Abstract
In humans and animal models of herpes simplex virus infection, zosteriform skin lesions have been described which result from anterograde spread of the virus following invasion of the nervous system. Such routes of viral spread have not been fully examined following corneal infection, and the possible pathologic consequences of such spread are unknown. To investigate this, recombinant viruses expressing reporter genes were generated to quantify and correlate gene expression with replication in eyes, trigeminal ganglia, and periocular tissue. Reporter activity peaked in eyes 24 h postinfection and rapidly fell to background levels by 48 h despite the continued presence of viral titers. Reporter activity rose in the trigeminal ganglia at 60 h and peaked at 72 h, concomitant with the appearance and persistence of infectious virus. Virus was present in the periocular skin from 24 h despite the lack of significant reporter activity until 84 h postinfection. This detection of reporter activity was followed by the onset of periocular disease on day 4. Corneal infection with a thymidine kinase-deleted reporter virus displayed a similar profile of reporter activity and viral titer in the eyes, but little or no detectable activity was observed in trigeminal ganglia or periocular tissue. In addition, no periocular disease symptoms were observed. These findings demonstrate that viral infection of periocular tissue and subsequent disease development occurs by zosteriform spread from the cornea to the periocular tissue via the trigeminal ganglion rather than by direct spread from cornea to the periocular skin. Furthermore, clinical evidence is discussed suggesting that a similar mode of spreading and disease occurs in humans following primary ocular infection.
Following infection, herpes simplex virus (HSV) replicates in the epithelium, gains access to axonal terminae, and is retrogradely transported to sensory ganglia. A short period of viral replication within infected sensory ganglia is concomitant with the establishment of a nonproductive latent infection (22). During acute infection, HSV may reemerge from the nervous system and cause disease within the same dermatome at a location distal from the initial site of infection (4, 7, 18, 24, 32). This phenomenon, termed zosteriform spread, has been shown in mouse models to progress from flank to flank, snout to eye, mouth to eye, and neck to pinna. Spread requires an intact nerve supply and has been likened to viral reactivation in that the virus must traverse the nervous system to cause disease at a distal site (26–28). Zosteriform models have been used to determine the effects of antivirals, vaccines, or the immune response on disease (2, 8, 11, 15, 17, 21, 27, 28, 34).
The mouse eye model of HSV infection has provided much information regarding eye diseases such as stromal keratitis (13, 31). Little is known, however, about the progression to and cause of periocular diseases, such as blepharitis and conjunctivitis, despite their high prevalence in HSV-infected individuals (19). Primary corneal infection of mice with HSV-1 results in severe corneal and periocular disease (20, 32). Abundant progeny virus is observed in the eyes and periocular skin throughout acute infection, but due to the close anatomical proximity of these tissues the primary site of viral replication is unknown. Blepharoconjunctivitis, periocular hair loss, and ulcerative lesions become apparent 4 days postinfection and progress in severity until day 15, after which they resolve (29). Interestingly, recombinant viruses that replicate in the cornea but not in the nervous system fail to cause periocular disease (5). We hypothesize, therefore, that periocular disease occurs by viral spread from the cornea to the periocular tissue via the trigeminal ganglia rather than by direct spread from the cornea to the skin.
In this study, recombinant reporter viruses were constructed which express luciferase or β-galactosidase under the control of immediate-early (IE) or early (E) gene promoters. These viruses were used to monitor and quantify viral gene expression and replication in the mouse ocular model. Reporter gene activity peaked in the eyes at 24 h, in trigeminal ganglia at 72 h, and in periocular tissue at 84 to 96 h. Periocular disease followed shortly thereafter. A thymidine kinase-deleted reporter virus expressed high levels of reporter activity in eyes, but no reporter gene activity was detectable in the trigeminal ganglia or periocular tissue. This demonstrates a requirement for viral replication within the nervous system for delivery to the periocular tissue and demonstrates that zosteriform spread from the cornea to the eyelids leads to periocular disease. In addition, we discuss clinical evidence that a similar progression results in human periocular disease.
MATERIALS AND METHODS
Cells and virus.
African green monkey kidney (Vero) cells were propagated as previously described (23) and were used for the in vitro growth and reporter assays of all viruses. Growth curve experiments were performed at the appropriate multiplicity of infection (MOI) on 24-well plates seeded 18 to 20 h previously with 105 Vero cells/well. All virus stocks were propagated on Vero cells as previously described (23). All viruses were constructed in the context of the KOS strain of HSV-1. The thymidine kinase null mutant, dl8.36tk, referred to in this study as KOS6βΔtk, has been previously described (16).
Plasmids and generation of recombinant viruses.
