Abstract
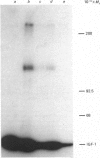
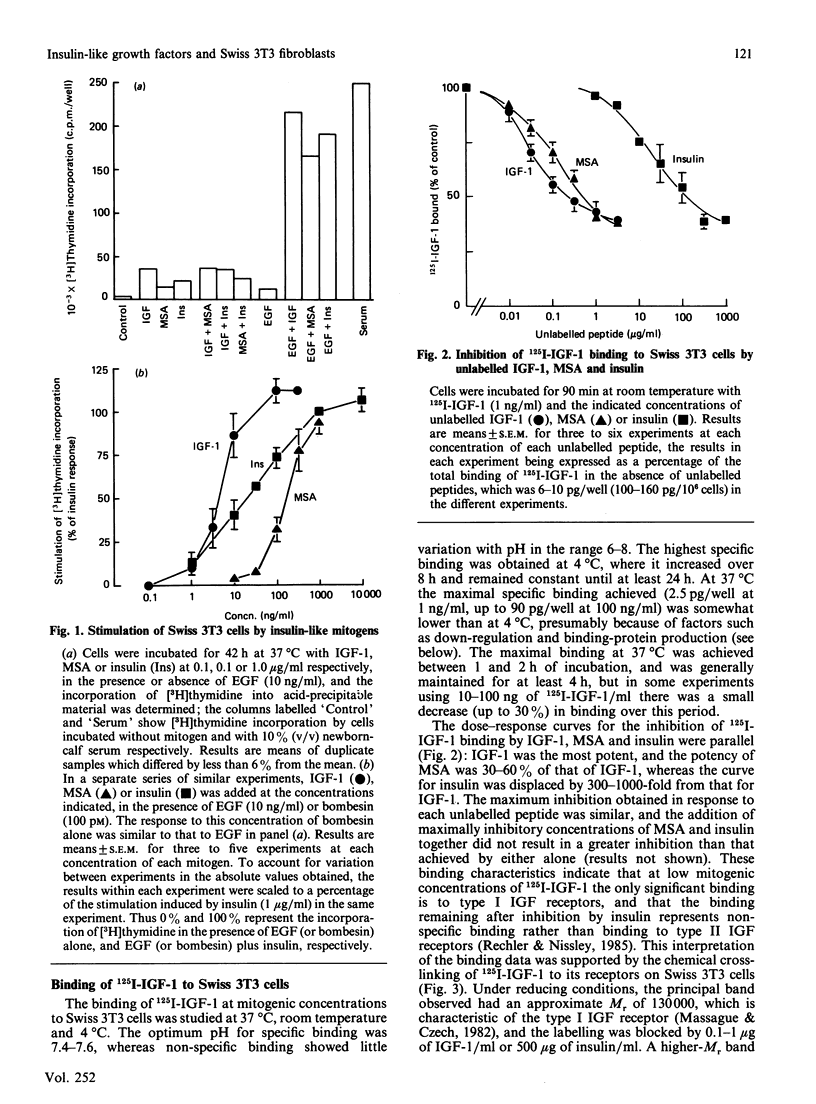
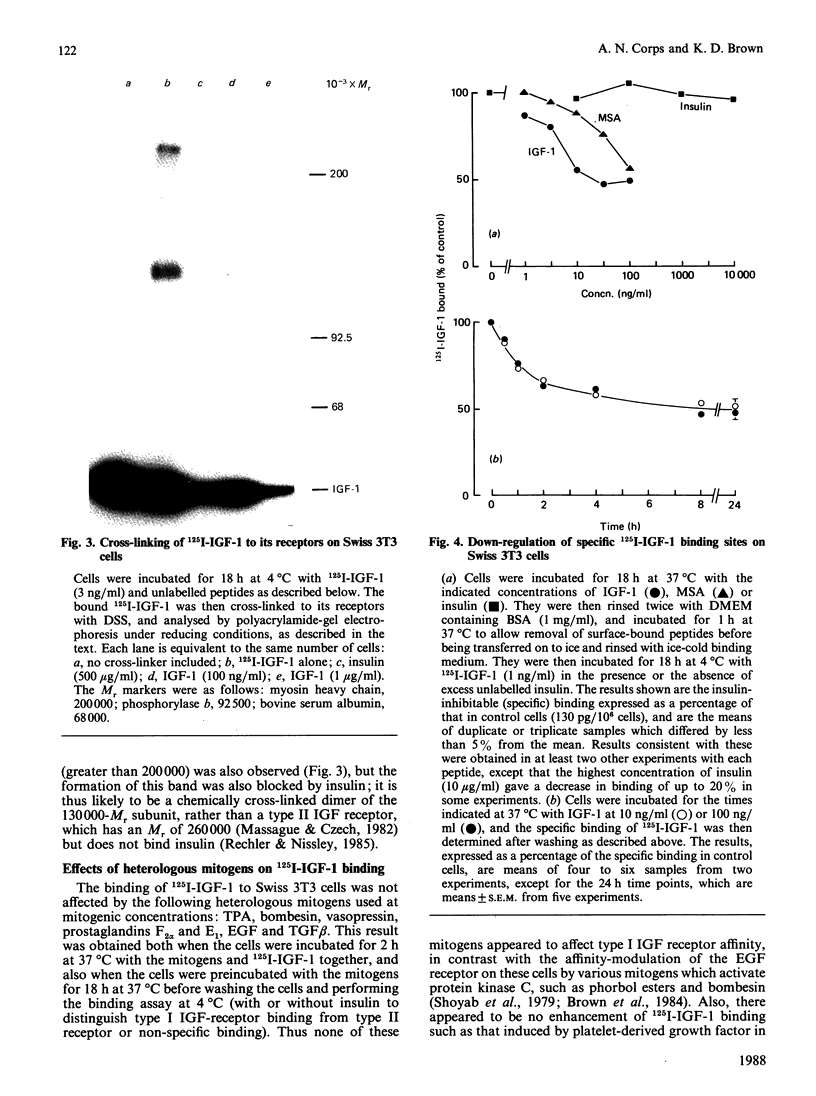
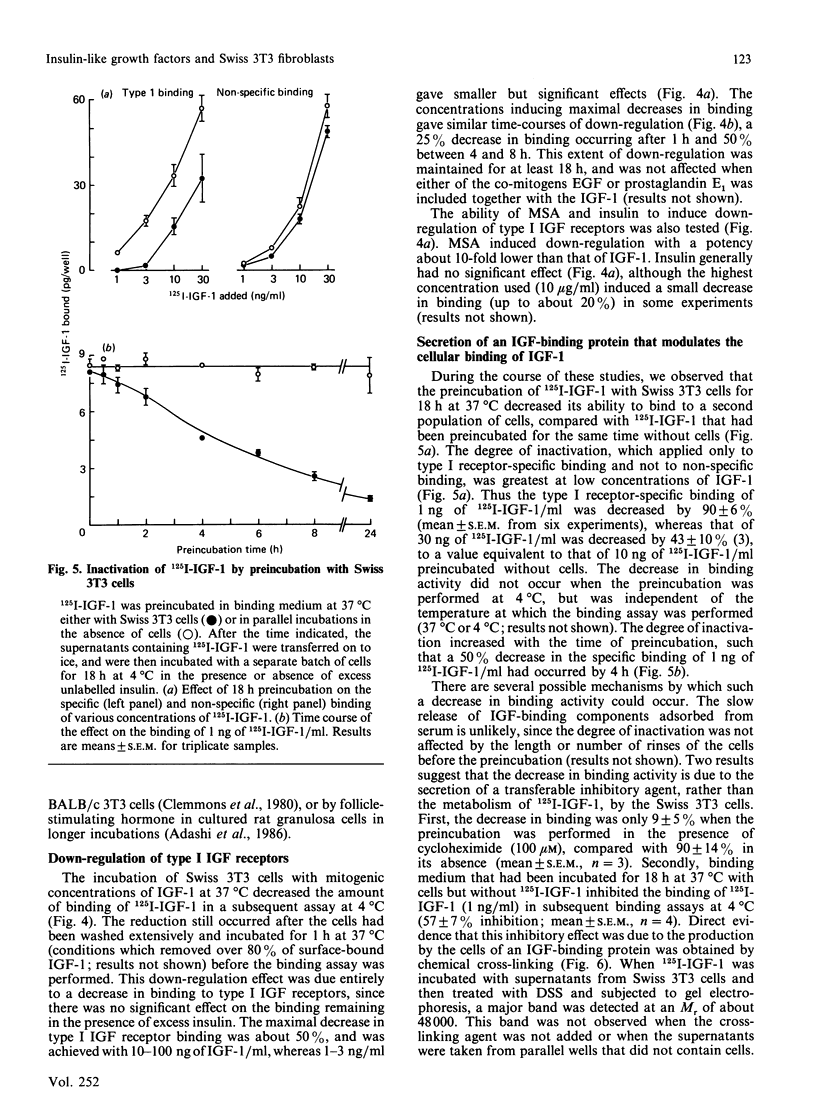
1. The binding of 125I-labelled insulin-like growth factor 1 (125I-IGF-1) to Swiss mouse 3T3 fibroblasts was time- and concentration-dependent. Unlabelled IGF-1 had a slightly higher potency than multiplication-stimulating activity (MSA) in inhibiting the binding of 125I-IGF-1, and insulin gave a parallel inhibition curve at 300-1000-fold lower potency. Chemical cross-linking of bound 125I-IGF-1 to its receptors, followed by polyacrylamide-gel electrophoresis under reducing conditions, revealed a major band of Mr 130,000, the labelling of which was inhibited by IGF-1 or high concentrations of insulin. 2. The binding of 125I-IGF-1 was not affected by either co-incubation or preincubation of the cells with a range of heterologous growth factors and mitogens. However, IGF-1 and MSA each induced down-regulation of 125I-IGF-1 binding sites. 3. The maximal stimulations of DNA synthesis induced by IGF-1, MSA and insulin, in the presence of a synergizing mitogen, were similar. The dose-response curve for insulin was not parallel to those for IGF-1 and MSA; in particular, low concentrations of insulin induced a greater stimulation than expected on the basis of its potency in the inhibition or down-regulation of 125I-IGF-1 binding. 4. The preincubation of 125I-IGF-1 with Swiss 3T3 cells at 37 degrees C decreased its ability to bind to a second batch of cells. This inactivation did not occur when the preincubation was performed at 4 degrees C or in the presence of cycloheximide. Chemical cross-linking revealed that the cells released an IGF-binding protein, giving a complex of Mr about 48,000. 