Abstract
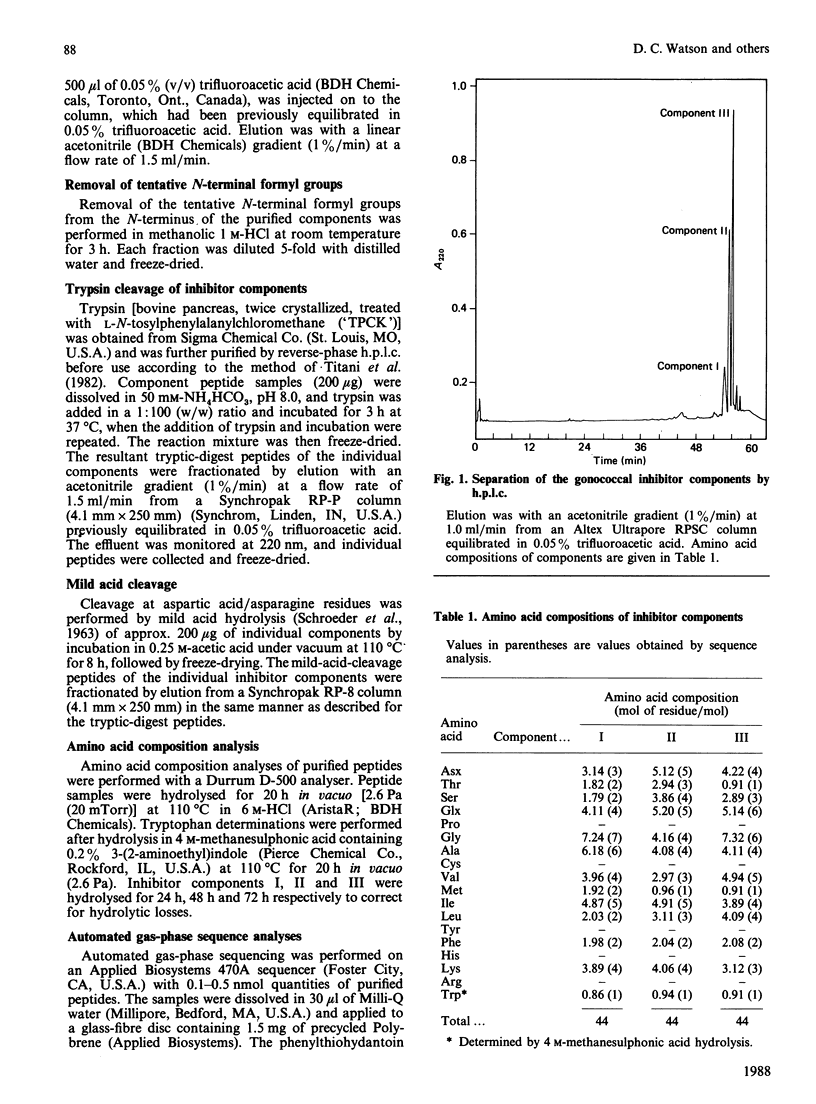
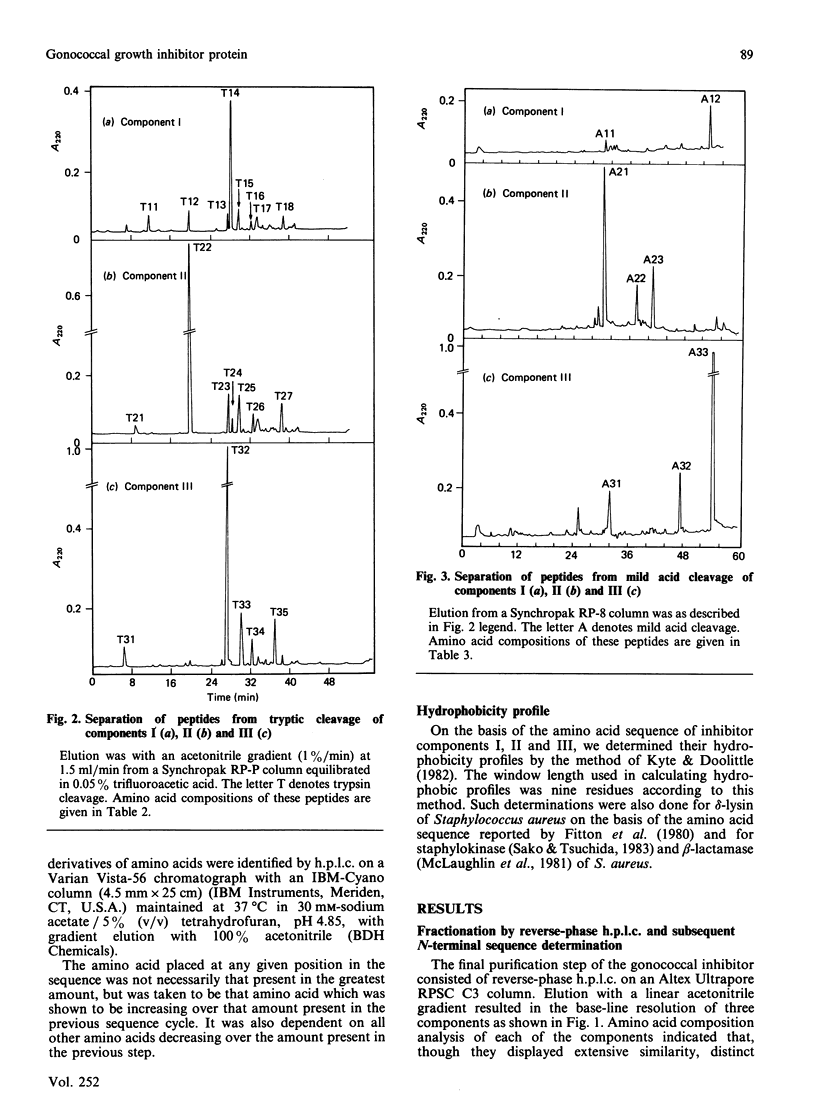
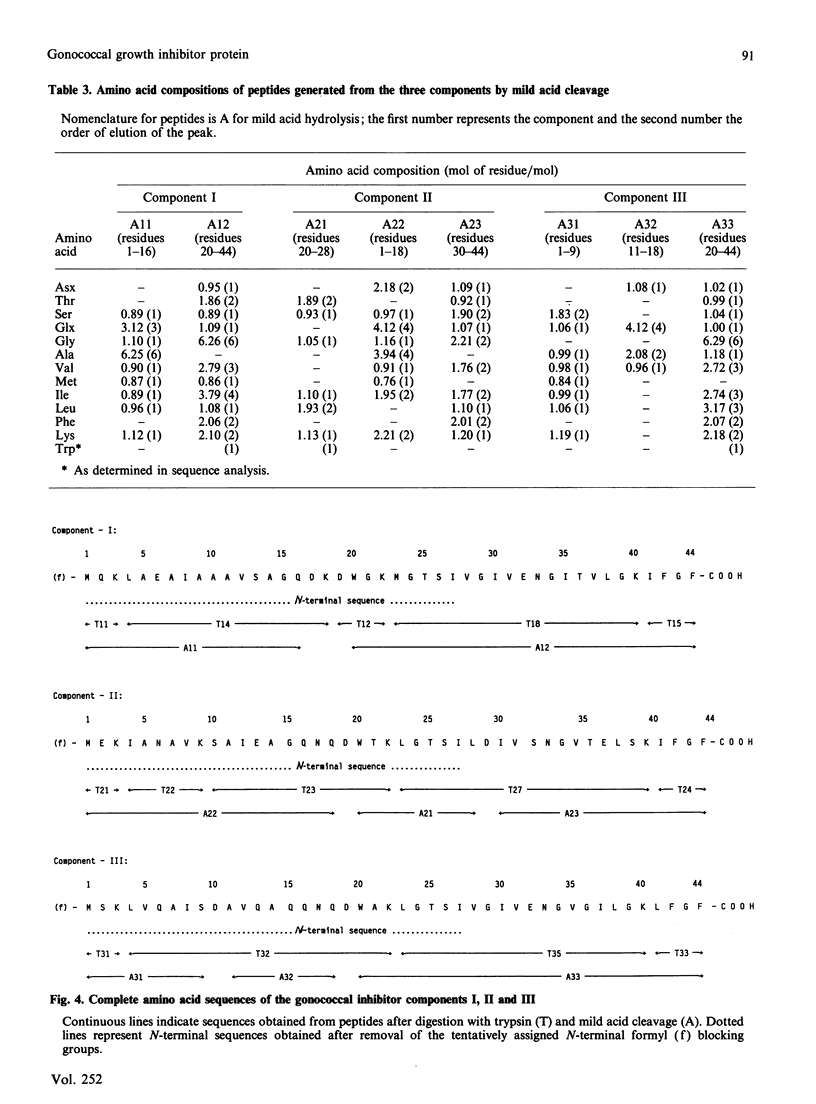
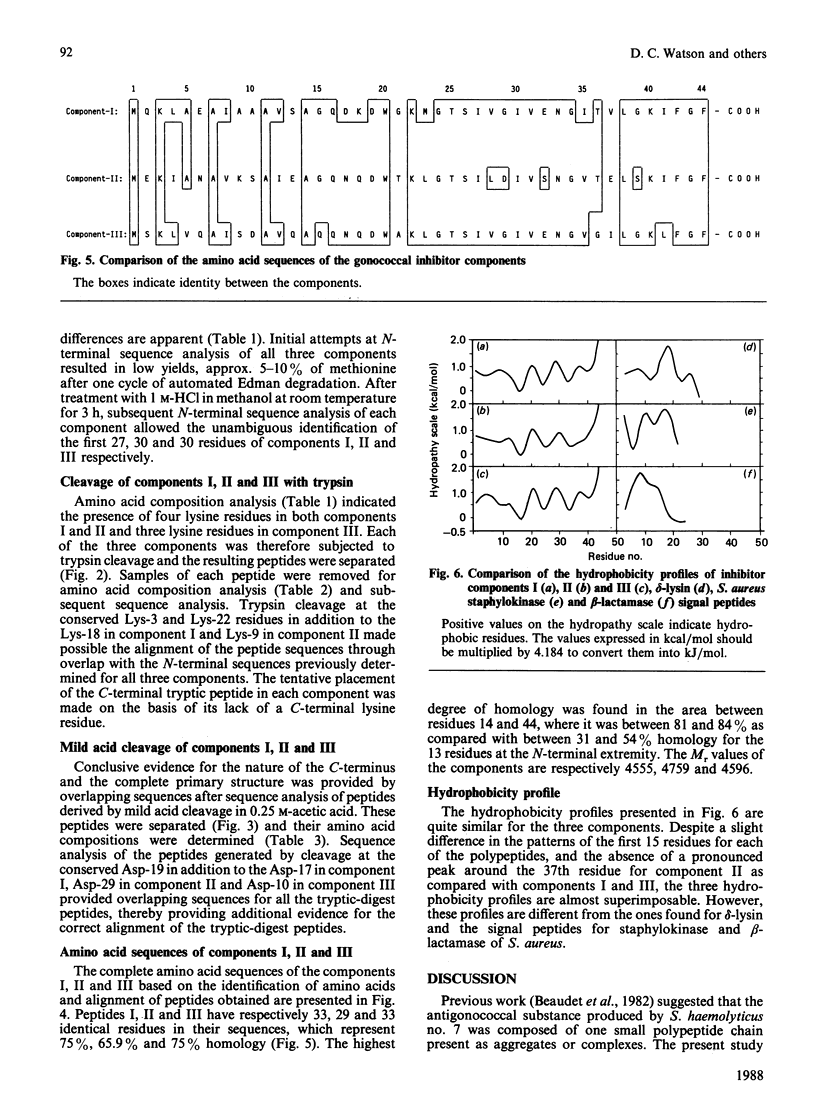
A gonococcal inhibitor produced by Staphylococcus haemolyticus was separated into three components by reverse-phase h.p.l.c. The amino acid composition analysis of each of the three components indicated extensive similarities. N-Terminal sequence analysis of all three components allowed the identification of the first 27-30 residues of each. The complete primary structure of each component was determined from the sequence analysis of trypic peptides and peptides generated by mild acid hydrolysis. Each component is composed of 44 amino acid residues, with evidence suggesting the presence of an N-terminal formylmethionine residue in each. The components I, II and III have respectively 33, 29 and 33 identical amino acid residues in their sequences, which represents 75%, 65.9% and 75% homology. These components contain a high proportion of hydrophobic amino acids, and their hydrophobicity profiles are closely related. Also, each of the three components contains a positively charged residue (lysine) as the third residue, followed by a core of hydrophobic residues. These results suggest that the three components are