A reporter plasmid, pDlux, was generated with both firefly and Renilla luciferase genes in a divergent orientation from a single multicloning site. The plasmid was constructed by NheI/SalI digestion of pGL3-Basic and pRL-null plasmids (Promega, Madison, Wis.) followed by isolation and ligation of regions containing the firefly and Renilla genes. A BamHI/NruI 822-bp fragment encoding the origin of replication S (oriS) and flanking ICP4 and ICP22/47 promoters was isolated and blunt-end ligated into the NheI site pDlux to yield pDlux/oriS. The Dlux/oriS cassette and the previously characterized pD6p cassette (10) encoding β-galactosidase under the regulation of the ICP6 promoter were cloned into the BglII site at position 106750 of plasmid pUIC to yield pUIC/Dlux/oriS and pUIC6β (D. J. Davido, D. A. Leib, and P. A. Schaffer, submitted for publication). pUIC-based clones were linearized with PstI and cotransfected with KOS infectious DNA. Resulting viruses were selected based on their ability to express either β-galactosidase or luciferase. Putative recombinant viruses were plaque purified three times, and identity was confirmed via Southern blot hybridization (data not shown).
Growth curve and gene expression analysis.
Vero cells were seeded at 105 cells/well in 24-well plates. Eighteen to twenty hours postseeding, cells were infected with virus at an MOI of 0.1 or 5. After adsorption for 1 h at 37°C, virus was aspirated, cells were washed, and 1 ml of medium was replaced. Medium was removed at indicated hours postinfection, frozen, thawed, and titered on Vero cells. At the time of harvest, cells were lysed in 100 μl of Passive Lysis Buffer (Promega), frozen, thawed, and assayed for firefly and Renilla luciferase activity using the Dual Luciferase Assay kit (Promega) or for β-galactosidase activity using previously published methods (25). Cycloheximide reversal experiments were performed as described previously (30) with minor alterations. Briefly, pretreatment of cells with 100 mg of cycloheximide per ml for 1 h was followed by infection as above at an MOI of 5 in the presence of drug. Eight hours postinfection. cells were washed and medium was replaced with 10 mg of actinomycin D per ml. Twelve hours postinfection cells were harvested in 100 μl of Passive Lysis Buffer, and luciferase of β-galactosidase activity was measured.
Animal procedures.
Outbred CD-1 female mice (body weight, 21 to 25 g; Charles River Breeding Laboratories, Inc., Kingston, N.Y.) were anesthetized with ketamine and xylazine, their corneas were bilaterally scarified, and they were inoculated with 2 × 106 PFU of virus in a volume of 5 μl as previously described (23). Tear film material was assayed for virus as previously described (23). Whole eyeballs, trigeminal ganglia, and 6-mm biopsy punches of periocular tissue were removed, placed in dry tubes containing 1-mm diameter beads, and frozen. Upon harvest, 100 μl of reporter buffer (50 mM HEPES [pH 7.5], 1 mM EDTA, 1% gelatin, 5% glycerol, 5 mM dithiothreitol) was added, and tissue was homogenized via beadbeating (Mini-Beadbeater-8; Biospec Products, Bartlesville, Okla.) and sonication. Ten microliters of homogenate was removed, added to 990 μl of medium, frozen, and thawed, and titers were determined on Vero cells. Twenty microliters of the same lysate was assayed for firefly and Renilla luciferase activity or β-galactosidase activity. Data were analyzed throughout using Student's t-test.
Periocular disease was measured in a masked fashion on a semiquantitative scale as previously described (29). To measure growth in isolated periocular skin, mice were corneally infected with virus and sacrificed 12 h later. Periocular skin was harvested as described above and explanted in culture. Supernatants were harvested and titers were determined every 12 h postexplant.
RESULTS
In vitro replication and reporter gene expression kinetics.