5. It is concluded that type I IGF receptors mediate the stimulation of Swiss 3T3 cells by insulin-like mitogens, but that insulin probably stimulates the cells through insulin receptors. The cells can modulate the amount of ligand binding, both by down-regulation of the receptors and by the secretion of an IGF-binding protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adashi E. Y., Resnick C. E., Svoboda M. E., Van Wyk J. J. Follicle-stimulating hormone enhances somatomedin C binding to cultured rat granulosa cells. Evidence for cAMP dependence. J Biol Chem. 1986 Mar 25;261(9):3923–3926. [PubMed] [Google Scholar]
- Ballard F. J., Read L. C., Francis G. L., Bagley C. J., Wallace J. C. Binding properties and biological potencies of insulin-like growth factors in L6 myoblasts. Biochem J. 1986 Jan 1;233(1):223–230. doi: 10.1042/bj2330223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay J., Brown K. D. Functional receptors for epidermal growth factor in an epithelial-cell line derived from the rat small intestine. Biochem J. 1985 Jan 1;225(1):85–94. doi: 10.1042/bj2250085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blakeley D. M. Inhibition of the binding of 125I-labelled epidermal growth factor to mouse cells by a mitogen in goat mammary secretions. Biochem J. 1983 May 15;212(2):465–472. doi: 10.1042/bj2120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Blay J., Irvine R. F., Heslop J. P., Berridge M. J. Reduction of epidermal growth factor receptor affinity by heterologous ligands: evidence for a mechanism involving the breakdown of phosphoinositides and the activation of protein kinase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):377–384. doi: 10.1016/0006-291x(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Casella S. J., Han V. K., D'Ercole A. J., Svoboda M. E., Van Wyk J. J. Insulin-like growth factor II binding to the type I somatomedin receptor. Evidence for two high affinity binding sites. J Biol Chem. 1986 Jul 15;261(20):9268–9273. [PubMed] [Google Scholar]