possible signal sequences of one or more secreted or membrane-associated proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudet R., Bisaillon J. G., Saheb S. A., Sylvestre M. Production, purification, and preliminary characterization of a gonococcal growth inhibitor produced by a coagulase-negative staphylococcus isolated from the urogenital flora. Antimicrob Agents Chemother. 1982 Aug;22(2):277–283. doi: 10.1128/aac.22.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaillon J. G., Beaudet R., Lafond L., Saheb S. A., Sylvestre M. Antigonococcal and antibacterial spectra of some bacterial isolates of the urogenital flora. Rev Can Biol. 1981 Jun;40(2):215–227. [PubMed] [Google Scholar]
- Bisaillon J. G., Beaudet R., Saheb S. A., Morisset R. Interference of Neisseria gonorrhoeae growth by aerobic bacterial representatives of the urogenital flora. Rev Can Biol. 1980 Dec;39(4):201–208. [PubMed] [Google Scholar]
- Bisaillon J. G., Lafond L., Beaudet R., Saheb S. A., Sylvestre M. Comparison of bacteriostatic and bactericidal inhibitors of Neisseria gonorrhoeae growth produced in vitro by urogenital staphylococci. Exp Biol. 1985;43(4):231–242. [PubMed] [Google Scholar]
- Chow A. W., Gribble M. J., Bartlett K. H. Characterization of the hemolytic activity of Staphylococcus aureus strains associated with toxic shock syndrome. J Clin Microbiol. 1983 Mar;17(3):524–528. doi: 10.1128/jcm.17.3.524-528.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton J. E., Dell A., Shaw W. V. The amino acid sequence of the delta haemolysin of Staphylococcus aureus. FEBS Lett. 1980 Jun 30;115(2):209–212. doi: 10.1016/0014-5793(80)81170-7. [DOI] [PubMed] [Google Scholar]
- Frenette M., Beaudet R., Bisaillon J. G., Sylvestre M., Portelance V. Chemical and biological characterization of a gonococcal growth inhibitor produced by Staphylococcus haemolyticus isolated from urogenital flora. Infect Immun. 1984 Nov;46(2):340–345. doi: 10.1128/iai.46.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Bock S. C., Kaiser E. T. Idealization of the hydrophobic segment of the alkaline phosphatase signal peptide. Nature. 1986 Jun 12;321(6071):706–708. doi: 10.1038/321706a0. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Kim K. S., Zaboretzky F., Bernheimer A. W. Purification and properties of staphylococcal delta hemolysin. Infect Immun. 1971 Mar;3(3):449–465. doi: 10.1128/iai.3.3.449-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lafond L., Bisaillon J. G., Saheb S. A., Beaudet R., Sylvestre M. In vitro antigonococcal activity of urogenital coagulase-negative staphylococci. Can J Microbiol. 1981 Jan;27(1):138–141. doi: 10.1139/m81-021. [DOI] [PubMed] [Google Scholar]