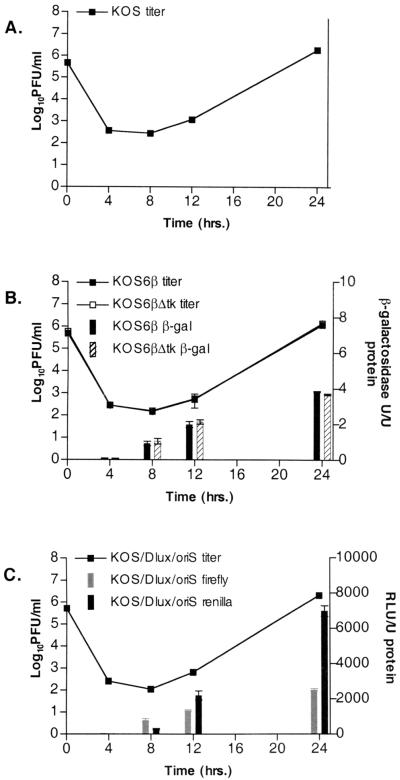
To validate the growth of the viruses used in this study, KOS, KOS6β, KOS6βΔtk, and KOS/Dlux/oriS were examined in Vero cells in both single- and multiple-step growth curves (Fig. 1A through D and 2A through C). Monolayers were infected at MOIs of 0.1 (data not shown) and 5 PFU/cell, and at various times postinfection supernatants were titered for infectious virus and cell monolayers were harvested to measure luciferase or β-galactosidase activity. The kinetics of viral growth and egress were similar among all viruses tested (Fig. 2A through C). In addition, reporter gene activity was detectable early in infection and rose concomitantly and proportionally with the detection of viral progeny.
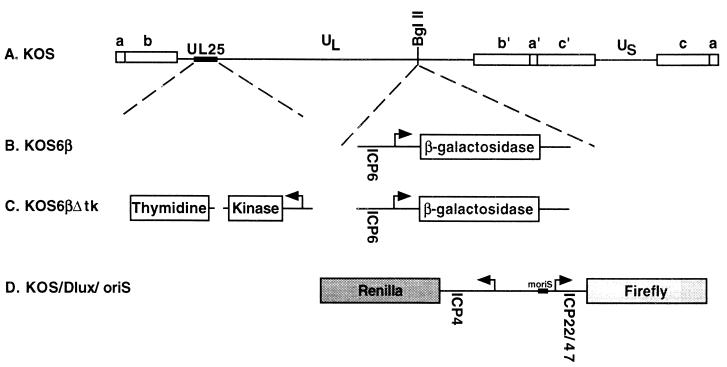
FIG. 1.
Maps of reporter gene viruses used in this study. (A) Prototypical arrangement of the HSV-1 genome, showing the unique long (UL) and unique short (US) segments flanked by internal (a′, b′, c′) and terminal (a, b, c) repeats. The locations of reporter cassette insertions and the thymidine kinase deletion are shown. (B) KOS6β was constructed by insertion of a cassette containing the early ICP6 promoter regulating the expression of β-galactosidase (10) into the BglII site at map position 106750. (C) KOS6βΔtk was constructed by a 360-bp deletion of the thymidine kinase optical reading frame within the context of KOS6β (16). (D) KOS/Dlux/oriS was constructed by insertion of a cassette carrying the ICP4/22/47 and oriS regulatory region driving firefly and Renilla luciferase expression into the BglII site.
FIG. 2.
Single-step growth kinetics and reporter gene activity for KOS (A), KOS6β and KOS6βΔtk (B), and KOS/Dlux/oriS (C). Vero cells were infected at an MOI of 5. Data represent standard errors of the means of three independent experiments. The limit of detection is 10 PFU/ml. RLU, relative light units.
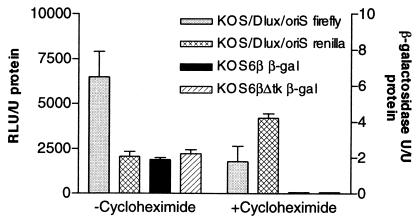
In order to validate the expression kinetics of the recombinant viruses, monolayers were infected in the presence or absence of cycloheximide (Fig. 3). All recombinant viruses exhibited expression of luciferase or β-galactosidase 12 h postinfection in the absence of cycloheximide. Infection in the presence of cycloheximide resulted in high levels of luciferase activity, but β-galactosidase activity was reduced to background levels. These data demonstrate that the luciferases in KOS/Dlux/oriS are regulated with IE kinetics, whereas β-galactosidase expressed by KOS6β and KOS6βΔtk is regulated as an E gene.
FIG. 3.
Reporter gene activity under cycloheximide reversal or control conditions. Vero cells were pretreated for 1 h and then were infected at an MOI of 5 in the presence or absence of cycloheximide (100 μg/ml) for 8 h, after which time cells were washed and plated in the presence of actinomycin D (10 μg/ml) for 4 h. Monolayers were harvested and assayed for reporter activity. RLU, relative light units.
Clinical disease in infected animals.
Mice were infected with KOS/Dlux/oriS, KOS6β, or KOS6βΔtk and scored for periocular disease using a masked, semiquantitative scale (Fig. 4). Little or no clinical disease was observed until day 5 despite detectable viral titers within the periocular tissue from 24 to 96 h postinfection. Clinical scores of mice infected with KOS/Dlux/oriS and KOS6β increased from days 5 to 8 and in accordance with previously published studies involving wild-type virus (29). KOS6βΔtk-infected mice, however, displayed no clinical disease at any time scored (Fig. 4).