- Clemmons D. R., Elgin R. G., Han V. K., Casella S. J., D'Ercole A. J., Van Wyk J. J. Cultured fibroblast monolayers secrete a protein that alters the cellular binding of somatomedin-C/insulinlike growth factor I. J Clin Invest. 1986 May;77(5):1548–1556. doi: 10.1172/JCI112470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J., Pledger W. J. Sequential addition of platelet factor and plasma to BALB/c 3T3 fibroblast cultures stimulates somatomedin-C binding early in cell cycle. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6644–6648. doi: 10.1073/pnas.77.11.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover C. A., Misra P., Hintz R. L., Rosenfeld R. G. Effect of an anti-insulin-like growth factor I receptor antibody on insulin-like growth factor II stimulation of DNA synthesis in human fibroblasts. Biochem Biophys Res Commun. 1986 Sep 14;139(2):501–508. doi: 10.1016/s0006-291x(86)80019-5. [DOI] [PubMed] [Google Scholar]
- Corps A. N., Rees L. H., Brown K. D. A peptide that inhibits the mitogenic stimulation of Swiss 3T3 cells by bombesin or vasopressin. Biochem J. 1985 Nov 1;231(3):781–784. doi: 10.1042/bj2310781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Usher P., Moses A. C. Monoclonal antibody to the type I insulin-like growth factor (IGF-I) receptor blocks IGF-I receptor-mediated DNA synthesis: clarification of the mitogenic mechanisms of IGF-I and insulin in human skin fibroblasts. Proc Natl Acad Sci U S A. 1986 Feb;83(3):664–668. doi: 10.1073/pnas.83.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froesch E. R., Schmid C., Schwander J., Zapf J. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- Gammeltoft S., Van Obberghen E. Protein kinase activity of the insulin receptor. Biochem J. 1986 Apr 1;235(1):1–11. doi: 10.1042/bj2350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Cordera R., Olefsky J. M. Substrate specificities of insulin and epidermal growth factor receptor kinases. Biochem Biophys Res Commun. 