Abstract
Background
Hybrid coronary revascularization (HCR) is a well-established technique for treating multi-vessel coronary disease. There remains a paucity of discussion assessing the efficacy of HCR with respect to the timing of the surgical component relative to that of the percutaneous coronary intervention (PCI).
Methods
A retrospective review was undertaken of our prospectively collected database from January 2009 to December 2019. Of 395 HCR patients analyzed, we examined the outcomes of 109 pairs of propensity-matched patients who either underwent robotic-assisted minimally-invasive direct coronary artery bypass (MIDCAB) first, or who had PCI prior to surgery.
Results
Thirty-day mortality was 0.25% (1 death) for the entire cohort. Mid-term survival for the total ‘MIDCAB-first’ group was 94.1% (17 deaths), not significantly different to that for the ‘PCI-first’ cohort (8 deaths, 92.7%), and this was also statistically comparable after propensity matching. Perioperative morbidity was not different between patient groups. Freedom from major adverse cardiac and cerebrovascular events (MACCE) and the incidence of repeat revascularization was similar between the two groups at up to 11-year follow-up. Elevated serum creatinine independently predicted increased MACCE for all patients, irrespective of the sequence of HCR revascularization employed.
Conclusions
In appropriately selected patients with multi-vessel coronary disease, HCR is associated with excellent short and longer-term results, irrespective of whether the MIDCAB or PCI procedure is performed first.
Keywords: Hybrid, left anterior descending (LAD), minimally-invasive direct coronary artery bypass (MIDCAB), minimally-invasive, percutaneous coronary intervention (PCI)
Introduction
Hybrid coronary revascularization (HCR) has emerged as a safe and viable option for patients with multi-vessel coronary artery disease (1). The 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guidelines state that HCR is a reasonable alternative to both coronary artery bypass grafting (CABG) and multi-vessel percutaneous coronary intervention (PCI) to improve the risk-benefit ratio of the procedures (2). The 2014 ESC/EACTS guidelines confirms that HCR is a useful option, particularly when multi-vessel PCI is deemed unsuitable, or the risk of traditional CABG is prohibitive (3). More recently, the 2018 ESC/EACTS guidelines affirm the benefits of an HCR strategy, stressing the importance of a collaborative multidisciplinary heart team approach (4). We have previously demonstrated the advantages of HCR compared with CABG, especially if complete revascularization can be achieved (5). Nevertheless, an analysis from the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database demonstrated that hybrid revascularization represented only 0.48% of all CABG volume in the United States between 2011 and 2013 (6). HCR encompasses minimally-invasive direct coronary artery bypass (MIDCAB) grafting of the left anterior descending (LAD) with an in-situ left internal mammary artery (LIMA), usually robotic-assisted, coupled with PCI to the other coronary territories. Mostly performed in a staged fashion, there remains a paucity of evidence assessing the optimal execution of an HCR strategy with specific reference to the timing of the MIDCAB component, relative to the PCI. We sought to evaluate the outcomes of patients who underwent HCR, analyzing our contemporary practice to discern any potential impacts of undertaking either the MIDCAB or the PCI elements first.
Methods
Study population
A retrospective review was undertaken of all patients who underwent revascularization for multi-vessel coronary disease between January 2009 and May 2023. Patients undergoing HCR between January 2009 and December 2019 are included in the full review described below, those patients undergoing HCR between January 2020 and May 2023 underwent analysis of perioperative and short-term outcomes only. Our study protocol was approved by the Northwell Health Institutional Review Board (approval number #16-835, November 29, 2016; requirement for patient consent was waived). We compared procedural data and short- and longer-term outcomes between those patients who underwent LIMA-LAD revascularization via MIDCAB followed by PCI (‘MIDCAB-first’), and those who had PCI prior to surgery (‘PCI-first’), both before and after propensity-matching. The indications for HCR were the presence of significant disease in the LAD with a suitable distal target vessel for bypass grafting and at least one other vessel that was amenable to PCI. Relative contraindications to HCR were the need for emergency revascularization and severe pulmonary disease prohibiting single-lung ventilation. Some investigators have tended not to offer HCR to more obese patients, as MIDCAB is more challenging in these individuals and has been associated with longer operative times, however, obesity was not prohibitive as our experience with MIDCAB in this sub-group has been positive (7,8). Patients unable to tolerate dual anti-platelet therapy after PCI were also not considered suitable for HCR.
Patients were excluded from analysis if they required any concomitant non-coronary surgery, if they had prior cardiothoracic surgery, if they were unstable, and if emergency intervention was required. Patients undergoing HCR from January 2020–May 2023 were not propensity-matched given limitations in data acquisition and limited long-term outcomes.
Surgical and interventional techniques
Our technique for robotic-assisted MIDCAB has been described previously (9). The Da Vinci Intuitive robot system (Intuitive Surgical Inc., Sunnyvale, CA, USA) is used to mobilize the LIMA. The LIMA-LAD anastomosis is completed off-pump, through an anterior muscle-sparing mini-thoracotomy, facilitated by a low-profile compression myocardial stabilizer. An intra-coronary shunt is utilized. Interrogation of all completed LIMA-LAD grafts is undertaken using transit-time flow probes (Medistim USA Inc., Plymouth, MN, USA). The PCI component was performed using standard catheter techniques. Most patients received drug-eluting stents (DES). We did not consider ‘complex PCI’ to be a contraindication to HCR.