FIG. 4.
Periocular disease scores in mice following corneal scarification and infection with 2 × 106 PFU per eye of KOS/Dlux/oriS, KOS6β, and KOS6βΔtk. Animals were scored as follows: 0, no lesions; 1, minimal eyelid swelling; 2, moderate swelling and crusty ocular discharge; 3, severe swelling, moderate periocular hair loss, and skin lesions; 4, severe swelling with eyes crusted shut, severe periocular hair loss, and skin lesions. Data represent combined averages of at least 10 mice per time point.
In vivo growth kinetics and gene expression.
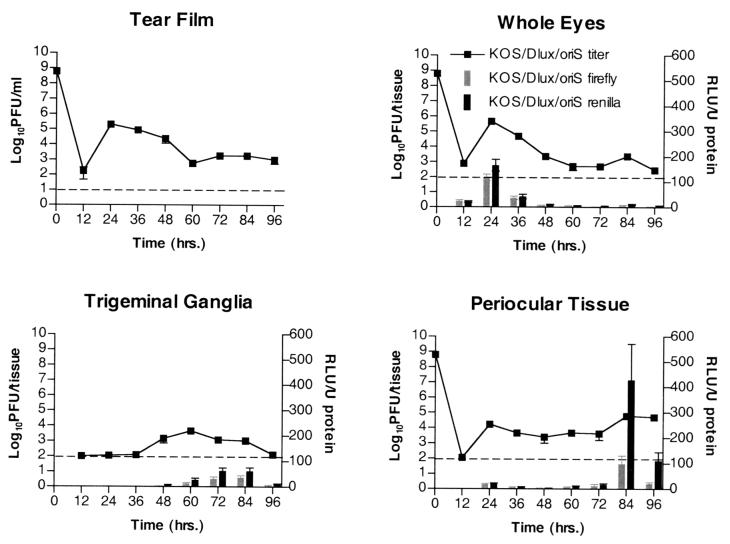
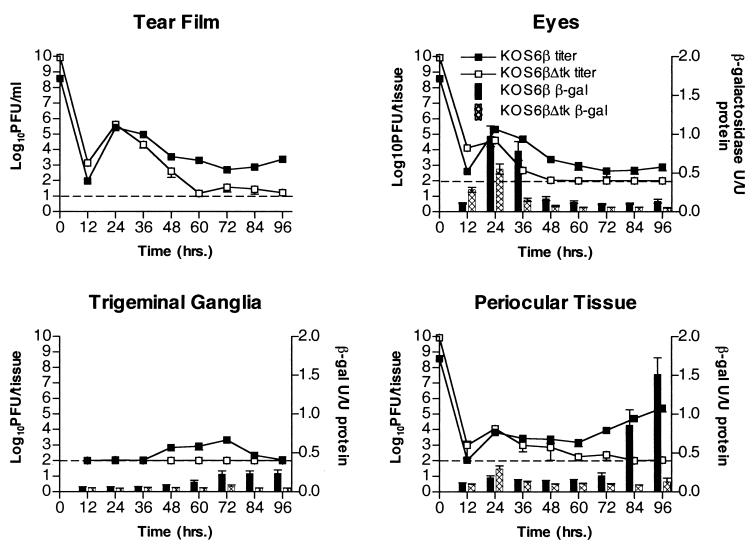
To determine viral IE and E gene expression patterns during acute infection, mice were infected via the scarified cornea with KOS/Dlux/oriS and KOS6β (Fig. 5 and 6). At various times postinfection mice were sacrificed and titers were measured in whole globes, trigeminal ganglia, periocular skin, and the tear film via corneal swab. Reporter gene expression was also measured in the same samples harvested for viral titration in order to correlate the presence of virus with infectious centers within adjacent tissues.
FIG. 5.
In vivo growth and reporter gene expression of KOS/Dlux/oriS after ocular infection. Mice were infected via corneal scarification and inoculation of 2 × 106 PFU per eye. At various times postinfection tissues were harvested and assayed for infectious virus and luciferase activity. Data points represent 12 tissues from two independent experiments with three mice. In periocular skin, both luciferase activity and titer were significantly increased (P < .05) at 84 h relative to earlier time points. RLU, relative light units.
