806-3.304) | 3.679 (2.564-5.279) | 2.057 (1.547-2.733) | 3.39 (2.303-4.991) |
| With effusion | 1.477 (1.103-1.977) | 2.49 (1.742-3.561) | 1.697 (1.277-2.255) | 2.017 (1.383-2.942) |
| With ROA | 2.238 (1.655-3.027) | 8.495 (5.663-12.744) | 2.668 (1.999-3.56) | 6.541 (4.235-10.1) |
| College education | 1.061 (0.78-1.445) | 0.476 (0.335-0.677) | 0.766 (0.571-1.027) | 0.422 (0.291-0.611) |
| comorbidity | 1.389 (0.957-2.017) | 2.078 (1.376-3.138) | 1.229 (0.869-1.74) | 2.212 (1.474-3.319) |
Notes: Bold denotes statistically significant results. *RR (95% CI): relative risk (95% confidence interval) for belonging in each trajectory relative to reference trajectory (No pain).
Abbreviations: NWBP, Non-weight-bearing pain; WBP, Weight-bearing pain; BMI, Body mass index; ROA, Radiographic knee osteoarthritis; BML Bone marrow lesion.
Multivariate Logistic Regression
The results of multivariate analyses are presented in Table 4. For WBP, BML, multisite pain, high BMI, and ROA were positively associated with mild and moderate pain trajectories, whereas older age was negatively associated with them. Effusion synovitis, depression, and the male sex were positively associated with the moderate pain trajectory, whereas quadriceps strength was negatively associated with it. Education was not associated with any trajectory. For NWBP, depression, multisite pain, and ROA were positively associated with mild moderate pain trajectories. Education level and older age were negatively correlated with the moderate pain trajectory, whereas higher BMI was positively associated with it. Effusion synovitis was positively associated with the mild pain trajectory. Quadriceps and hamstring strength, sex, and BML were not associated with any trajectory. Overall, the common predictors of WBP and NWBP trajectories included BMI, depression, multisite pain, ROA, and comorbidities. Quadriceps strength and BML were specific predictors of WBP trajectories, whereas low education was a specific predictor of NWBP trajectories.
Table 4.
Adjusted Associations of Predictors with the WBP and NWBP Trajectory Groups
| Factors | RR of WBP (95% CI)* | RR of NWBP (95% CI)* | ||
|---|---|---|---|---|
| Mild vs No | Moderate vs No | Mild vs No | Moderate vs No | |
| age | 0.97 (0.95-0.989) | 0.956 (0.932-0.981) | 0.983 (0.964-1.002) | 0.957 (0.932-0.982) |
| BMI | 1.04 (1.003-1.078) | 1.064 (1.017-1.113) | 1.027 (0.993-1.062) | 1.046 (1-1.094) |
| quadriceps | 0.998 (0.996-1) | 0.996 (0.993-0.999) | 1 (0.998-1.002) | 0.998 (0.995-1.001) |
| hamstrings | 0.999 (0.995-1.003) | 0.998 (0.993-1.003) | 0.997 (0.993-1.001) | 0.998 (0.993-1.003) |
| Sex (male) | 1.229 (0.811-1.863) | 2.148 (1.262-3.654) | 0.996 (0.67-1.481) | 1.37 (0.796-2.356) |
| With BML | 2.361 (1.598-3.487) | 3.787 (2-7.167) | 1.111 (0.744-1.659) | 1.404 (0.745-2.646) |
| depression | 1.363 (0.711-2.612) | 2.648 (1.284-5.465) | 1.887 (1.071-3.324) | 2.66 (1.356-5.218) |
| Multisite pain | 2.622 (1.887-3.642) | 4.023 (2.631-6.15) | 1.972 (1.451-2.681) | 3.193 (2.067-4.933) |
| With effusion | 1.164 (0.844-1.607) | 1.628 (1.069-2.478) | 1.401 (1.028-1.911) | 1.507 (0.981-2.317) |
| With ROA | 1.868 (1.327-2.629) | 5.995 (3.776-9.517) | 2.477 (1.786-3.434) | 5.76 (3.518-9.43) |
| College education | 1.267 (0.899-1.785) | 0.665 (0.435-1.015) | 0.911 (0.66-1.258) | 0.597 (0.389-0.917) |
| comorbidity | 1.346 (0.903-2.007) | 1.919 (1.181-3.118) | 1.177 (0.811-1.709) | 2.013 (1.261-3.215) |
Notes: Bold denotes statistically significant results. *RR (95% CI): relative risk (95% confidence interval) for belonging in each trajectory relative to reference trajectory (No pain).