Data analysis
Patient characteristics, perioperative variables, and post-procedural outcomes were obtained from the New York State Cardiac Surgery Reporting System (https://www.health.ny.gov/forms/cardiac_surgery) and the STS Adult Cardiac Surgery Database (http://www.sts.org/registries-research-center/sts-national-database/adult-cardiac-surgery-database/data-collection). Study data was collected and managed using REDCap (Research Electronic Data Capture) software hosted at Lenox Hill Hospital.
Observed covariates at baseline included age, gender, body mass index, diabetes mellitus, cerebrovascular disease, peripheral artery disease, chronic obstructive pulmonary disease, pre-procedural creatinine, dialysis-dependent renal failure, prior myocardial infarction, heart failure, and left ventricular ejection fraction. Variables identified by step-wise regression to impact major adverse cardiac and cerebrovascular events (MACCE; defined as composite end-point of mortality, stroke, myocardial infarction, and repeat revascularization), and thus utilized for propensity matching, included gender, left ventricular ejection fraction, serum creatinine, heart failure, and chronic obstructive pulmonary disease. We calculated propensity scores using multivariable logistic regression for each patient. Patients who underwent MIDCAB first were matched with those who underwent PCI first in a 1:1 ratio through a nearest neighbor-matching algorithm with a caliper of 0.2 of the standard deviation (SD) of the logit of the propensity score. The area under the curve for the receiver operating characteristic (ROC) of the propensity model was 0.62. The matched sample included 218 patients, evenly distributed between ‘MIDCAB-first’ and ‘PCI-first’ groups.
Patient characteristics, procedural data, and clinical outcomes were compared using χ2, Fisher’s exact test, and/or Student’s t-test, as appropriate. A χ2 test was used for categorical variables where the expected value for each cell was 5 or higher; if this assumption was not met, then we used Fisher’s exact test. A P value of less than 0.05 was considered statistically significant. Longitudinal outcomes, including freedom from MACCE, was estimated using Kaplan-Meier analysis and Cox proportional hazard regression models. All statistical analysis, including those for matched patients, were performed with SPSS for Windows, version 26.0 (IBM Corporation, Armonk, NY, USA), SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and with R software, version 3.6.2 (https://www.r-project.org/).
All-cause mortality up to 11 years was obtained by querying the National Death Index (NDI) to determine dates of death up to and including December 31, 2016. Additional information was accrued via interrogation of our health system electronic medical record (EMR), and of our prospectively-collated robotic surgical registry, up to December 31, 2019. Patients were also contacted directly, and, with their permission, other physicians involved in their care were called as well, so as not to miss any repeat LAD revascularization procedures that may have been performed at other institutions. Mean ± SD follow-up was 9.25±0.15 years and was 100% complete.
Results
Study population
From January 2009 through December 2019, 11,452 patients underwent revascularization for multi-vessel coronary disease. An HCR strategy was employed in 416 cases, of whom 395 met inclusion criteria for analysis, constituting our final study cohort. MIDCAB was performed prior to PCI in 286 cases (72.4%), as compared with 109 cases (27.6%) who underwent PCI first (Figure 1). Preoperative patient characteristics are summarized in Table 1.
Figure 1.

Patient flow diagram. HCR, hybrid coronary revascularization; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; MIDCAB, minimally-invasive direct coronary artery bypass.
Table 1. Patient demographics.
| Variable | All patients [2009–2019] | Propensity-matched patients [2009–2019] | Recent data [2020–2023] |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIDCAB-first (n=286) | PCI-first (n=109) | P value | SMD | MIDCAB-first (n=109) | PCI-first (n=109) | MIDCAB-first (n=96) | PCI-first (n=42) | P value | |||