The patterns of viral replication and reporter gene activity of KOS6β were comparable to those of KOS/Dlux/oriS in all tissues tested (Fig. 5 and 6). Titers peaked in the eyes at 24 h and in the trigeminal ganglia at 72 h. Reporter activity of both luciferase and β-galactosidase followed the same trend of peak and kinetics. The relationship between reporter activity and viral replication in the periocular tissue differed from that seen in the eyes and ganglia. The initial high titers observed 24 h postinfection were accompanied by a small amount of reporter activity in the skin, which dropped to background levels after 36 to 72 h. At 84 and 96 h a significant increase of viral titer was accompanied by a precipitous peak of reporter activity coincident with early signs of periocular disease for both KOS6β and KOS/Dlux/oriS. The appearance of viral progeny, reporter activity, and disease symptoms is consistent with zosteriform spread of the virus from the cornea to the periocular tissue via the trigeminal ganglia.
FIG. 6.
In vivo growth and reporter gene expression of KOS6β and KOS6βΔtk after ocular infection. Mice were infected via corneal scarification and inoculation of 2 × 106 PFU of KOS6β or 8.5 × 107 PFU of KOS6βΔtk per eye. At various times postinfection tissues were harvested and assayed for infectious virus and β-galactosidase activity. Data points represent 12 tissues from two independent experiments with three mice. In KOS6β infections, β-galactosidase activity and titer were significantly increased (P < 0.05) at 84 h relative to earlier time points and significantly higher than those of KOS6βΔtk (P < 0.05) from 72 h onward.
An alternative route considered was direct spread of virus from the cornea to the periocular skin. Previous studies have used nerve transection to define pathways of viral spread in vivo (26, 27). Due to the delicate nature and inherent problems with such surgery on cranial and facial nerves, we utilized a genetic lesion at the thymidine kinase locus within the virus to rule out direct spread. Thymidine kinase mutants of HSV-1 grow to slightly reduced levels in the cornea but are unable to replicate in trigeminal ganglia (6). If direct spread is sufficient for acute infection in the periocular skin and development of disease therein, then a tk null virus will resemble wild-type virus during infection. If, on the other hand, replication in the trigeminal ganglia and anterograde return to the skin are required for disease, the tk null virus will be absent in the skin late during the assay period and, consequently, will be incapable of causing disease.
Patterns of KOS6βΔtk reporter activity and replication were similar to those of KOS6β in the tear film and whole eyes until 24 h, after which both rapidly dropped to background levels (Fig. 6). As expected, little or no replication and reporter activity was observed in the trigeminal ganglia of KOS6βΔtk- infected animals (6). In stark contrast to KOS6β in the periocular tissue, KOS6βΔtk failed to show any increase in levels of gene expression or titer between 72 and 96 h postinfection. In addition, no periocular disease was observed, despite inoculation of 30-fold more KOS6βΔtk than KOS6β. This lack of disease, β-galactosidase activity, and detectable titers strongly supports the hypothesis that replication in trigeminal ganglia and retrograde return to the skin is required for the replication and disease observed in the periocular tissue late in the assay period.
Previous reports have demonstrated that tk null viruses grow to slightly reduced levels in the cornea (6). Consequently, a growth defect of KOS6βΔtk within the periocular tissue could result in a lack of progeny virus and gene expression late in the assay period. To ensure both viruses could replicate to equivalent levels within the periocular tissue, mice were infected with KOS6β or KOS6βΔtk and periocular tissue was harvested 12 h postinfection, washed, and explanted. At times postexplant, medium was harvested and titers were determined to quantify progeny virus (Fig. 7). Both viruses replicated to similar levels ex vivo in the periocular tissue explants. These data support the hypothesis that the inability of KOS6βΔtk to generate reporter activity, replicate detectably at late times, and cause disease is not due to an inherent growth defect in the periocular skin but rather an inability to replicate in, and return from, the trigeminal ganglion.
FIG. 7.
Growth of KOS6β and KOS6βΔtk in periocular tissue explants after ocular infection. Mice were infected via corneal scarification and inoculation of 2 × 106 PFU of KOS6β or 8.5 × 107 PFU of KOS6βΔtk per eye. Twelve hours postinfection tissue was harvested and explanted in medium. Supernatants were titers at various times postinfection. Data represent the mean of data from four mice from two independent experiments.
