Abstract
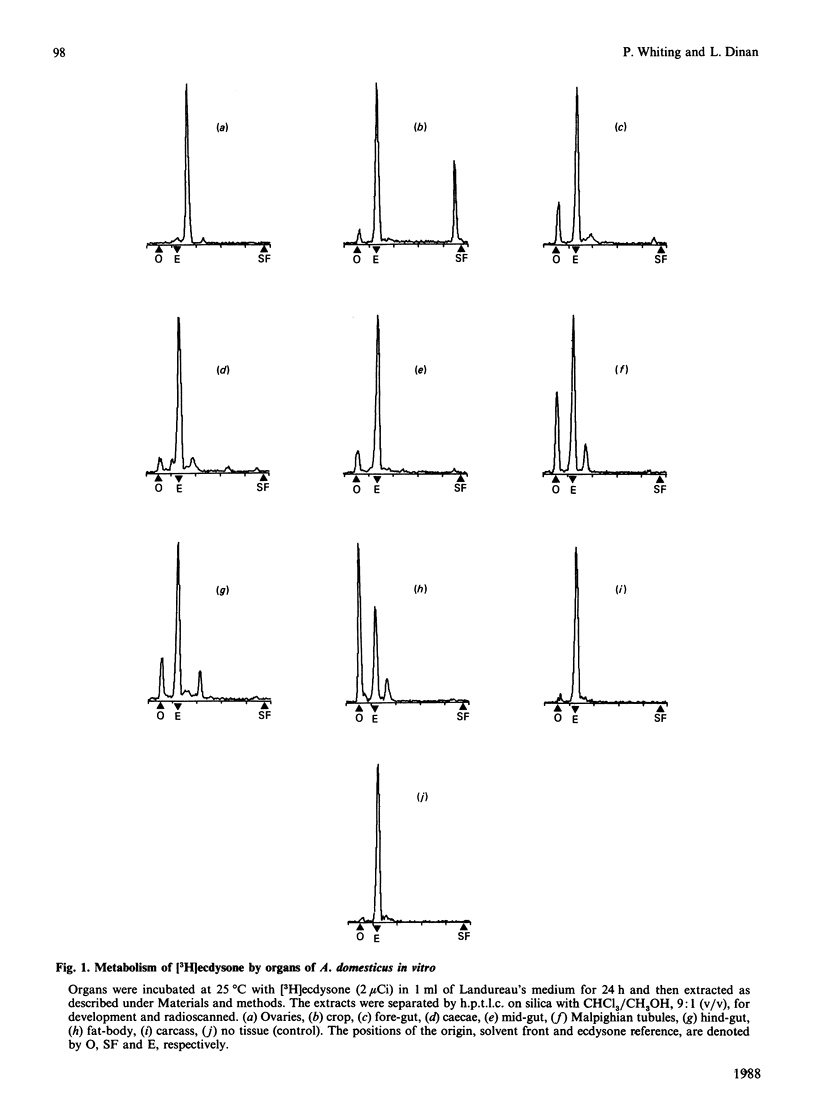
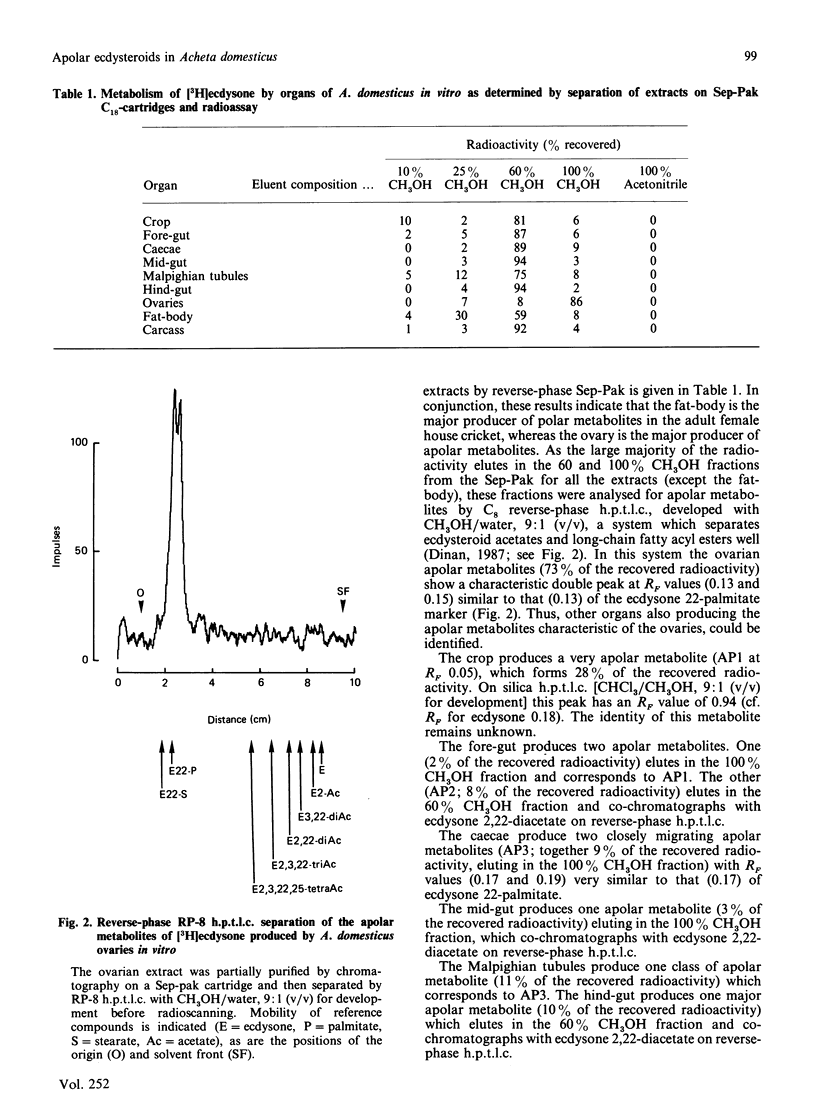
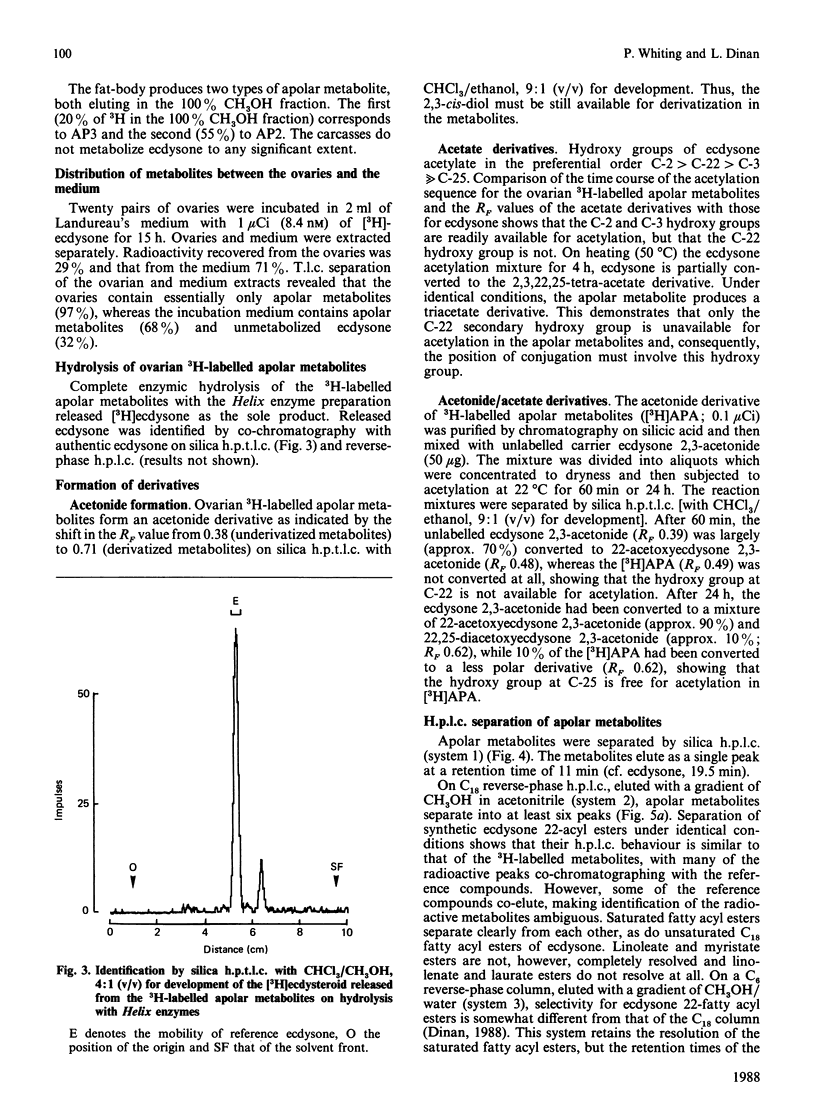
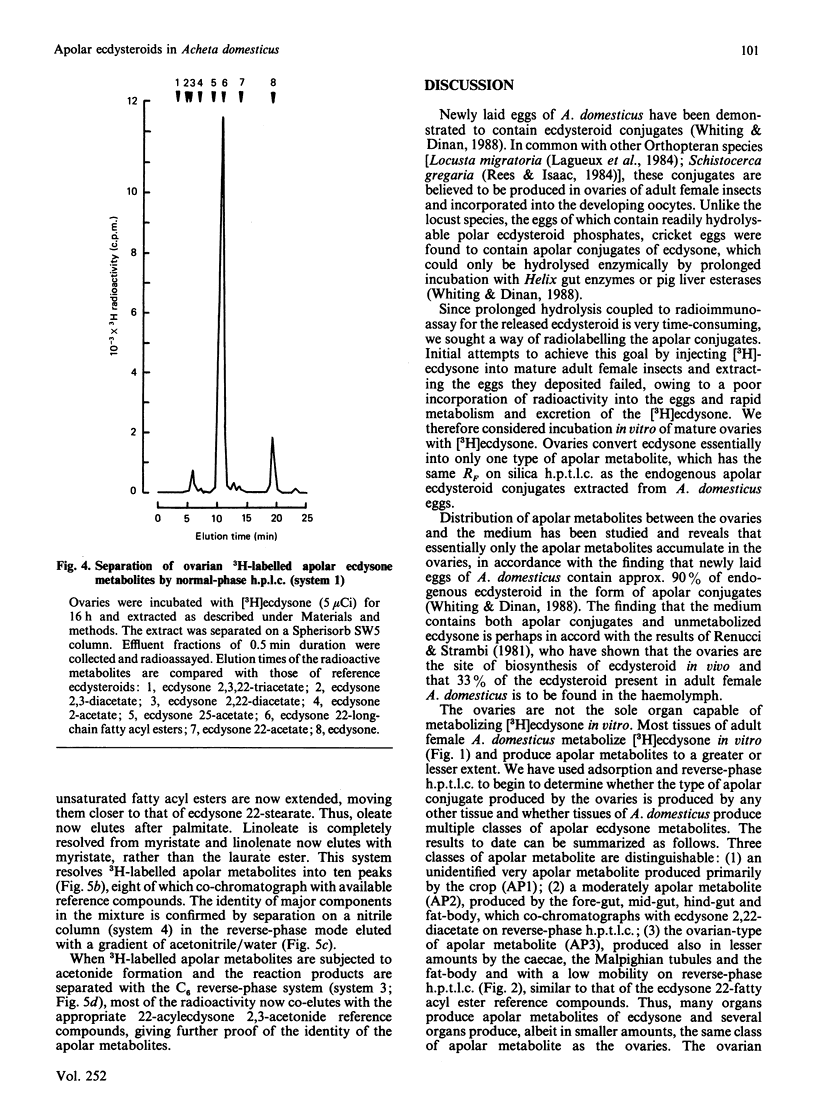
The newly laid eggs of the house cricket Acheta domesticus contain apolar ecdysteroid conjugates, which we have hypothesized to be ecdysone long-chain fatty acyl esters [Whiting & Dinan (1988) J. Insect Physiol., in the press]. The ovaries of mature adult female A. domesticus in vitro convert [3H]ecdysone into apolar conjugates identical with those found in newly laid eggs. Comparison of the radioactive metabolites produced on incubation of [3H]ecdysone with various organs of adult female A. domesticus in vitro indicate that the fat-body is the major producer of polar ecdysteroid metabolites at this stage of development, whereas the ovaries are the major site of production of apolar metabolites. Apolar metabolites are also produced to a lesser extent by the crop, gut