| Age, years | 67.84±10.48 | 67.88±10.86 | 0.088 | 0.191 | 66.16±10.93 | 67.88±10.86 | 69.28±10.32 | 67.60±9.35 | 0.215 | ||
| Female gender | 68 (23.8) | 36 (33.0) | 0.082 | 0.206 | 35 (32.1) | 36 (33.0) | 19 (19.8) | 14 (33.3) | 0.128 | ||
| BMI, kg/m2 | 28.04±4.88 | 26.67±4.00 | 0.026 | 0.262 | 27.98±5.35 | 26.67±4.0 | 27.88±5.3 | 29.10±4.9 | 0.060 | ||
| Diabetes mellitus | 135 (47.2) | 56 (51.4) | 0.529 | 0.084 | 55 (50.5) | 56 (51.4) | 42 (43.8) | 21 (50.0) | 0.578 | ||
| Cerebrovascular disease | 13 (11.9) | 10 (9.2) | 0.659 | 0.090 | 23 (21.1) | 10 (9.2) | 3 (7.1) | 8 (8.3) | >0.999 | ||
| Peripheral arterial disease | 29 (10.1) | 17 (15.6) | 0.182 | 0.163 | 13 (11.9) | 17 (15.6) | 8 (8.3) | 4 (9.5) | 0.756 | ||
| Chronic obstructive pulmonary disease | 48 (16.8) | 19 (17.4) | 0.997 | 0.017 | 20 (18.3) | 19 (17.4) | 25 (26.1) | 11 (26.1) | 0.342 | ||
| Pre-procedural creatinine, mg/dL | 1.16±1.14 | 1.42±1.98 | 0.106 | 0.160 | 1.33±1.44 | 1.42±1.98 | 1.01±0.51 | 0.98±0.22 | 0.147 | ||
| Dialysis-dependent renal failure | 10 (3.5) | 4 (3.7) | >0.999 | 0.009 | 7 (6.4) | 4 (3.7) | 4 (4.2) | 0 | 0.314 | ||
| Previous myocardial infarction | 86 (30.1) | 42 (38.5) | 0.137 | 0.179 | 36 (33.0) | 42 (38.5) | 12 (12.5) | 16 (38.1) | 0.001 | ||
| Congestive heart failure (NYHA III/IV) | 34 (11.9) | 24 (22.0) | 0.017 | 0.272 | 21 (19.3) | 24 (22.0) | 13 (13.5) | 8 (19.0) | 0.444 | ||
| Left ventricular ejection fraction, % | 54.23±10.93 | 53.79±10.74 | 0.716 | 0.041 | 53.92±10.64 | 53.79±10.74 | 58.33±7.22 | 54.64±12.98 | 0.343 | ||
| STS PROM, % | 1.42±1.85 | 8.16±2.03 | 0.076 | 0.236 | 1.71±2.01 | 8.16±2.03 | 7.70±4.48 | 10.47±7.44 | 0.172 | ||
Values are expressed as n (%) or mean ± standard deviation. P values are within each group; propensity matched patients excluded as no category was statistically different between groups. MIDCAB, minimally-invasive direct coronary artery bypass; PCI, percutaneous coronary intervention; SMD, standardized mean difference; BMI, body mass index; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; PROM, predicted risk of mortality.
Operative and procedural data
The overall duration of the MIDCAB was comparable between both patient groups, before and after propensity-matching (Table 2). Significantly more patients in the matched PCI-first group had a stent to their right coronary artery (RCA) than those who had MIDCAB first (75.2% vs. 35.8%, P<0.001). PCI to the left main coronary artery (LMCA) was more prevalent in the matched ‘MIDCAB-first’ cohort (19.3% vs. 0.91%, P<0.001).
Table 2. Interventional and operative data.
| Variable | All patients | Propensity-matched patients | |||||
|---|---|---|---|---|---|---|---|
| MIDCAB-first (n=286) | PCI-first (n=109) | P value | MIDCAB-first (n=109) | PCI-first (n=109) | P value | ||
| MIDCAB surgical times | |||||||
| Total operative time, minutes | 154.53±37.83 | 148.82±46.63 | 0.287 | 156.13±35.24 | 148.82±46.63 | 0.269 | |
| Total robotic time, minutes | 48.3±10.31 | 46.27±15.52 | 0.307 | 49.65±15.44 | 46.27±15.52 | 0.215 | |
| LIMA mobilization time, minutes | 33.95±9.21 | 33.67±12.17 | 0.897 | 36.51±15.62 | 34.7±10.9 | 0.387 | |
| Non-LAD vessels diseased | |||||||
| Left main coronary artery | 56 (19.6) | 1 (0.91) | <0.001 | 21 (19.3) | 1 (0.91) | <0.001 | |
| Circumflex coronary artery | 142 (49.7) | 42 (38.5) | 0.055 | 58 (53.2) | 42 (38.5) | 0.041 | |
| Right coronary artery | 113 (39.5) | 82 (75.2) | <0.001 | 39 (35.8) | 82 (75.2) | <0.001 | |
| Stents used for PCI to non-LAD vessels | |||||||
| Bare metal | 8 (2.8) | 8 (7.3) | 0.049 | 2 (1.8) | 8 (7.3) | 0.101 | |
| Paclitaxel-eluting | 14 (4.9) | 3 (2.8) | 0.420 | 6 (5.5) | 3 (2.8) | 0.499 | |
| Sirolimus-eluting | 2 (0.7) | 0 | 0.247 | 1 (0.9) | 0 | >0.999 | |
| Everolimus-eluting | 114 (39.9) | 46 (42.2) | 0.731 | 35 (32.1) | 46 (42.2) | 0.161 | |
| Zotarolimus-eluting | 162 (56.6) | 74 (67.9) | 0.880 | 71 (65.1) | 74 (67.9) | 0.774 | |
| Bio-absorbable (everolimus-eluting) | 12 (4.2) | 3 (2.8) | 0.769 | 1 (0.91) | 3 (2.8) | 0.622 | |
Values are expressed as n (%) or mean ± standard deviation. MIDCAB, minimally-invasive direct coronary artery bypass; PCI, percutaneous coronary intervention; LIMA, left internal mammary artery; LAD, left anterior descending (coronary artery).