DISCUSSION
Reporter gene viruses have been previously utilized to monitor and elucidate viral promoter regulation (3, 14, 33). Recombinant viruses containing promoter-reporter cassette insertions into genes dispensable for growth (e.g., tk and gC) have been useful for elucidating promoter elements during the lytic life cycle in vitro. Such viruses, however, face limitations with respect to pathogenesis in vivo due to insertions into loci which may be virulence determinants. To address these limitations, we utilized a locus whose disruption did not significantly affect growth in vitro and pathogenesis in vivo (this study and data not shown). This locus was a BglII site (map position 106750) which interrupts the C-terminal 12 amino acids of the UL49.5 gene (1). Insertion at this site has no effect on protein synthesis, signal sequence cleavage, protein targeting of UL49.5, or viral growth in vitro. Insertions at this site have only a limited effect on in vivo parameters, including acute replication within the eyes, trigeminal ganglia, and periocular tissue, disease kinetics and severity, as well as reactivation from explanted trigeminal ganglia (this study and Davido et al., submitted). These viruses have clear utility for the study of viral spread and gene expression during acute infection as well as for future experiments examining the efficacy of antivirals and immunity in limiting viral gene expression.
Utilizing these reporter gene viruses, we have demonstrated that HSV-1 progresses from the corneal surface to the periocular tissue via the trigeminal ganglion. This progression was observed irrespective of reporter gene measured or the kinetic class of promoter regulating its expression. The onset of periocular disease correlated with increased viral titer and reporter gene activity within this tissue. The pattern of viral titer, reporter gene activity, and disease development is consistent with periocular disease being a manifestation of zosteriform spread through the nervous system rather than direct spread from cornea to skin.
Although zosteriform spread appeared to be the primary mode of dissemination, the close anatomical proximity of the eye and skin does allow limited direct infection of the periocular tissue. Indeed, small amounts of reporter activity and viral titer are detectable within the periocular tissue at 24 h. In addition, mice develop a mild, transient blepharoconjunctivitis 24 to 36 h postinfection. Infection with a thymidine kinase-deleted virus, however, did not cause disease despite its demonstrated ability to directly infect and replicate within the periocular tissue. In agreement with these results, ribonucleotide reductase mutants are also unable to cause appreciable periocular disease after corneal infection (5). We therefore conclude that limited direct spread does occur, but viral replication within the sensory nervous system and centrifugal spread are necessary for the development of periocular disease.
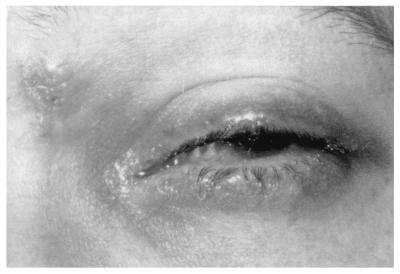
The experiments above have detailed a requirement for zosteriform spread of HSV from the cornea to the periocular tissue via the sensory nerves to cause disease in mice. Despite the obvious contribution of the mouse model to our understanding of ocular disease, the relevance of such observations to human herpetic eye disease should be considered. For humans, a number of reports have detailed periocular skin involvement after ocular infection with HSV (9, 12 and S. Yamamoto, Y. Shimomura, S. Kinoshita, and Y. Tano, Letter, Arch. Ophthalmol. 112:1515–1516, 1994). In a case study of 122 HSV ocularly infected individuals, 54% exhibited eyelid and conjunctival disease during their first episode and 31% during recurrent episodes (19). This previous study suggests that periocular involvement following ocular infection is highly prevalent in HSV-infected individuals. We detail here a case of human ocular HSV disease in which the timing of periocular involvement relative to corneal infection strongly agrees with our observations in the mouse ocular model. An 18-year-old male presented to the San Francisco General Hospital Ophthalmology Clinic with an ocular infection which was characterized by a geographic corneal ulcer. A diagnosis of primary HSV geographic epithelial keratitis was made, and he was prescribed topical trifluoridine. He was seen again 5 days later, and upon examination his corneal ulcer had reduced in size by 50%, but he now had periorbital swelling and redness with discrete vesicles, characteristic of HSV, on the lid margin and brow (Fig. 8). The patient was treated with oral acyclovir and had an uneventful recovery. The timing and progression of symptoms in this case of primary ocular infection strongly correlate with the mouse data presented in this study, suggesting a similar mode of viral spread. This suggests parallel patterns of zosteriform spread within mice and humans and that treatment of primary infection with systemic, rather than topical, antivirals may serve to better control the spread of infection.
FIG. 8.
Human clinical correlate to periocular disease in mice. Five days following initial presentation with primary corneal disease, the subject shown had periorbital swelling and redness. Discrete vesicles and lesions, characteristic of HSV, are visible on the lid margin and brow.
ACKNOWLEDGMENTS
We thank Skip Virgin, Sam Speck, Lynda Morrison, and members of their laboratories for helpful discussions.
This study was supported by NIH grants RO1EY09083 to David A. Leib, EY10008 and EY02162 to Todd P. Margolis, and P30-EY02687 to the Department of Ophthalmology and Visual Sciences. Support from Research to Prevent Blindness to the department and a Robert E. McCormick Scholarship to David A. Leib are gratefully acknowledged.