Abbreviations: NWBP, Non-weight-bearing pain; WBP, Weight-bearing pain; BMI, Body mass index; ROA, Radiographic knee osteoarthritis; BML, Bone marrow lesion.
In the sensitivity analysis, 242 participants without OA and pain were excluded, and pain trajectories were reconstructed. Three distinct trajectories for both WBP and NWBP were identified, consistent with the primary analysis. The developmental trends and most predictors were largely in line with the main analysis, as detailed in Supplementary Figures 2 and 3, and Supplementary Table 5.
Discussion
This study applied GBTM to identify developmental trajectories of WBP and NWBP and investigated their predictors in individuals who had or were at risk of knee OA. We identified three trajectories in WBP and NWBP, and all of them remained stable over time. WBP and NWBP trajectories had common predictors, such as BMI, depression, multisite pain, ROA and comorbidities. Quadriceps strength and BML were specific predictors of WBP trajectories, whereas education level was a specific predictor of NWBP trajectories.
In our study, we observed that the developmental course of pain was heterogeneous in both WBP and NWBP. This heterogeneity suggests that different individuals experience varying trajectories of pain development. Furthermore, once the sensation of pain was established, the intensity of pain tended to remain relatively stable over time, indicating that fluctuations were minimal within the observed period. One study reported findings that align with ours. Pan and colleagues22 identified three stable pain trajectories on the basis of 10.7-year follow-up data of another cohort. A study based on the English Longitudinal Study of Ageing (ELSA) reached a different conclusion. James et al modeled the trajectory of overall pain severity in self-reported arthritis patients and found that around 20% experienced significant fluctuations.29 The differences in results may be due to variations in ethnicity, inclusion criteria, and assessment methods. The OAI recruited Americans with or at risk of knee OA and used the WOMAC scale specifically designed for knee OA patients. In contrast, the ELSA recruited European elderly individuals, with most assessments being self-reported, and used the VAS to evaluate overall pain levels. Our observations support that pain is a chronic rather than a progressive symptom in patients with knee OA, and the possible reason for this is that patients may proactively circumvent pain by modifying their daily activities or consuming gradually increasing analgesia doses.11 Participants enrolled in this study had relatively mild pain intensity, and only 52% of the participants were diagnosed with ROA, implying that most of the participants were in the early stage of knee OA; thus, a 9-year follow-up period is relatively short to observe significant progression.
It has been proposed that multisite pain and psychological distress may act as indicators of pain sensitization underlying chronic pain.30 Supporting this, our findings indicated that multisite pain and depression were risk factors for belonging to worse pain trajectories, suggesting that sensitization may play a role in worse pain trajectories, whether for WBP or NWBP. Pain sensitization results from neuronal plasticity, leading to pain hypersensitivity by lowering the response threshold, increasing spontaneous neuronal activity, and expanding the receptive field. This means that even subthreshold stimuli, such as daily activities, can trigger pain, while noxious stimuli elicit an exaggerated pain response. This, in turn, exacerbates sensitization, creating a vicious cycle. These mechanisms may help explain the contribution of pain sensitization to worse trajectories.
BML was a WBP-specific predictor, and previous studies have revealed that it plays an important role in generating knee pain in OA; however, the exact pathophysiological mechanisms are still debatable The sensitizing nerves and release of chemical factors within the subchondral bone may contribute to the process.8 To figure out whether there exist specially localized BML that mediate worse outcomes of WBP, we further analyzed the influence of BML of different regions8(Supplementary Figure 4), on WBP trajectories and found that BMLs in the lateral patellofemoral (LPF) joint and medial femorotibial (MFT) joint were responsible for worse WBP outcomes (Supplementary Table 6). Some studies have found that a higher mechanical load was a predictor of the development and progression of BML in the MFT31 and LPF32 joints. This may explain why BML (especially in the MFT and LPF joints) exerts an adverse impact on WBP prognosis.