Clinical and angiographic outcomes
Thirty-day mortality for the matched cohort was 0.25% (1). Stroke was also infrequent at 0.25% (1). There was a trend towards a greater transfusion requirement (10.1% vs. 7.3%) and a higher incidence of reoperation for bleeding in the matched ‘PCI-first’ group (5.5% vs. 1.8%). Overall, 11-year mortality was 6.3% (n=25), not significantly different between groups. After matching, all late deaths occurred with PCI first. Incidence of MACCE and repeat revascularization was comparable. Clinical outcomes are outlined in Table 3 and time-related freedom from MACCE is depicted in Figures 2,3. In total, 96.2% (n=275) of patients who had MIDCAB first underwent LIMA angiography at PCI, which identified three grafts (1.1%) as having a significant stenosis at the LIMA-LAD anastomosis. All were managed with ballooning with excellent results. One patient (0.36%) had a focal stenosis in the mid-portion of the LIMA graft that was rectified. Two patients (0.73%) had atretic or occluded LIMA grafts that were unable to be salvaged and underwent PCI of their native LAD. Angiographically-proven patency of the LIMA-LAD graft (Fitzgibbon A), when assessed during the interval PCI procedure, was 269/275 (97.8%). LIMA graft was not selectively engaged in 11 patients; in these cases, patency was inferred from competitive flow in the native LAD.
Table 3. Clinical outcomes.
| Variable | All patients | Propensity-matched patients | |||||
|---|---|---|---|---|---|---|---|
| MIDCAB-first (n=286) | PCI-first (n=109) | P value | MIDCAB-first (n=109) | PCI-first (n=109) | P value | ||
| Short-term outcomes | |||||||
| 30-day mortality | 1 (0.3) | 0 | >0.999 | 0 | 0 | >0.999 | |
| Stroke | 0 | 1 (0.9) | >0.999 | 0 | 1 (0.9) | >0.999 | |
| Postoperative chest tube output, mL | 648.48±812.70 | 494.89±371.12 | 0.223 | 693.21±999.73 | 494.89±371.12 | 0.209 | |
| Transfusion required | 19 (6.6) | 11 (10.1) | 0.288 | 8 (7.3) | 11 (10.1) | 0.632 | |
| Reoperation for bleeding | 10 (3.5) | 6 (5.5) | 0.395 | 2 (1.8) | 6 (5.5) | 0.280 | |
| Perioperative myocardial infarction | 0 | 0 | >0.999 | 0 | 0 | >0.999 | |
| New dialysis-dependent renal failure | 1 (0.3) | 1 (0.9) | >0.999 | 1 (0.9) | 1 (0.9) | >0.999 | |
| Postoperative length of stay, days | 5.25±6.23 | 5.21±3.86 | 0.949 | 5.15±3.08 | 5.21±3.86 | 0.892 | |
| Postoperative length of stay, days | 4 (3 to 6) | 4 (3 to 5) | – | 5 (4 to 6) | 4 (3 to 5) | – | |
| Mid to longer-term outcomes | |||||||
| Mortality | 17 (5.9) | 8 (7.3) | 0.781 | 0 | 8 (7.3) | 0.567 | |
| Stroke | 2 (0.7) | 2 (1.8) | 0.656 | 1 (0.9) | 2 (1.8) | >0.999 | |
| Myocardial infarction | 4 (1.4) | 1 (0.9) | >0.999 | 0 | 1 (0.9) | >0.999 | |
| Repeat revascularization procedure | 41 (14.3) | 16 (14.7) | >0.999 | 14 (12.8) | 16 (14.7) | 0.844 | |
| MACCE | 57 (19.9) | 22 (20.2) | >0.999 | 19 (17.4) | 22 (20.2) | 0.729 | |
Values are expressed as n (%), mean ± standard deviation, or median (IQR). MIDCAB, minimally-invasive direct coronary artery bypass; PCI, percutaneous coronary intervention; MACCE, major adverse cardiovascular and cerebrovascular event; IQR, interquartile range.
Figure 2.
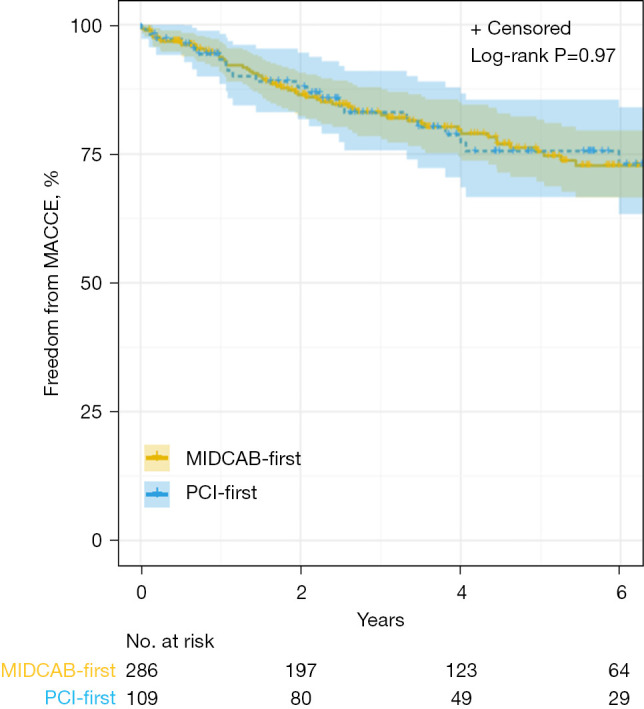
Kaplan-Meier estimates for freedom from MACCE with number of subjects at risk and 95% Hall-Wellner bands (all patients). MACCE, major adverse cardiac and cerebrovascular events; MIDCAB, minimally-invasive direct coronary artery bypass; PCI, percutaneous coronary intervention.