REFERENCES
- 1.Adams R, Cunningham C, Davison M D, MacLean C A, Davison A J. Characterization of the protein encoded by gene UL49A of herpes simplex virus type 1. J Gen Virol. 1998;79:813–823. doi: 10.1099/0022-1317-79-4-813. [DOI] [PubMed] [Google Scholar]
- 2.Awan A R, Harmenberg J, Flink O, Field H J. Combinations of antiviral and anti-inflammatory preparations for the topical treatment of herpes simplex virus assessed using a murine zosteriform infection model. Antivir Chem Chemother. 1998;9:19–24. doi: 10.1177/095632029800900101. [DOI] [PubMed] [Google Scholar]
- 3.Bloom D C, Jarman R G. Generation and use of recombinant reporter viruses for study of herpes simplex virus infections in vivo. Methods. 1998;16:117–125. doi: 10.1006/meth.1998.0649. [DOI] [PubMed] [Google Scholar]
- 4.Blyth W A, Harbour D A, Hill T J. Pathogenesis of zosteriform spread of herpes simplex virus in the mouse. J Gen Virol. 1984;65:1477–1486. doi: 10.1099/0022-1317-65-9-1477. [DOI] [PubMed] [Google Scholar]
- 5.Brandt C R, Kintner R L, Pumfery A M, Visalli R J, Grau D R. The herpes simplex virus ribonucleotide reductase is required for ocular virulence. J Gen Virol. 1991;72:2043–2049. doi: 10.1099/0022-1317-72-9-2043. [DOI] [PubMed] [Google Scholar]
- 6.Coen D M, Kosz-Vnenchak M, Jacobson J G, Leib D A, Bogard C L, Schaffer P A, Tyler K L, Knipe D M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci USA. 1989;86:4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyson H, Shimeld C, Hill T J, Blyth W A, Easty D L. Spread of herpes simplex virus within ocular nerves of the mouse: demonstration of viral antigen in whole mounts of eye tissue. J Gen Virol. 1987;68:2989–2995. doi: 10.1099/0022-1317-68-12-2989. [DOI] [PubMed] [Google Scholar]
- 8.Erturk M, Hill T J, Shimeld C, Jennings R. Acute and latent infection of mice immunised with HSV-1 ISCOM vaccine. Arch Virol. 1992;125:87–101. doi: 10.1007/BF01309630. [DOI] [PubMed] [Google Scholar]
- 9.Forrest W M, Kaufman H E. Zosteriform herpes simplex. Am J Ophthalmol. 1976;81:86–88. doi: 10.1016/0002-9394(76)90196-3. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein D J, Weller S K. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J Virol. 1988;62:2970–2977. doi: 10.1128/jvi.62.8.2970-2977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon Y J, Cheng K P, Araullo-Cruz T, Romanowski E, Johnson B J, Blough H A. Efficacy of glycoprotein inhibitors alone and in combination with trifluridine in the treatment of murine herpetic keratitis. Curr Eye Res. 1986;5:93–99. doi: 10.3109/02713688609015097. [DOI] [PubMed] [Google Scholar]
- 12.Heskel N S, Hanifin J M. “Recurrent herpes zoster”: an unproved entity? J Am Acad Dermatol. 1984;10:486–490. doi: 10.1016/s0190-9622(84)80099-7. [DOI] [PubMed] [Google Scholar]
- 13.Hill T J. Ocular pathogenicity of herpes simplex virus. Curr Eye Res. 1987;6:1–7. doi: 10.3109/02713688709020060. [DOI] [PubMed] [Google Scholar]
- 14.Ho D Y, Mocarski E S. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology. 1988;167:279–283. doi: 10.1016/0042-6822(88)90079-7. [DOI] [PubMed] [Google Scholar]
- 15.Ijichi K, Ashida N, Varia S, Machida H. Topical treatment with BV-araU of immunosuppressed and immunocompetent shaved mice cutaneously infected with herpes simplex virus type 1. Antivir Res. 1993;21:47–57. doi: 10.1016/0166-3542(93)90066-r. [DOI] [PubMed] [Google Scholar]
- 16.Kaplitt M G, Tjuvajev J G, Leib D A, Berk J, Pettigrew K D, Posner J B, Pfaff D W, Rabkin S D, Blasberg R G. Mutant herpes simplex virus induced regression of tumors growing in immunocompetent rats. J Neurooncol. 1994;19:137–147. doi: 10.1007/BF01306455. [DOI] [PubMed] [Google Scholar]
- 17.Kristofferson A, Ericson A C, Sohl-Akerlund A, Datema R. Limited efficacy of inhibitors of herpes simplex virus DNA synthesis in murine models of recrudescent disease. J Gen Virol. 1988;69:1157–1166. doi: 10.1099/0022-1317-69-6-1157. [DOI] [PubMed] [Google Scholar]