In our previous study,33 we found a negative correlation between quadriceps strength and pain in patients with advanced OA by a cross-sectional method. A recent meta-analysis also showed that quadriceps weakness may contribute to incident symptomatic knee OA.15 In this study, we further found that stronger quadriceps strength was a protective predictor of WBP prognosis, perhaps because quadriceps muscles can help to attenuate mechanical loading during weight-bearing tasks34 and maintain knee joint stability during lower extremity dynamic activities.35 At the molecular level, myokines (such as IL-6 and TNF-ɑ) secreted from skeletal muscle cells are considered to regulate inflammation in knee OA.36,37 Rainbow et al38 also reported that muscle tissues can modulate inflammatory pathways and influence local metabolism in knee OA. However, less attention has been paid on the involvement of quadriceps in the pathogenesis of knee inflammation. Thus, this warrants further investigation in the future.
Education has been regarded as one of the core nonpharmacological interventions for patients with knee OA.39 We support this idea by identifying education level as an independent predictor of NWBP trajectories. Notably, the CIs for education level in WBP and NWBP showed partial overlap. This suggests that education level may reflect a trend rather than a strong independent predictor. Education level is an indicator of a person’s socioeconomic position.9 Timely detection and intervention are difficult for patients with lower socioeconomic positions, which may contribute to the rapid pain progression. Low education is also associated with unfavorable daily life practices, such as smoking and alcohol consumption, which may also adversely affect pain outcomes.40 Additionally, a higher education level is associated with better cognitive function, which helps to decrease pain perception41 and increases the therapeutic effects of psychotherapy, such as cognitive behavioral therapy.42 Overall, most of the existing studies focus on the relationship between education and overall pain rather than detailed NWBP; Therefore, more studies are required to further validate our findings.
Older age appeared to be a protective factor. Other studies11,43 also reported similar results. The possible reasons are as follows: (1) the OAI cohort recruited relatively healthier participants and excluded those with severe conditions, which mainly occurred in the elderly. The health-based selection bias may contribute to the protective effect.43 (2) Younger participants are more physically active and therefore more likely to experience overuse-related pain.11
Our study has several clinical implications. (1) Differences in nature and predictors have been observed between WBP and NWBP; thus, the assessment of knee pain in clinical practice should be more accurate so that the therapy strategies can be more targeted; (2) For patients with mainly WBP, biomechanics-related treatment, such as weight loss, quadriceps training, and knee braces, that reduce loading of LPF and MFT joints should be considered first; and (3) For patients with WBP and NWBP, antidepressants and psychotherapies can be attempted in addition to biomechanics-related treatment.
The strengths of this study are as follows: (1) To the best of our knowledge, this study is the first to identify WBP and NWBP trajectories and examine their predictors in knee OA. (2) This study included a relatively large sample. However, this study also has several limitations: (1) The participants of this study were free to choose the treatment, which may influence the shape of the trajectories. (2) The model selection process was relatively subjective, albeit we used comprehensive evaluation criteria to reduce selection bias. (3) The OAI cohort recruited relatively healthier participants; thus, our study results should be extrapolated cautiously. (4) While the key confounders have been considered, there are still potential biases that remain unadjusted.
Conclusion
The developmental course of pain in WBP and NWBP is heterogeneous. Quadriceps strength and bone marrow lesions may be specific predictors for WBP, while education level may be a specific predictor for NWBP. More precise assessment of knee pain is crucial, as it may aid in identifying appropriate therapeutic targets.
Acknowledgment
We thank the participants and stuff of OAI for generating the valuable data set.
Funding Statement
This work was supported by the National Natural Science Foundation of China grant number 82072544, and Dongguan Science and Technology of Social Development Program grant number 20231800935612 and 20231800932492.
Data Sharing Statement
The OAI database can be assessed at https://nda.nih.gov/oai/. Collated data files can be accessed upon request to corresponding author.