Figure 3.
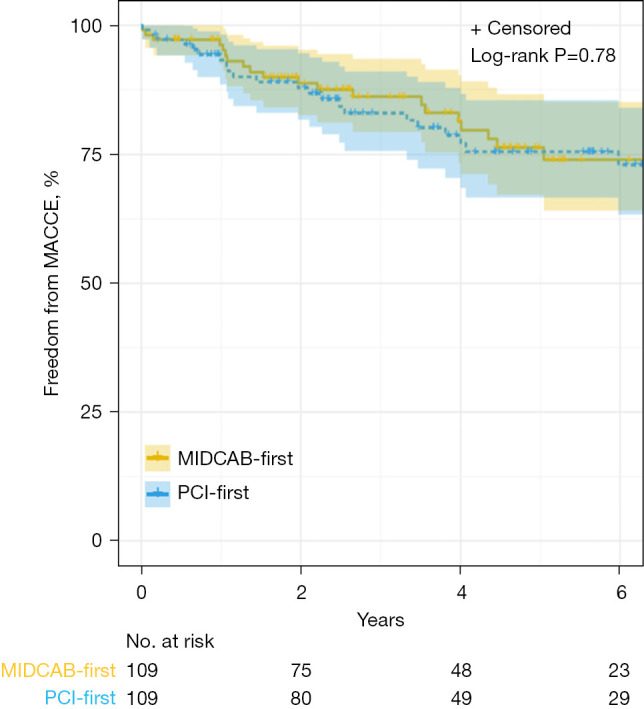
Kaplan-Meier estimates for freedom from MACCE with number of subjects at risk and 95% Hall-Wellner bands (propensity-matched patients). MACCE, major adverse cardiac and cerebrovascular events; MIDCAB, minimally-invasive direct coronary artery bypass; PCI, percutaneous coronary intervention.
Predictors of MACCE
Elevated serum creatinine was independently associated with a greater incidence of MACCE before and after matching in both cohorts (Table 4).
Table 4. Predictors of MACCE, multivariate analysis.
| Variable | All patients | Propensity-matched patients | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| All patients | |||||||
| Female gender | 1.88 | 1.18–2.99 | 0.008 | 1.55 | 0.82–2.91 | 0.176 | |
| Chronic obstructive pulmonary disease | 0.37 | 0.09–1.54 | 0.17 | 0.72 | 0.17–3.04 | 0.652 | |
| Pre-procedural creatinine | 1.24 | 1.13–1.37 | <0.001 | 1.29 | 1.15–1.45 | <0.001 | |
| Congestive heart failure (NYHA III/IV) | 0.40 | 0.18–0.89 | 0.025 | 0.39 | 0.15–1.06 | 0.064 | |
| Left ventricular ejection fraction | 0.98 | 0.96–1.00 | 0.097 | 0.99 | 0.96–1.02 | 0.582 | |
| PCI-first strategy | 0.90 | 0.54–1.49 | 0.670 | 1.02 | 0.55–1.88 | 0.961 | |
| MIDCAB-first patients | |||||||
| Female gender | 2.23 | 1.30–3.82 | 0.004 | 1.81 | 0.73–4.50 | 0.201 | |
| Chronic obstructive pulmonary disease | 0.26 | 0.04–1.91 | 0.185 | 1.01 | 0.12–8.37 | 0.990 | |
| Pre-procedural creatinine | 1.26 | 1.10–1.44 | <0.001 | 1.48 | 1.23–1.80 | <0.001 | |
| Congestive heart failure (NYHA III/IV) | 0.26 | 0.07–0.97 | 0.044 | 0.25 | 0.03–2.21 | 0.210 | |
| Left ventricular ejection fraction | 0.98 | 0.96–1.01 | 0.198 | 1.02 | 0.97–1.08 | 0.467 | |
| PCI-first patients | |||||||
| Female gender | 1.29 | 0.52–3.17 | 0.583 | 1.29 | 0.52–3.17 | 0.583 | |
| Chronic obstructive pulmonary disease | 0.67 | 0.09–5.28 | 0.706 | 0.67 | 0.09–5.28 | 0.706 | |
| Pre-procedural creatinine | 1.19 | 1.03–1.38 | 0.015 | 1.19 | 1.03–1.38 | 0.015 | |
| Congestive heart failure (NYHA III/IV) | 0.75 | 0.25–2.28 | 0.609 | 0.75 | 0.25–2.28 | 0.609 | |
| Left ventricular ejection fraction | 0.97 | 0.93–1.01 | 0.174 | 0.97 | 0.93–1.01 | 0.174 | |
MACCE, major adverse cardiac and cerebrovascular events; HR, hazard ratio; CI, confidence interval; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; MIDCAB, minimally-invasive direct coronary artery bypass.