- 18.Labetoulle M, Kucera P, Ugolini G, Lafay F, Frau E, Offret H, Flamand A. Neuronal propagation of HSV1 from the oral mucosa to the eye. Investig Ophthalmol Vis Sci. 2000;41:2600–2606. [PubMed] [Google Scholar]
- 19.Liesegang T J. A community study of ocular herpes simplex. Curr Eye Res. 1991;10:111–115. doi: 10.3109/02713689109020366. [DOI] [PubMed] [Google Scholar]
- 20.Maggs D J, Chang E, Nasisse M P, Mitchell W J. Persistence of herpes simplex virus type 1 DNA in chronic conjunctival and eyelid lesions of mice. J Virol. 1998;72:9166–9172. doi: 10.1128/jvi.72.11.9166-9172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mester J C, Glorioso J C, Rouse B T. Protection against zosteriform spread of herpes simplex virus by monoclonal antibodies. J Infect Dis. 1991;163:263–269. doi: 10.1093/infdis/163.2.263. [DOI] [PubMed] [Google Scholar]
- 22.Preston C M. Repression of viral transcription during herpes simplex virus latency. J Gen Virol. 2000;81:1–19. doi: 10.1099/0022-1317-81-1-1. [DOI] [PubMed] [Google Scholar]
- 23.Rader K A, Ackland-Berglund C E, Miller J K, Pepose J S, Leib D A. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J Gen Virol. 1993;74:1859–1869. doi: 10.1099/0022-1317-74-9-1859. [DOI] [PubMed] [Google Scholar]
- 24.Robinson T W, Dover J R. Experimental zosteriform herpes simplex virus infection in mouse skin. Br J Dermatol. 1972;86:40–48. doi: 10.1111/j.1365-2133.1972.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 25.Rouet P, Raguenez G, Salier J P. Optimized assays for quantifying transient expressions of co-transfected beta-galactosidase and CAT reporter genes. BioTechniques. 1992;13:700–701. [PubMed] [Google Scholar]
- 26.Shimeld C, Dyson H, Lewkowicz-Moss S, Hill T J, Blyth W A, Easty D L. Spread of HSV-1 to the mouse eye after inoculation in the skin of the snout requires an intact nerve supply to the inoculation site. Curr Eye Res. 1987;6:9–12. doi: 10.3109/02713688709020061. [DOI] [PubMed] [Google Scholar]
- 27.Simmons A, Nash A A. Role of antibody in primary and recurrent herpes simplex virus infection. J Virol. 1985;53:944–948. doi: 10.1128/jvi.53.3.944-948.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simmons A, Nash A A. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J Virol. 1984;52:816–821. doi: 10.1128/jvi.52.3.816-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith T J, Ackland-Berglund C E, Leib D A. Herpes simplex virus virion host shutoff (vhs) activity alters periocular disease in mice. J Virol. 2000;74:3598–3604. doi: 10.1128/jvi.74.8.3598-3604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sze P, Herman R C. The herpes simplex virus type 1 ICP6 gene is regulated by a ‘leaky’ early promoter. Virus Res. 1992;26:141–152. doi: 10.1016/0168-1702(92)90153-z. [DOI] [PubMed] [Google Scholar]
- 31.Thomas J, Rouse B T. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 32.Tullo A B, Shimeld C, Blyth W A, Hill T J, Easty D L. Ocular infection with herpes simplex virus in nonimmune and immune mice. Arch Ophthalmol. 1983;101:961–964. doi: 10.1001/archopht.1983.01040010961023. [DOI] [PubMed] [Google Scholar]
- 33.Wagner E K, Petroski M D, Pande N T, Lieu P T, Rice M. Analysis of factors influencing kinetics of herpes simplex virus transcription utilizing recombinant virus. Methods. 1998;16:105–116. doi: 10.1006/meth.1998.0648. [DOI] [PubMed] [Google Scholar]
- 34.Watkins A M, Dunford P J, Moffatt A M, Wong-Kai-In P, Holland M J, Pole D S, Thomas G M, Martin J, Roberts N A, Mulqueen M J. Inhibition of virus-encoded thymidine kinase suppresses herpes simplex virus replication in vitro and in vivo. Antivir Chem Chemother. 1998;9:9–18. [PubMed] [Google Scholar]