Ethics Approval and Consent to Participate
This study performed secondary analysis using OAI database, thus the Ethics Department of Dongguan people’s hospital exempted the ethical review. Institutional review board of OAI Coordinating Center reviewed and approved the OAI study (approval number 10-00532), and each participant signed informed consent. The OAI study was conducted in accordance with the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Duong V, Oo WM, Ding C, Culvenor AG, Hunter DJ. Evaluation and treatment of knee pain: a review. JAMA. 2023;330(16):1568–1580. doi: 10.1001/jama.2023.19675 [DOI] [PubMed] [Google Scholar]
- 2.Zhang W, Robertson WB, Zhao J, Chen W, Xu J. Emerging trend in the pharmacotherapy of osteoarthritis. Front Endocrinol. 2019;10:431. doi: 10.3389/fendo.2019.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 4.Veronese N, Cooper C, Bruyère O, et al. Multimodal multidisciplinary management of patients with moderate to severe pain in knee osteoarthritis: a need to meet patient expectations. Drugs. 2022;82(13):1347–1355. doi: 10.1007/s40265-022-01773-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neelapala YVR, Neogi T, Kumar D, et al. Exploring different models of pain phenotypes and their association with pain worsening in people with early knee osteoarthritis: the MOST cohort study. Osteoa Cartilage. 2024;32(2):210–219. doi: 10.1016/j.joca.2023.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han W, Aitken D, Zhu Z, et al. Signal intensity alteration in the infrapatellar fat pad at baseline for the prediction of knee symptoms and structure in older adults: a cohort study. Ann Rheum Dis. 2016;75(10):1783–1788. doi: 10.1136/annrheumdis-2015-208360 [DOI] [PubMed] [Google Scholar]
- 7.Power JD, Perruccio AV, Gandhi R, et al. Neuropathic pain in end-stage hip and knee osteoarthritis: differential associations with patient-reported pain at rest and pain on activity. Osteo Cartilage. 2018;26(3):363–369. doi: 10.1016/j.joca.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Aso K, Shahtaheri SM, McWilliams DF, Walsh DA. Association of subchondral bone marrow lesion localization with weight-bearing pain in people with knee osteoarthritis: data from the osteoarthritis initiative. Arthritis Res Therapy. 2021;23(1). doi: 10.1186/s13075-021-02422-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesseling J, Bastick AN, Ten Wolde S, et al. Identifying trajectories of pain severity in early symptomatic knee osteoarthritis: a 5-year followup of the cohort hip and cohort knee (CHECK) study. J Rheumatol. 2015;42(8):1470–1477. doi: 10.3899/jrheum.141036 [DOI] [PubMed] [Google Scholar]
- 10.Nicholls E, Thomas E, van der Windt DA, Croft PR, Peat G. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: findings from the knee clinical assessment study and the osteoarthritis initiative. Osteo Cartilage. 2014;22(12):2041–2050. doi: 10.1016/j.joca.2014.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteo Cartilage. 2014;22(5):622–630. doi: 10.1016/j.joca.2014.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteo Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruhdorfer A, Wirth W, Eckstein F. Relationship between isometric thigh muscle strength and minimum clinically important differences in knee function in osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res. 2015;67(4):509–518. doi: 10.1002/acr.22488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stratford PW, Kennedy DM, Woodhouse LJ, Spadoni GF. Measurement properties of the WOMAC LK 3.1 pain scale. Osteo Cartilage. 2007;15(3):266–272. doi: 10.1016/j.joca.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 15.Øiestad BE, Juhl CB, Culvenor AG, Berg B, Thorlund JB. Knee extensor muscle weakness is a risk factor for the development of knee osteoarthritis: an updated systematic review and meta-analysis including 46 819 men and women. Br J Sports Med. 2022;56(6):349–355. doi: 10.1136/bjsports-2021-104861 [DOI] [PubMed] [Google Scholar]
- 16.Singh JA, Mehta B, Mirza SZ, et al. When has a knee or hip replacement failed? A patient perspective. J Rheumatol. 2021;48(3):447–453. doi: 10.3899/jrheum.191024 [DOI] [PubMed] [Google Scholar]
- 17.Radloff LS. The CES-D Scale. Appl Psychol Meas. 2016;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 18.Amris K, Waehrens EE, Bliddal H, Danneskiold-Samsoe B. How widespread should pain be to be defined as widespread? Pain. 2016;157(8):1831–1832. doi: 10.1097/j.pain.0000000000000585 [DOI] [PubMed] [Google Scholar]
- 19.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006 [DOI] [PubMed] [Google Scholar]
- 20.Rantanen T, Era P, Heikkinen E. Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing. 1994;23(2):132–137. doi: 10.1093/ageing/23.2.132 [DOI] [PubMed] [Google Scholar]