Recent data
A total of 138 patients undergoing revascularization between January 2020 and May 2023 were assessed for short-term and operative outcomes. The mean ± SD age was 69.28±10.32 years with 76% (n=105) being male. Comorbidities were similar to the earlier demographics as seen in Table 1. Patients received ‘MIDCAB first’ 70% (n=96) of the time with the remainder receiving ‘PCI first’. Within this subset of patients, the duration of operative MIDCAB was similar between groups at 170.8 minutes within ‘MIDCAB first’ and 172.3 minutes if ‘PCI first’. Thirty-day mortality was 0%, and no postoperative strokes were observed in either cohort. The average ± SD length of stay for the MIDCAB operation was 4.81±1.52 days for patients undergoing ‘MIDCAB first’ and 4.18±1.60 days for patients undergoing ‘PCI first’ (P=0.025). The remainder of short-term outcomes can be found in Table 5. Temporally, more HCR were performed in 2022 than in 2009 (5% vs. 8%) and a temporal trend can be found in Figure 4.
Table 5. Recent experience—operative and short-term outcomes.
| Values | MIDCAB-first (n=96) | PCI-first (n=42) | P value |
|---|---|---|---|
| Operative outcomes | |||
| Total operative time, minutes | 170.76±40.4 | 172.33±53.4 | 0.745 |
| Intraoperative mortality | 0 | 0 | – |
| Non-LAD vessels diseased | |||
| Left main coronary artery | 37 (38.5) | 9 (21.4) | 0.072 |
| Circumflex coronary artery | 61 (63.5) | 20 (47.6) | 0.144 |
| Right coronary artery | 61 (63.5) | 21 (50.0) | 0.083 |
| Short-term outcomes | |||
| 30-day mortality | 0 | 0 | |
| Stroke | 0 | 0 | |
| Transfusion required | 1 (1.0) | 1 (2.4) | 0.499 |
| Reoperation for bleeding | 1 (1.0) | 2 (4.8) | 0.220 |
| Perioperative myocardial infarction | 0 | 0 | – |
| New dialysis-dependent renal failure | 0 | 0 | – |
| Postoperative length of stay days | 4.81±1.52 | 4.18±1.60 | 0.025 |
Values are expressed as n (%) or mean ± standard deviation. MIDCAB, minimally-invasive direct coronary artery bypass; PCI, percutaneous coronary intervention; LAD, left anterior descending.
Figure 4.

Temporal trend of HCR performed throughout the study period. HCR, hybrid coronary revascularization.
Discussion
The advantages of robotic MIDCAB, both as a stand-alone procedure for patients with isolated LAD stenosis, as well as part of an HCR strategy for patients with multi-vessel disease continues to be affirmed in the literature. Excellent mid to longer-term patency of a robotic MIDCAB as well as low perioperative mortality and morbidity continues to be reported (10-12). Furthermore, MIDCAB has been previously demonstrated to have a lower reintervention rate when compared to PCI alone for LAD disease, albeit with similar short- and intermediate-term survival (13). When compared to traditional CABG, robotic MIDCAB and HCR have been associated with shorter intensive care and hospital lengths of stay, reduced transfusion, less postoperative pain, and faster functional recovery (14-19). Some reports have even suggested a long-term mortality advantage to HCR compared with traditional CABG (20). Despite this, a comprehensive discussion about the timing of individual components of HCR remains lacking. Most HCR procedures reported in the literature are performed in stages, not simultaneously. The 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery recommended the surgical component be completed first, an approach we favor and have adopted for most of our patients (21).
Importantly, the ‘MIDCAB-first’ approach allows the LIMA-LAD graft to be interrogated during the PCI procedure and affords an element of myocardial protection during the non-LAD PCI, particularly if these catheter-based interventions are higher-risk or involve the left main coronary artery. Undertaking the LIMA-LAD graft first also enables the surgery to be performed without concern for excess bleeding that may be associated with dual antiplatelet therapy. We previously described our satisfactory experience with MIDCAB in patients taking dual antiplatelet agents, however, these results were not necessarily echoed by others (22,23). We did note a trend towards increased transfusion requirements in the ‘PCI-first’ group, although this did not translate into a significantly higher incidence of reoperation for bleeding. We pursue a ‘PCI-first’ strategy primarily in patients who present with an acute coronary syndrome whereby the non-LAD lesion is deemed the culprit, or in those in whom the severity and significance of the non-LAD lesion is thought to be greater than the LAD itself. In these patients, MIDCAB is performed on continued dual antiplatelet therapy.
To our knowledge, this is one of the largest studies with the long-term reported follow-up, which directly assesses perioperative and longitudinal outcomes of contemporary HCR practice, with specific reference as to whether MIDCAB or PCI is undertaken first. Not only did we demonstrate comparable short-term results between the two groups, but mid-term survival and freedom from MACCE was also similar, both before and after propensity-matching. Choi and associates found no significant difference in outcomes between 12 patients who underwent MIDCAB first, as compared with 68 patients who had PCI first, at a mean follow-up of just over 2 years (24). Furthermore, Repossini and colleagues reported no differences in short-term outcomes or in 5-year MACCE-free survival when they compared 106 ‘MIDCAB-first’ patients with 60 ‘PCI-first’ cases (25).