sections and the fat-body. Hydrolysis of radioactive metabolites produced by the ovaries with Helix enzymes releases only [3H]ecdysone, and thus ecdysone is not metabolized before conjugation by the ovaries. Formation of chemical derivatives (acetonide and acetates) of these 3H-labelled apolar conjugates strongly indicates that the position of conjugation is through the hydroxy group at C-22 of ecdysone. Extensive chromatographic analysis of the 3H-labelled apolar metabolites produced by the ovaries by t.l.c. and h.p.l.c. and comparison with authenticated reference compounds have conclusively demonstrated that the conjugates consist of ecdysone esterified at C-22 to a mixture of common long-chain fatty acids. The major fatty acyl esters have been identified and their percentage contribution to the mixture determined: laurate (0.5%), myristate (2.8%), palmitate (25.8%), stearate (8.4%), arachidate (1.0%), oleate (15.7%), linoleate (38.8%) and linolenate (2.1%). In addition there are three minor unidentified peaks, one of which has been tentatively identified as ecdysone 22-palmitoleate (2.6%). Comparison of this percentage composition with the previously published fatty acid composition of A. domesticus haemolymph [Wang & Patton (1969) J. Insect Physiol. 15, 851-860] reveals remarkable similarities, indicating that the acyl transferase(s) forming the conjugates have a broad specificity with regard to the fatty acyl substrate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Connat J. L., Diehl P. A., Morici M. Metabolism of ecdysteroids during the vitellogenesis of the tick Ornithodoros moubata (Ixodoidea, Argasidae): accumulation of apolar metabolites in the eggs. Gen Comp Endocrinol. 1984 Oct;56(1):100–110. doi: 10.1016/0016-6480(84)90066-2. [DOI] [PubMed] [Google Scholar]
- Crosby T., Evershed R. P., Lewis D., Wigglesworth K. P., Rees H. H. Identification of ecdysone 22-long-chain fatty acyl esters in newly laid eggs of the cattle tick Boophilus microplus. Biochem J. 1986 Nov 15;240(1):131–138. doi: 10.1042/bj2400131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K. H., Bulenda D., Thiry E., Schmid E. Apolar ecdysteroid esters in adult female crickets, Gryllus bimaculatus. Life Sci. 1985 Jul 15;37(2):185–192. doi: 10.1016/0024-3205(85)90422-9. [DOI] [PubMed] [Google Scholar]
- Isaac R. E., Rose M. E., Rees H. H., Goodwin T. W. Identification of the 22-phosphate esters of ecdysone, 2-deoxyecdysone, 20-hydroxyecdysone and 2-deoxy-20-hydroxyecdysone from newly laid eggs of the desert locust, Schistocerca gregaria. Biochem J. 1983 Aug 1;213(2):533–541. doi: 10.1042/bj2130533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson P. Eighth Adolf Butenandt lecture. Why are so many hormones steroids? Hoppe Seylers Z Physiol Chem. 1983 Sep;364(9):1067–1087. [PubMed] [Google Scholar]