- 21.Hunter DJ, Guermazi A, Lo GH, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteo Cartilage. 2011;19(8):990–1002. doi: 10.1016/j.joca.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan F, Tian J, Aitken D, Cicuttini F, Jones G. Predictors of pain severity trajectory in older adults: a 10.7-year follow-up study. Osteo Cartilage. 2018;26(12):1619–1626. doi: 10.1016/j.joca.2018.08.002 [DOI] [PubMed] [Google Scholar]
- 23.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheumatic Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886–1893. doi: 10.1007/s11999-016-4732-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6(1):109–138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 26.Nagin DS. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychological Methods. 1999;4(2):139–157. doi: 10.1037/1082-989X.4.2.139 [DOI] [PubMed] [Google Scholar]
- 27.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):716–723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 28.Westreich D, Greenland S. The table 2 fallacy: presenting and interpreting confounder and modifier coefficients. Am J Epidemiol. 2013;177(4):292–298. doi: 10.1093/aje/kws412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.James RJE, Walsh DA, Ferguson E. General and disease-specific pain trajectories as predictors of social and political outcomes in arthritis and cancer. BMC Med. 2018;16(1):51. doi: 10.1186/s12916-018-1031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lluch E, Nijs J, Courtney CA, et al. Clinical descriptors for the recognition of central sensitization pain in patients with knee osteoarthritis. Disabil Rehabil. 2018;40(23):2836–2845. doi: 10.1080/09638288.2017.1358770 [DOI] [PubMed] [Google Scholar]
- 31.Beckwee D, Vaes P, Shahabpour M, Muyldermans R, Rommers N, Bautmans I. The influence of joint loading on bone marrow lesions in the knee: a systematic review with meta-analysis. Am J Sports Med. 2015;43(12):3093–3107. doi: 10.1177/0363546514565092 [DOI] [PubMed] [Google Scholar]
- 32.Otsuki S, Nakajima M, Okamoto Y, et al. Correlation between varus knee malalignment and patellofemoral osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24(1):176–181. doi: 10.1007/s00167-014-3360-3 [DOI] [PubMed] [Google Scholar]
- 33.He ZJ, Li SL, Zou JH, et al. Pain related risk factors among radiologic stages of knee osteoarthritis: data from the osteoarthritis initiative. Arthrit Care Res. 2023;75(6):1333–1339. doi: 10.1002/acr.24997 [DOI] [PubMed] [Google Scholar]
- 34.Bennell KL, Wrigley TV, Hunt MA, Lim B-W, Hinman RS. Update on the role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am. 2013;39(1):145–176. doi: 10.1016/j.rdc.2012.11.003 [DOI] [PubMed] [Google Scholar]
- 35.Takagi S, Omori G, Koga H, et al. Quadriceps muscle weakness is related to increased risk of radiographic knee OA but not its progression in both women and men: the matsudai knee osteoarthritis survey. Knee Surg Sports Traumatol Arthrosc. 2017;26(9):2607–2614. doi: 10.1007/s00167-017-4551-5 [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Wang L, You W, Shan T. Myokines mediate the cross talk between skeletal muscle and other organs. J Cell Physiol. 2020;236(4):2393–2412. doi: 10.1002/jcp.30033 [DOI] [PubMed] [Google Scholar]
- 37.Krishnasamy P, Hall M, Robbins SR. The role of skeletal muscle in the pathophysiology and management of knee osteoarthritis. Rheumatology. 2018;57(suppl_4):iv22–iv33. doi: 10.1093/rheumatology/kex515 [DOI] [PubMed] [Google Scholar]
- 38.Rainbow RS, Kwon H, Foote AT, Preda RC, Kaplan DL, Zeng L. Muscle cell-derived factors inhibit inflammatory stimuli-induced damage in hMSC-derived chondrocytes. Osteo Cartilage. 2013;21(7):990–998. doi: 10.1016/j.joca.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis. JAMA. 2021;325(6):568–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Previtali D, Andriolo L, Di Laura Frattura G, et al. Pain trajectories in knee osteoarthritis—a systematic review and best evidence synthesis on pain predictors. J Clin Med. 2020;9(9):2828. doi: 10.3390/jcm9092828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oosterman JM, Gibson SJ, wlja P, Veldhuijzen DS. On the moderating role of age in the relationship between pain and cognition. Eur J Pain. 2012;17(5):735–741. doi: 10.1002/j.1532-2149.2012.00235.x [DOI] [PubMed] [Google Scholar]
- 42.Rodrigo H, Beukes EW, Andersson G, Manchaiah V. Internet-based cognitive–behavioural therapy for tinnitus: secondary analysis to examine predictors of outcomes. BMJ Open. 2021;11(8):e049384. doi: 10.1136/bmjopen-2021-049384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiphof D, Kerkhof HJ, Damen J, et al. Factors for pain in patients with different grades of knee osteoarthritis. Arthritis Care Res. 2013;65(5):695–702. doi: 10.1002/acr.21886 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The OAI database can be assessed at https://nda.nih.gov/oai/. Collated data files can be accessed upon request to corresponding author.