A small number of centers have reported satisfactory results with the simultaneous execution of both the surgical and PCI components of HCR (26,27). Although this may appear to be an attractive option, we do not favor this approach. Concurrent MIDCAB and PCI necessitates the use of a hybrid operating suite, a luxury not available at all institutions. Performing both procedures concurrently can complicate the use of dual antiplatelet therapy as not all surgeons are comfortable administering a loading dose a potent P2Y12 antagonist upon completion of a robotic MIDCAB. Additionally, interrogating the LIMA-LAD graft by angiography minutes after it has been constructed is frequently not representative of its patency due to local edema and vasospasm; a fresh, immature anastomosis may indeed appear to be imperfect, only to be seen to be widely patent when re-imaged 4–6 weeks postoperatively.
Limitations
Our data is derived from a retrospective review in a single institution; a multi-center approach, with a larger sample size, would undoubtedly be more adequately powered to analyze further subtle differences between patient groups. Our patients were not randomized—the decision regarding technique to implement first was made by a multi-disciplinary team and consequently selection bias can therefore not be eliminated. Our institution has quite extensive experience with minimal-access robotic-assisted LIMA-LAD grafting, as well as with complex PCI techniques; our results may thus not be directly generalizable to other institutions who may be earlier in their learning curves. We utilized the NDI as our primary source for long-term survival data; this may somewhat underestimate true mortality (28). Lastly, our most recent data was limited to operative and short-term data and therefore not included in the propensity match with our older, more complete dataset.
Conclusions
Our results affirm that HCR is associated with excellent short and mid-term results, in appropriately selected patients, and these outcomes appear to be independent of whether the MIDCAB or PCI component is completed first. We found no significant difference in longer-term survival, freedom from MACCE, or in the need for repeat revascularization between patient groups. Elevated serum creatinine is independently predictive of MACCE, irrespective of the sequence of HCR revascularization. We suggest that, in those patients deemed suitable candidates for HCR, the decision as to whether to initially proceed with MIDCAB or to pursue non-LAD PCI be made by a multidisciplinary heart team. In all cases, complete revascularization should remain the goal.
Acknowledgments
The authors are grateful to Sridhar Uttara BS, Efstathia A. Mihelis MBA, and to Antonio J. Guirola, for their assistance in data collection and management, and in the overall preparation of this manuscript.
Funding: None.
Footnotes
Conflicts of Interest: N.C.P. MD is on the scientific advisory board of Vascular Graft Solutions. M.C.K. is a consultant for Boston Scientific, Cardiovascular Systems Inc., Heartflow, and Medtronic, Inc. V.P.S. is a consultant for Abbott, Boston Scientific, and Medtronic, Inc. The other authors have no conflicts of interest to declare.
References
- 1.Harskamp RE, Vassiliades TA, Mehta RH, et al. Comparative Effectiveness of Hybrid Coronary Revascularization vs Coronary Artery Bypass Grafting. J Am Coll Surg 2015;221:326-34.e1. 10.1016/j.jamcollsurg.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012;126:e354-471. 10.1161/CIR.0b013e318277d6a0 [DOI] [PubMed] [Google Scholar]
- 3.Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2014;46:517-92. 10.1093/ejcts/ezu366 [DOI] [PubMed] [Google Scholar]
- 4.Sousa-Uva M, Neumann FJ, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2019;55:4-90. 10.1093/ejcts/ezy289 [DOI] [PubMed] [Google Scholar]
- 5.Patel NC, Hemli JM, Kim MC, et al. Short- and intermediate-term outcomes of hybrid coronary revascularization for double-vessel disease. J Thorac Cardiovasc Surg 2018;156:1799-1807.e3. 10.1016/j.jtcvs.2018.04.078 [DOI] [PubMed] [Google Scholar]
- 6.Harskamp RE, Brennan JM, Xian Y, et al. Practice patterns and clinical outcomes after hybrid coronary revascularization in the United States: an analysis from the society of thoracic surgeons adult cardiac database. Circulation 2014;130:872-9. 10.1161/CIRCULATIONAHA.114.009479 [DOI] [PubMed] [Google Scholar]
- 7.Rosenblum JM, Harskamp RE, Hoedemaker N, et al. Hybrid coronary revascularization versus coronary artery bypass surgery with bilateral or single internal mammary artery grafts. J Thorac Cardiovasc Surg 2016;151:1081-9. 10.1016/j.jtcvs.2015.10.061 [DOI] [PubMed] [Google Scholar]
- 8.Hemli JM, Darla LS, Panetta CR, et al. Does body mass index affect outcomes in robotic-assisted coronary artery bypass procedures? Innovations (Phila) 2012;7:350-3. 10.1097/IMI.0b013e31827e1ea9 [DOI] [PubMed] [Google Scholar]