1985 Feb 28;127(1):254–263. doi: 10.1016/s0006-291x(85)80152-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- Pilch P. F., Czech M. P. The subunit structure of the high affinity insulin receptor. Evidence for a disulfide-linked receptor complex in fat cell and liver plasma membranes. J Biol Chem. 1980 Feb 25;255(4):1722–1731. [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Romanus J. A., Yang Y. W., Nissley S. P., Rechler M. M. Biosynthesis of the low molecular weight carrier protein for insulin-like growth factors in rat liver and fibroblasts. Endocrinology. 1987 Sep;121(3):1041–1050. doi: 10.1210/endo-121-3-1041. [DOI] [PubMed] [Google Scholar]
- Rosenfeld R. G., Dollar L. A. Characterization of the somatomedin-C/insulin-like growth factor I (SM-C/IGF-I) receptor on cultured human fibroblast monolayers: regulation of receptor concentrations by SM-C/IGF-I and insulin. J Clin Endocrinol Metab. 1982 Sep;55(3):434–440. doi: 10.1210/jcem-55-3-434. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Mendoza S. A. Synergistic signals in mitogenesis: role of ion fluxes, cyclic nucleotides and protein kinase C in Swiss 3T3 cells. J Cell Sci Suppl. 1985;3:229–242. doi: 10.1242/jcs.1985.supplement_3.20. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Van Wyk J. J., Graves D. C., Casella S. J., Jacobs S. Evidence from monoclonal antibody studies that insulin stimulates deoxyribonucleic acid synthesis through the type I somatomedin receptor. J Clin Endocrinol Metab. 1985 Oct;61(4):639–643. doi: 10.1210/jcem-61-4-639. [DOI] [PubMed] [Google Scholar]
- de Vroede M. A., Romanus J. A., Standaert M. L., Pollet R. J., Nissley S. P., Rechler M. M. Interaction of insulin-like growth factors with a nonfusing mouse muscle cell line: binding, action, and receptor down-regulation. Endocrinology. 1984 May;114(5):1917–1929. doi: 10.1210/endo-114-5-1917. [DOI] [PubMed] [Google Scholar]