- Lee K. Y., Birkbeck T. H. In vitro synthesis of the delta-lysin of Staphylococcus aureus. Infect Immun. 1984 May;44(2):434–438. doi: 10.1128/iai.44.2.434-438.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- SCHROEDER W. A., SHELTON J. R., SHELTON J. B., CORMICK J., JONES R. T. THE AMINO ACID SEQUENCE OF THE GAMMA CHAIN OF HUMAN FETAL HEMOGLOBIN. Biochemistry. 1963 Sep-Oct;2:992–1008. doi: 10.1021/bi00905a016. [DOI] [PubMed] [Google Scholar]
- Sako T., Tsuchida N. Nucleotide sequence of the staphylokinase gene from Staphylococcus aureus. Nucleic Acids Res. 1983 Nov 25;11(22):7679–7693. doi: 10.1093/nar/11.22.7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloma A., Gross M. Molecular cloning and nucleotide sequence of the type I beta-lactamase gene from Bacillus cereus. Nucleic Acids Res. 1983 Jul 25;11(14):4997–5004. doi: 10.1093/nar/11.14.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Sasagawa T., Resing K., Walsh K. A. A simple and rapid purification of commercial trypsin and chymotrypsin by reverse-phase high-performance liquid chromatography. Anal Biochem. 1982 Jul 1;123(2):408–412. doi: 10.1016/0003-2697(82)90465-1. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]