- 9.Hemli JM, Henn LW, Panetta CR, et al. Defining the learning curve for robotic-assisted endoscopic harvesting of the left internal mammary artery. Innovations (Phila) 2013;8:353-8. 10.1097/IMI.0000000000000017 [DOI] [PubMed] [Google Scholar]
- 10.Gaudino M, Bakaeen F, Davierwala P, et al. New Strategies for Surgical Myocardial Revascularization. Circulation 2018;138:2160-8. 10.1161/CIRCULATIONAHA.118.035956 [DOI] [PubMed] [Google Scholar]
- 11.Kitahara H, Nisivaco S, Balkhy HH. Graft Patency after Robotically Assisted Coronary Artery Bypass Surgery. Innovations (Phila) 2019;14:117-23. 10.1177/1556984519836896 [DOI] [PubMed] [Google Scholar]
- 12.Kofler M, Stastny L, Reinstadler SJ, et al. Robotic Versus Conventional Coronary Artery Bypass Grafting: Direct Comparison of Long-Term Clinical Outcome. Innovations (Phila) 2017;12:239-46. 10.1097/IMI.0000000000000393 [DOI] [PubMed] [Google Scholar]
- 13.Patel NC, Hemli JM, Seetharam K, et al. Minimally invasive coronary bypass versus percutaneous coronary intervention for isolated complex stenosis of the left anterior descending coronary artery. J Thorac Cardiovasc Surg 2022;163:1839-1846.e1. 10.1016/j.jtcvs.2020.04.171 [DOI] [PubMed] [Google Scholar]
- 14.Sardar P, Kundu A, Bischoff M, et al. Hybrid coronary revascularization versus coronary artery bypass grafting in patients with multivessel coronary artery disease: A meta-analysis. Catheter Cardiovasc Interv 2018;91:203-12. 10.1002/ccd.27098 [DOI] [PubMed] [Google Scholar]
- 15.Zhu P, Zhou P, Sun Y, et al. Hybrid coronary revascularization versus coronary artery bypass grafting for multivessel coronary artery disease: systematic review and meta-analysis. J Cardiothorac Surg 2015;10:63. 10.1186/s13019-015-0262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harskamp RE, Bagai A, Halkos ME, et al. Clinical outcomes after hybrid coronary revascularization versus coronary artery bypass surgery: a meta-analysis of 1,190 patients. Am Heart J 2014;167:585-92. 10.1016/j.ahj.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 17.Harskamp RE, Walker PF, Alexander JH, et al. Clinical outcomes of hybrid coronary revascularization versus coronary artery bypass surgery in patients with diabetes mellitus. Am Heart J 2014;168:471-8. 10.1016/j.ahj.2014.06.027 [DOI] [PubMed] [Google Scholar]
- 18.Halkos ME, Walker PF, Vassiliades TA, et al. Clinical and angiographic results after hybrid coronary revascularization. Ann Thorac Surg 2014;97:484-90. 10.1016/j.athoracsur.2013.08.041 [DOI] [PubMed] [Google Scholar]
- 19.Dixon LK, Akberali U, Di Tommaso E, et al. Hybrid coronary revascularization versus coronary artery bypass grafting for multivessel coronary artery disease: A systematic review and meta-analysis. Int J Cardiol 2022;359:20-7. 10.1016/j.ijcard.2022.04.030 [DOI] [PubMed] [Google Scholar]
- 20.Yu L, Zhu K, Du N, et al. Comparison of hybrid coronary revascularization versus coronary artery bypass grafting in patients with multivessel coronary artery disease: a meta-analysis. J Cardiothorac Surg 2022;17:147. 10.1186/s13019-022-01903-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2011;124:e652-735. 10.1161/CIR.0b013e31823c074e [DOI] [PubMed] [Google Scholar]
- 22.Hemli JM, Darla LS, Panetta CR, et al. Does dual antiplatelet therapy affect blood loss and transfusion requirements in robotic-assisted coronary artery surgery? Innovations (Phila) 2012;7:399-402. 10.1177/155698451200700605 [DOI] [PubMed] [Google Scholar]
- 23.Daniel WT, Liberman HA, Kilgo P, et al. The impact of clopidogrel therapy on postoperative bleeding after robotic-assisted coronary artery bypass surgery. Eur J Cardiothorac Surg 2014;46:e8-13. 10.1093/ejcts/ezu160 [DOI] [PubMed] [Google Scholar]
- 24.Choi HJ, Kang J, Song H, et al. Comparison of Coronary Artery Bypass Graft-First and Percutaneous Coronary Intervention-First Approaches for 2-Stage Hybrid Coronary Revascularization. Korean J Thorac Cardiovasc Surg 2017;50:247-54. 10.5090/kjtcs.2017.50.4.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Repossini A, Tespili M, Saino A, et al. Hybrid revascularization in multivessel coronary artery disease. Eur J Cardiothorac Surg 2013;44:288-93; discussion 293-4. 10.1093/ejcts/ezt016 [DOI] [PubMed] [Google Scholar]
- 26.Shen L, Hu S, Wang H, et al. One-stop hybrid coronary revascularization versus coronary artery bypass grafting and percutaneous coronary intervention for the treatment of multivessel coronary artery disease: 3-year follow-up results from a single institution. J Am Coll Cardiol 2013;61:2525-33. 10.1016/j.jacc.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 27.Adams C, Burns DJ, Chu MW, et al. Single-stage hybrid coronary revascularization with long-term follow-up. Eur J Cardiothorac Surg 2014;45:438-42; discussion 442-3. 10.1093/ejcts/ezt390 [DOI] [PubMed] [Google Scholar]
- 28.Peterss S, Charilaou P, Ziganshin BA, et al. Assessment of survival in retrospective studies: The Social Security Death Index is not adequate for estimation. J Thorac Cardiovasc Surg 2017;153:899-901. 10.1016/j.jtcvs.2016.09.014 [DOI] [PubMed] [Google Scholar]


